Abstract
We addressed the potential effects of changes in ambient temperature on the profiles of volatile emissions from flowers and tested whether warming could induce significant quantitative and qualitative changes in floral emissions, which would potentially interfere with plant-pollinator chemical communication. We measured the temperature responses of floral emissions of various common species of Mediterranean plants using dynamic headspace sampling and used GC-MS to identify and quantify the emitted terpenes. Floral emissions increased with temperature to an optimum and thereafter decreased. The responses to temperature modeled here predicted increases in the rates of floral terpene emission of 0.03-1.4-fold, depending on the species, in response to an increase of 1 °C in the mean global ambient temperature. Under the warmest projections that predict a maximum increase of 5 °C in the mean temperature of Mediterranean climates in the Northern Hemisphere by the end of the century, our models predicted increases in the rates of floral terpene emissions of 0.34-9.1-fold,depending on the species. The species with the lowest emission rates had the highest relative increases in floral terpene emissions with temperature increases of 1-5 °C. The response of floral emissions to temperature differed among species and among different compounds within the species. Warming not only increased the rates of total emissions, but also changed the ratios among compounds that constituted the floral scents, i.e. increased the signal for pollinators, but also importantly altered the signal fidelity and probability of identification by pollinators, especially for specialists with a strong reliance on species-specific floral blends.
Keywords: chemical communication, emission profiles, flower volatile emissions, flower physiology, global warming, monoterpenes, physicochemical properties, sesquiterpenes, temperature-response curve, volatility
Introduction
Plants use biogenic volatile organic compounds (BVOCs) to interact with both beneficial (pollinators, seed dispersers and carnivores) and detrimental (herbivores, parasites and competitors) organisms (Dudareva et al., 2006; Fineschi et al., 2013; Trowbridge & Stoy, 2013). Floral blends of volatiles constitute private communication channels between emitter plants and those animal receivers to which the volatiles are directed (Raguso, 2008). Constitutively emitted BVOCs become specific signatures that allow organisms to identify the plant species and the tissue from which the scents are emitted. BVOCs may serve, for example, to promote reproductive isolation among compatible, sympatric, closely related species by providing pollinators with distinguishable floral scents (Füssel et al., 2007). Plants present a diverse array of volatile compounds to attract pollinators to their flowers for assuring pollination (Knudsen et al., 2006), and pollinators use the scent trails of floral emissions to locate flowers (Cardé & Willis, 2008). Mixtures of floral BVOCs allow pollinators to identify the plant species emitting the scent and provide diverse information about the flowers, such as their developmental stage (Mactavish & Menary, 1997; Proffit et al., 2008; Goodrich & Raguso, 2009) and the availability and quality of their rewards (Howell & Alarcón, 2007; Wright et al., 2009). In many cases, floral chemical messages directed at pollinators contain specific mixtures of compounds with specific ratios of each emitted volatile (Raguso, 2008).
Environmental conditions can affect BVOC emissions from plants. In particular, temperature is an abiotic factor that strongly affects plant emissions (Peñuelas, 2008; Peñuelas & Staudt, 2010; Grote et al., 2013). Temperature can affect emissions in two ways: first, through its effects on the physicochemical properties of BVOCs, such as volatility, solubility and diffusivity; and second, by affecting various plant physiological traits that play a role in some of the phases of BVOC emission, e.g. biosynthesis of BVOCs, stomatal resistance or regulated processes of release (Niinemets et al., 2004). The effect of temperature on physicochemical properties is clearer than the effect on plant physiology, which depends on the species (Kesselmeier & Staudt, 1999), the effects of past and present stresses on the physiological state of a plant (Fortunati et al., 2008; Niinemets, 2010) and environmental conditions such as temperature and light that modify the rate of BVOC synthesis (Penuelas & Llusia, 2001; Niinemets et al., 2010a). Higher temperatures enhance the activities of enzymes involved in BVOC biosynthesis, reduce BVOC solubilities and increase BVOC volatilities (vapor pressure and partitioning to the gas phase) and diffusivities along cellular phases and thereby decrease the resistance of emission pathways, thus promoting an increase in the rates of emission (Niinemets et al., 2004; Harley, 2013). Different compounds have different chemical properties and volatilities, which affect the rate of release from internal tissues. Compounds with higher volatilities will be more rapidly released, while those with lower volatilities will need to accumulate in higher amounts in intratissular non-specific storage pools and reach higher internal concentrations to be released at similar rates (Niinemets et al., 2004; Noe et al., 2006).
Environmental conditions are changing globally due to human activities, and the main drivers of global change are likely to increase emissions of BVOCs by plants (Peñuelas & Staudt, 2010). The mean surface temperature in the Mediterranean Basin is projected to increase by approximately 1-5 °C by the end of the century relative to the period 1850-1900 (IPCC, 2013). A temperature increase of this magnitude will induce several effects on the physiology and physicochemistry of living organisms. The rate of the current warming will exceed the ability of most plant populations and species to migrate (Neilson et al., 2005), so they will not be able to move toward cooler areas to counteract the effects of global warming. Plants will thus inevitably be submitted to warmer temperatures that will cause various physiological changes and unavoidable derived effects on various functions.
The volatility of each compound has a compound-specific dependence on temperature (Llusia & Penuelas, 2000; Copolovici & Niinemets, 2005; Copolovici et al., 2005). Warming may therefore not only induce a general increase in BVOC emissions, it may also induce differential changes in the rates of compound emissions due to differences in the physicochemical properties of the compounds (Niinemets & Reichstein, 2002; Noe et al., 2006) and may therefore affect the ratios of the compounds in the floral blends (Niinemets & Reichstein, 2002). Staudt & Bertin (1998) observed significant changes in the relative composition of terpenes in the foliar emissions from Quercus ilex along a temperature gradient of 5-45 °C. Major changes in the emission profile were due to a stronger response of the acyclic monoterpenes cis- and trans-β-ocimene from 35 to 45 °C, compared to that of mono- and bicyclic monoterpenes that stabilized near 35 °C, and to the induction of sesquiterpene caryophyllene emissions (Staudt & Bertin, 1998). Induced emissions due to heat stress at extreme temperatures (Joó et al., 2011; Copolovici et al., 2012) may also induce qualitative changes in floral scents. All these changes in the amount and relative composition of plant emissions can affect the correct establishment of specific communication channels between plants and mutualists.
Changes in temperature and other accompanying factors associated with global change are thus expected to induce quantitative and qualitative changes in floral BVOC emissions (Peñuelas, 2008; Peñuelas & Staudt, 2010) that could affect plant-pollinator interactions in several ways (Farré-Armengol et al., 2013). Our goals were to assess the effects of warming on floral emissions and to test our hypothesis that increases in ambient temperature would induce quantitative and qualitative variations in floral terpene emissions. We also quantified these variations in seven widespread species of Mediterranean plants with differing flowering phenologies.
Materials and Methods
Measurement of temperature responses
Seven common Mediterranean species (Globularia alypum (L.) Greuter, Erica multiflora L., Q. ilex L., Dorycnium pentaphyllum Scop., Spartium junceum L., Sonchus tenerrimus L. and Dittrichia viscosa L.) growing in the field were selected for the experiments. The plants were chosen from various locations in the province of Barcelona (Catalonia, Spain). We chose the species taking into consideration their commonness and ecological representativeness. We chose species that flower at different seasons of the year: Globularia alypum and Erica multiflora flowered in winter, Quercus ilex and Dorycnium pentaphyllum in spring, Sparium junceum and Sonchus tenerrimus in summer, and Dittrichia viscosa from late summer to early autumn. Additionally, Quercus ilex was chosen as a model of a typical anemophilous species. The measurements were conducted at periods of peak flowering, except for D. viscosa that was tested both in late summer and again in early autumn at the end of the flowering period. The experimental setup for the winter-flowering species G. alypum and E. multiflora only allowed for the measurement of temperature responses to 30 °C. In all other cases, the temperature responses were measured over a temperature range of 15-40 °C, at intervals of 5 °C. We measured 3-6 replicate temperature responses per species, and the response of each replicate was measured from a different plant.
Samples were collected under field conditions using a dynamic headspace technique. We employed a portable infrared gas analyzer (IRGA) system (LC-Pro+, ADC BioScieic Ltd., Great Amwell) to create the required conditions of temperature and to provide a constant light intensity of 1000 μmol m-2 s-1 for the sample tissue and to record periodic measurements of variables of gas exchange. One or several attached flowers for each sample were enclosed in the chamber of the IRGA. We used either a broad leaf chamber (12 cm3) or a conifer leaf chamber (175 cm3), depending on the size of the flowers of each species (but always the same size of chamber for all samples from each species). We collected the samples of terpene emissions after setting the required quantum flux density and temperature and after an acclimation period of approximately 10 min or the time needed to reach a steady-state exchange of CO2 and H2O. The air exiting the leaf cuvette, with a mean flux of air of approximately 200-250 ml min-1, was directed through a Teflon tube to a tube filled with the adsorbents Tenax (50% vol.) and Carbotrap (50% vol.), which collected the terpenes emitted by the flower(s) over a period of 10-15 minutes. The same process was repeated with empty leaf cuvettes that served as blanks of the system. At least two blank samples were collected for each curve, one at the beginning of the emission samplings and another at the end. After each sampling sequence we collected the flower samples to dry and weigh them for emission rate calculations.
Terpene analyses
The terpene samples in the adsorbent tubes were thermally desorbed, and the samples were analyzed by gas cromatography-mass spectrometry (GC-MS; GC: 7890A, MS: 5975C inert mass spectrometric detector with Triple-Axis Detector, Agilent Technologies, Palo Alto, CA, USA). Samples were injected into a 30 m x 0.25 mm x 0.25 μm capillary column (HP-5MS, Agilent Technologies, Palo Alto, CA, USA). Helium flow was 1 ml min-1, and total run time was 26 min. After injection, the sample was maintained for 1 min. at 35 °C, the temperature was then increased at 15 °C min-1 to 150 °C and maintained for 5 min, then increased at 50 °C min-1 to 250 °C and maintained for 5 min and then increased at 30 °C min-1 to 280 °C and maintained for 5 min.
The terpenes were identified by comparing the retention times with standards (Fluka, Buchs, Switzerland) that had been injected into clean adsorbent tubes, and the fractionation mass spectra were compared with standard spectra and spectra in the Nist05a and wiley7n mass spectral libraries. Calibration curves for the common terpenes α-pinene, β-pinene, D-limonene, γ-terpinene, linalool and α-humulene were determined each day of the analysis. The terpene calibration curves (n=4 different terpene concentrations) were always highly significant (r2>0.99 for the relationship between the signal and the amount of compound injected). Terpene concentrations were determined from the calibration curves.
Statistical treatment
We used the lme function of the nlme package of the R software (Pinheiro et al., 2013) to analyze the changes in the relative percentage ratios of the terpene compounds along temperature gradients. We considered plant individuals as a random factor in the analysis.
The temperature-response curves of floral terpene emissions were fitted by local polynomial functions using the loess function of R (Cleveland et al., 1992; R Development Core Team, 2011). The fitted models were used to calculate the predicted emission rates of floral terpenes at the mean maximum temperature of the month of the flowering peak of the species at the sampling location (Tpeak). Thereafter, we used the fitted models to predict the emission rates at temperatures of 1, 2, 3, 4 and 5 °C above Tpeak to explore the potential changes in floral terpene emissions in response to the temperature increases projected for the coming decades by global circulation models (IPCC 2013).
Results
Total terpene emissions
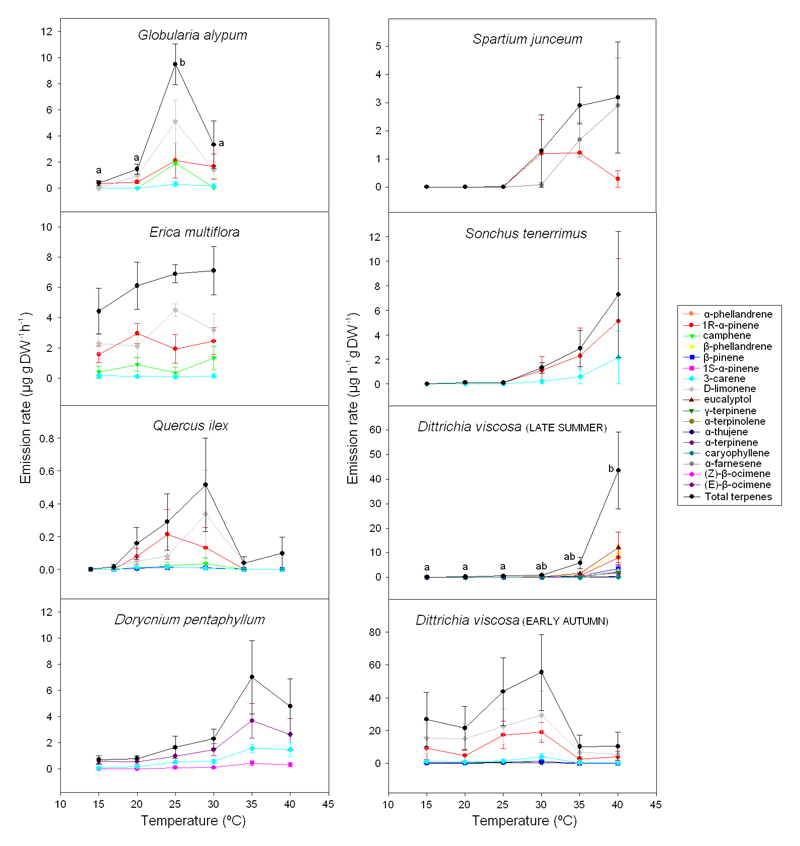
The rates of terpene emissions initially increased with temperature in all species to an optimum temperature and decreased in most species at higher temperatures (Figure 1). The temperature responses varied, depending on species and the spectrum of compounds emitted. The flowers of G. alypum and E. multiflora had maximum terpene emissions at 25-30 °C. Quercus ilex floral emission rates reached a maximum at approximately 30 °C. The rates of terpene emission from the flowers of D. pentaphyllum increased with temperature up to 35 °C and decreased slightly at 40 °C. The rates of terpene emission from the flowers of D. viscosa, S. junceum and S. tenerrimus increased with temperature even at the highest tested temperature of 40 °C, and the maximum increase was observed between temperatures of 30 to 40 °C. The measurements conducted on D. viscosa in early autumn, however, changed considerably compared with those in late summer. The emission maxima in early autumn occurred at 25-30 °C, while the maxima in late summer occurred at 40 °C or higher.
Figure 1.
Emission rates (μg g DW-1 h-1) of single and total terpenes from the flowers of seven Mediterranean species over a temperature gradient of 15-40 °C. The quantum flux density was maintained at 1000 μmol m-2 s-1 during the measurements. Error bars indicate SE (n=3-6). Letters indicate significant differences among the emission rates at different temperatures.
Relative terpene composition of floral scents
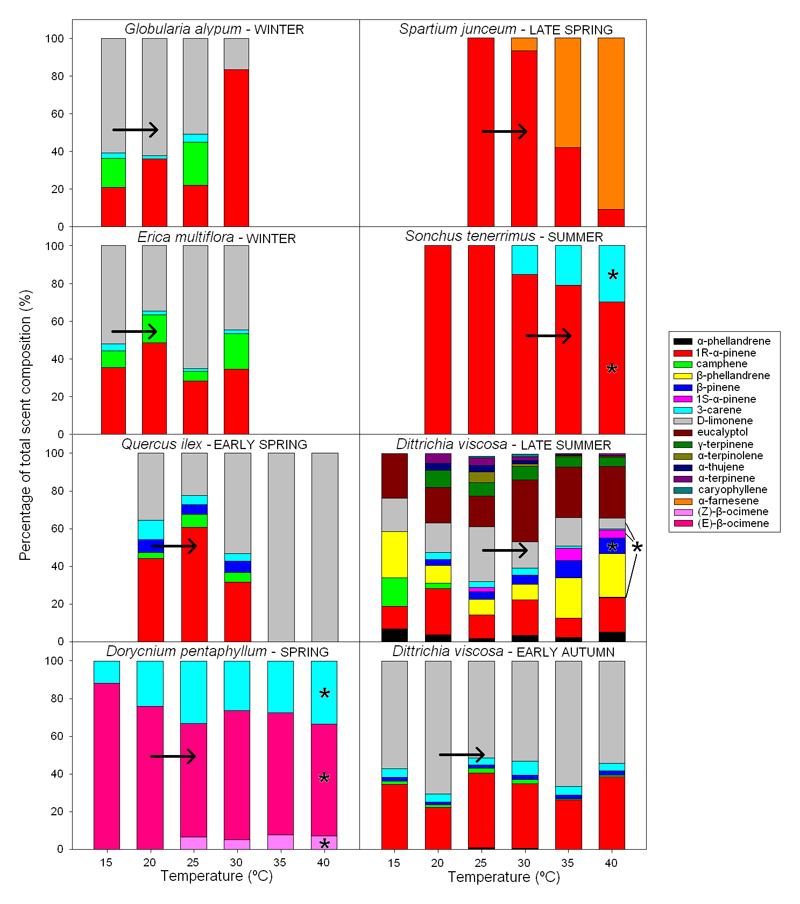
The relative composition of floral emissions varied with temperature (Figure 2). Only some compounds in some species, however, displayed significant trends in the relative abundance along temperature gradients. D-limonene, which was predominant at low temperatures, was partially substituted by1R-α-pinene at higher temperatures in the floral scent of G. alypum. The patterns of decrease and increase in the relative abundances of D-limonene and 1R-α-pinene, however, were not statistically significant. Terpene ratios in the floral scent of E. multiflora did not change significantly with temperature. The floral emissions from Q. ilex were entirely composed of D-limonene at high temperatures (35 and 40 °C) and contained other monoterpenes from 20 to 30 °C, but the percentages of each compound followed no significant trends. The relative composition of terpenes in the floral scent of D. pentaphyllum showed a gradual substitution of 3-carene, which experienced a reduction in its relative percentage (P<0.001) with increasing temperature, by the two isomers (E)- and (Z)-β-ocimene that increased their relative abundances (P<0.001 and P=0.001, respectively). In the floral scent of S. junceum, the monoterpene 1R-α-pinene was gradually substituted by the sesquiterpene α-farnesene as temperature increased, but the trends were not significant. The floral scent of S. tenerrimus was entirely composed at low temperatures of 1R-α-pinene, which decreased at higher temperatures (P=0.07) when levels of 3-carene increased (P=0.07).
Figure 2.
Ratios (%) of the rates of floral terpene emissions relative to the rates of total terpene emissions of each species over a temperature gradient of 15-40 °C. Arrows indicate the hypothetical change (assuming no acclimation of emission profiles) in the composition of floral scents when the mean maximum temperature of the flowering period of each species was increased by 5 °C, the maximum increase projected by IPCC (2013) by the end of the century.
The floral emissions from D. viscosa in late summer increased significantly with temperature and also changed in the relative composition of terpenes along the temperature gradient. β-pinene (P=0.004) and 1S-α-pinene (P=0.01) increased steadily in relative ratio, while D-limonene (P=0.07) and camphene (P=0.09) decreased. The relative composition of terpenes in the floral emissions from D. viscosa in early autumn did not vary significantly in the temperature-response curves, and the diversity of emitted volatiles was lower than in late summer. The emissions in early autumn particularly lacked eucalyptol and β-phellandrene, which were the most abundant terpenes in the floral scent of D. viscosa in late summer.
Predicted changes in total and relative floral emissions of terpenes with future warming
The magnitude of the stimulating effect of temperature on total emissions of floral terpenes varied among species. The modeled rates of floral terpene emission would increase 0.03-1.4-fold with a 1 °C increase in mean maximum temperature, depending on the species (Table 1). Under the warmest scenario projected by the IPCC (2013), which predicts a maximum increase of 5 °C in mean maximum temperatures for this century in the Mediterranean climates of the Northern Hemisphere, rates of floral terpene emissions would increase 0.34-9.1-fold. Under global warming ranging from +1 to +5 °C, S. junceum and Q. ilex would have the highest relative increases in the rates of floral terpene emissions (1.4-9.1- and 0.33-7-fold, respectively); G. alypum, D. pentaphyllum and S. tenerrimus would have moderate relative increases (0.1-2.55-, 0.18-1.02- and 0.34-2.22-fold, respectively) and D. viscosa in late summer and early autumn and E. multiflora would have smaller relative increases (0.10-0.69-, 0.12-0.68- and 0.03-0.34-fold, respectively).
Table 1.
Predicted values of floral terpene emission rates (μg g DW-1h-1) of the various species at the mean maximum temperature of the month of their flowering peaks (Tpeak) and at temperatures of 1, 2, 3, 4 and 5 °C higher than Tpeak. The values were predicted from the loess functions that fitted the measurements of floral terpene emissions at different temperatures.
| Species | Floral emission rates (μg g DW-1 h-1) | ||||||
|---|---|---|---|---|---|---|---|
| Tpeak | Mean max | +1 °C | +2 °C | +3 °C | +4 °C | +5 °C | |
| Globularia alypum | 14.3 | 0.4 | 0.44 | 0.66 | 0.94 | 1.23 | 1.42 |
| Erica multiflora | 14.3 | 4.41 | 4.54 | 4.94 | 5.31 | 5.63 | 5.92 |
| Quercus ilex | 18.5 | 0.03 | 0.04 | 0.08 | 0.13 | 0.19 | 0.24 |
| Dorycnium pentaphyllum | 20.5 | 0.84 | 0.99 | 1.18 | 1.38 | 1.56 | 1.7 |
| Spartium junceum | 26 | 0.1 | 0.24 | 0.4 | 0.59 | 0.79 | 1.01 |
| Sonchus tenerrimus | 29.9 | 1.02 | 1.37 | 1.76 | 2.20 | 2.71 | 3.28 |
| Dittrichia viscosa (late summer) | 23.5 | 0.51 | 0.56 | 0.58 | 0.65 | 0.75 | 0.86 |
| Dittrichia viscosa (early autumn) | 20.8 | 27.8 | 31.1 | 35 | 39.2 | 43 | 46.8 |
Discussion
Total terpene emissions
Our results confirm a generalized pattern of increase in the rates of floral terpene emissions with temperature, especially within the temperature range of 25-35 °C. The terpenes were emitted at low rates from the flowers of the anemophilous tree Q. ilex, as can be expected for a species that does not need to attract pollinators. We detected, however, some ubiquitous monoterpenes, whose emission rates reached a maximum at approximately 30 °C. These results support those from Hu et al. (2013) who found an increase in floral emissions from 10 to 30 °C, followed by a decrease at 40 °C. Our results are also similar to the well-characterized temperature response of BVOC emissions in leaves (Guenther et al., 1999; Penuelas & Llusia, 2001; Niinemets et al., 2010b; Peñuelas & Staudt, 2010).
The global pattern of increase in floral emissions with temperature may in part be due to the temperature dependencies of the physicochemical properties of terpenes and to the enzymatic activities of terpene synthases, all of which could enhance emissions with warming. Additionally, the physiology of flowers may affect the responses of floral emissions to temperature. In fact, the optimum temperatures for floral emissions varied among species even though these species shared most of the main compounds in their floral scents. These variations in the optimum temperatures among species, therefore, cannot be due to differences in the physicochemical properties of specific compounds and are also not driven by compound-specific optimal temperatures. These factors lead to the assumption that species-specific traits of floral physiology play an additional and important role in determining the responses of floral emissions to temperature. Floral physiology controls the production of each compound through the regulation of transcription, production and activity of enzymes and the concentrations of the substrates of these enzymes (Dudareva & Pichersky, 2000; van Schie et al., 2006). A broad array of terpene synthases are responsible for the formation of floral volatiles (Pichersky et al., 2006; Dudareva & Pichersky, 2008). Some of these synthases are highly specific, forming only one product, while others form multiple products (Dudareva et al., 1996, 2003; Nagegowda et al., 2008; Memari & Pazouki, 2013). In response to variable temperature conditions, floral physiology can thus modify biosynthetic activity to regulate the emission of each floral compound or of multiple compounds simultaneously, depending on synthase specificity.
Relative terpene compositions of floral scents
The magnitude of the changes to the relative composition of floral terpene blends driven by temperature also varied among species. The temperature-driven shifts observed in floral terpene composition (Figure 2) allow us to predict some compositional changes in the floral terpene blends in response to warming. The changes in relative floral terpene composition after increasing the temperature 5 °C were not significant (Figure 2), but they followed the significant trends of change over the entire temperature responses described in the previous section. For D. pentaphyllum (20-25 °C) and S. junceum (25-30 °C), additional compounds that are not emitted at the current mean maximum temperature of the flowering period are expected to be present in floral blends in warmer climates ((Z)-ß-ocimene and α-farnesene, respectively). The floral blend of G. alypum (15-20 °C) may not drastically change compositionally, except for the loss of camphene from the blend. The relative ratios of various compounds would change subtly in the floral terpene emissions from E. multiflora (15-20 °C), Q. ilex (20-25 °C), S. tenerrimus (30-35 °C) and D. viscosa (25-30 °C in late summer; 20-25 °C in early autumn). Relative increases in 1R-α-pinene over D-limonene are predicted for the floral emissions of E. multiflora and Q. ilex and the D. viscosa plants flowering in early autumn. A relative increase of 3-carene over 1R-α-pinene is predicted for the floral blend of S. tenerrimus. A relative increase of eucalyptol and 1R-α-pinene over α-terpinolene and D-limonene is predicted for the floral emissions of D. viscosa plants flowering in late summer. All these compositional changes are in agreement with the findings of Hu et al. (2013) showing that Lilium “siberia” plants emit different terpenes at different temperatures and also that the emission rates of different BVOC chemical groups (terpenes, aromatics, alkanes, aldehydes,…) show different temperature-response curves, leading to scent shifts not only in terpene composition but also in BVOC chemical groups.
Seasonal variation of the temperature response
We detected intraspecific seasonal changes in the temperature responses of total terpene emissions in D. viscosa, which was sampled in late summer and again in autumn. We also observed a reduction in the diversity of terpene signatures constituting the floral blend in autumn. Intraspecific seasonal differences in the responses of terpene emissions to temperature have also been observed in leaves (Llusia et al., 2006). These seasonal changes also point to physiology as a factor that not only determines the temperature response of floral emissions but also modulates the shape of this response at the intraspecific level, depending on the season. Such intraspecific variations demonstrate large temperature-driven plasticity of plant physiological traits and clearly emphasize the need to consider genotypic, epigenetic and phenotypic plasticity in estimating and modeling floral emissions.
Altered floral emissions in a warmer world and implications for pollinators
Projections of mean surface temperatures in the Mediterranean Basin predict an increase of approximately 1-5 °C relative to the period 1850-1900 by the end of the century (IPCC, 2013). If a direct projection is made from the temperature responses obtained here, the rates of floral terpene emission by the end of the century could increase 0.34-9.1-fold for a 5 °C increase in mean maximum temperature during the flowering peak of the species (Table 1). Such a broad range of variation in the magnitude of the increase in floral terpene emissions in response to global warming points to future significant differences among species in the intensity of floral olfactive signals. The species with the highest relative increases in floral terpene emissions were those with the lowest rates of emission, so we may expect the lightly scented flowers of these species to significantly increase the intensity of their olfactive signals, while increases in the signal intensity of strongly scented species will be low to moderate.
The relative composition of terpenes along temperature curves changed significantly in the floral blends of some species, especially at the highest temperature ranges and in those species that flower in the warmest seasons. Some of the observed changes were small, while some implied substitutions of the predominant compounds in the floral blend. The expected changes in the relative terpene composition of floral scents in response to an increase in temperature of 1-5 °C, which are likely to occur by the end of the century (IPCC, 2013), may imply changes to the composition of floral scents in some species (Figure 2). The changes in composition that we observed at higher temperatures included changes in the relative abundance of preexisting compounds, the appearance of new compounds or the disappearance of compounds that are emitted at current temperatures. Heat stress can cause variations in the composition of floral scents, such as the appearance or increase of some compounds only at high temperatures (Niinemets, 2010; Copolovici et al., 2012).
An increase in terpene emissions in response to the predicted warmer temperatures from global warming may extend the physical range of the signals that pollinators detect and follow (Peñuelas, 2008; Peñuelas & Staudt, 2010; Niinemets et al., 2013) but also implies the attraction of a broader group of pollinators with varying efficiencies of signal reception. Higher concentrations of floral scents may also increase the importance of the olfactory stimulus, thus leading to enhanced initial responses and learned performances of the pollinators (Katzenberger et al., 2013).
Increased floral emissions, however, may also have negative effects. A significant increase in the emission of floral terpenes, and in terpene emissions from other tissues that also respond positively to temperature increases, may imply higher metabolic costs and carbon consumption by secondary metabolic pathways that produce these compounds. The investment of carbon in terpene synthesis can account for up to 10% (Peñuelas & Llusià, 2003) or even 20% (Sharkey & Loreto, 1993) of the carbon fixed by photosynthesis, indicating that the cost to plants can be a significant fraction of total plant production. In addition to stimulating the biosynthesis of terpenes, higher temperatures can enhance photosynthesis, which may partially compensate for the relative carbon cost of terpene production in the absence of other limiting factors, such as drought. The positive effect of enhanced signals for pollinators combined with the negative effect of higher carbon costs of enhanced floral emissions would likely lead to changes in plant fitness.
Additionally, qualitative changes in floral scents such as those found here may potentially interfere with the chemical communication between plants and pollinators (Beyaert & Hilker, 2014). The effect of qualitative changes in floral scents on pollinators may depend on the learning capabilities and innate preferences of the pollinators (Cunningham et al., 2004; Schiestl & Johnson, 2013). Pollinators with a high capacity to learn the floral odors of the plant species in the community may be more plastic and would adapt better to qualitative changes in floral scents, while those that rely more on innate constitutive olfactive preferences, inherited through the coevolution of their sensory systems with the floral emissions of their host plants, may be affected more (Cunningham et al., 2004; Schiestl & Johnson, 2013). In effect, learning new signals could give insect pollinators the flexibility to visit species for which they do not have an innate attraction, and this capability could allow them to exploit a dynamic floral environment (Riffell et al., 2013). Moreover, olfactive learning could help pollinators to adapt to subtle differences in floral scents that occur within species, such as those caused by changing environmental conditions, and therefore to continue to identify the scents by their changed blends of volatiles. For pollinators that rely on olfactive learning, such as generalist social bees (Dötterl & Vereecken, 2010), changes in the ratios of floral emissions may thus not involve serious problems of identification, because these pollinators continuously learn the scents of the flowering species in the community and establish associations between their scents and the resources they offer. In contrast, specialist pollinators such as hawkmoths that visit nocturnally blooming flowers (Raguso et al., 2003; Riffell et al., 2008), or specialist bees visiting only one or a few host plant species (Filella et al., 2011), tend to rely to various degrees on innate preferences for the species-specific floral scents of the plants they visit, and these innate preferences may have a genetic basis that is much less dynamic than the acquired knowledge obtained by learning.
Concluding remarks and future perspectives
We demonstrated that temperature also has a general positive effect on terpene emissions that is well known in leaves. The relative increases calculated for floral terpene emissions indicate that very significant increases in the amount of floral scents will likely occur in a warmer world, although species can differ greatly in the rates of increase. We observed that the relative terpene ratios also vary with temperature, thus showing that temperature increase has the potential to induce qualitative changes in floral scent. We additionally observed seasonal changes in the effect of temperature on terpene emissions within a species. In summary, the amount of floral emissions may increase, with higher temperatures leading to enhanced olfactive signals for pollinators. The relative compositions of floral scents may also change to different degrees in different species, which could potentially interfere with the correct identification of flowers by pollinators.
The effect of temperature on foliar emissions has been extensively explored, but the effect on floral emissions has not, so further experiments to test the observed trends in other plant species are warranted. Parallel tests of pollinator responses for determining the effect of the observed changes in floral scent on the identification of flowers by pollinators are also warranted.
Acknowledgements
This research was supported by the Spanish government grants CGL2010-17172 and Consolider-Ingenio Montes CSD2008-00040, the Catalan government grant SGR2009-458, the European Research Council Synergy Grant (ERC-2013-SyG-610028 IMBALANCE-P) and Advanced Grant (322603, SIP-VOL+), the Estonian Ministry of Science and Education (institutional grant IUT-8-3) and the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation).
References
- Beyaert I, Hilker M. Plant odour plumes as mediators of plant-insect interactions. Biological reviews of the Cambridge Philosophical Society. 2014;89:68–81. doi: 10.1111/brv.12043. [DOI] [PubMed] [Google Scholar]
- Cardé RT, Willis MA. Navigational Strategies Used by Insects to Find Distant, Wind-Borne Sources of Odor. Journal of Chemical Ecology. 2008;34:854–866. doi: 10.1007/s10886-008-9484-5. [DOI] [PubMed] [Google Scholar]
- Cleveland WS, Grosse E, Shyu WM. Local regression models. In: Hastie JMC, TJ, editors. Statistical Models in S. Wadsworth edn. 1992. [Google Scholar]
- Copolovici LO, Niinemets U. Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere. 2005;61:1390–400. doi: 10.1016/j.chemosphere.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Filella I, Llusià J, Niinemets Ü, Peñuelas J. The Capacity for Thermal Protection of Photosynthetic Electron Transport Varies for Different Monoterpenes in Quercus ilex. Plant Physiology. 2005;139:485–496. doi: 10.1104/pp.105.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. Journal of plant physiology. 2012;169:664–72. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Moore CJ, Zalucki MP, West SA. Learning, odour preference and flower foraging in moths. doi: 10.1242/jeb.00733. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Moore CJ, Zalucki MP, West SA. Learning, odour preference and flower foraging in moths. The Journal of experimental biology. 2004;207:87–94. doi: 10.1242/jeb.00733. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Vereecken NJ. The chemical ecology and evolution of bee–flower interactions: a review and perspectives The present review is one in the special series of reviews on animal–plant interactions. Canadian Journal of Zoology. 2010;88:668–697. [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and Molecular Genetic Aspects of Floral Scents. Plant Physiology. 2000;122:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Metabolic engineering of plant volatiles. Current opinion in biotechnology. 2008;19:181–9. doi: 10.1016/j.copbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Cseke L, Blanc VM, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. The Plant cell. 1996;8:1137–48. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, et al. ( E ) - -Ocimene and Myrcene Synthase Genes of Floral Scent Biosynthesis in Snapdragon : Function and Expression of Three Terpene Synthase Genes of a New Terpene Synthase Subfamily. The Plant cell. 2003;15:1227–1241. doi: 10.1105/tpc.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Negre F, Nagegowda Da, Orlova I. Plant Volatiles: Recent Advances and Future Perspectives. Critical Reviews in Plant Sciences. 2006;25:417–440. [Google Scholar]
- Farré-Armengol G, Filella I, Llusia J, Peñuelas J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspectives in Plant Ecology, Evolution and Systematics. 2013;15:56–67. [Google Scholar]
- Filella I, Bosch J, Llusià J, Peñuelas A, Peñuelas J. Chemical cues involved in the attraction of the oligolectic bee Hoplitis adunca to its host plant Echium vulgare. Biochemical Systematics and Ecology. 2011;39:498–508. [Google Scholar]
- Fineschi S, Loreto F, Staudt M, Peñuelas J. Biology, controls and models of tree volatile organic compound emissions. Niinemets, edn. Springer; Berlin: 2013. Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives; pp. 1–20. [Google Scholar]
- Fortunati A, Barta C, Brilli F, Centritto M, Zimmer I, Schnitzler J-P, Loreto F. Isoprene emission is not temperature-dependent during and after severe drought-stress: a physiological and biochemical analysis. The Plant journal: for cell and molecular biology. 2008;55:687–697. doi: 10.1111/j.1365-313X.2008.03538.x. [DOI] [PubMed] [Google Scholar]
- Füssel U, Dötterl S, Jürgens A, Aas G. Inter- and Intraspecific Variation in Floral Scent in the Genus Salix and its Implication for Pollination. Journal of Chemical Ecology. 2007;33:749–765. doi: 10.1007/s10886-007-9257-6. [DOI] [PubMed] [Google Scholar]
- Goodrich KR, Raguso RA. The olfactory component of floral display in Asimina and Deeringothamnus (Annonaceae) New Phytologist. 2009;183:457–469. doi: 10.1111/j.1469-8137.2009.02868.x. [DOI] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer edn. Berlin: 2013. pp. 315–355. [Google Scholar]
- Guenther A, Baugh B, Brasseur G, et al. Isoprene emission estimates and uncertainties for the central African EXPRESSO study domain. Journal of Geophysical Research: Atmospheres. 1999;104:30625–30639. [Google Scholar]
- Harley PC. The roles of stomatal conductance and compound volatility in controlling the emission of volatile organic compounds from leaves. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer; Berlin: 2013. pp. 181–208. [Google Scholar]
- Howell AD, Alarcón R. Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Animal Behaviour. 2007;74:199–205. [Google Scholar]
- Hu Z, Zhang H, Leng P, Zhao J, Wang W, Wang S. The emission of floral scent from Lilium “siberia” in response to light intensity and temperature. Acta Physiologiae Plantarum. 2013;35:1691–1700. [Google Scholar]
- IPCC. Summary for Policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2013. [Google Scholar]
- Joó É, Dewulf J, Amelynck C, et al. Constitutive versus heat and biotic stress induced BVOC emissions in Pseudotsuga menziesii. Atmospheric Environment. 2011;45:3655–3662. [Google Scholar]
- Katzenberger TD, Lunau K, Junker RR. Salience of multimodal flower cues manipulates initial responses and facilitates learning performance of bumblebees. Behavioral Ecology and Sociobiology. 2013;67:1587–1599. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. Journal of Atmospheric Chemistry. 1999;33:23–88. [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. Diversity and Distribution of Floral Scent. The Botanical Review. 2006;72:1–120. [Google Scholar]
- Llusia J, Penuelas J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. American Journal of Botany. 2000;87:133–140. [PubMed] [Google Scholar]
- Llusia J, Penuelas J, Alessio Ga, Estiarte M. Seasonal contrasting changes of foliar concentrations of terpenes and other volatile organic compound in four dominant species of a Mediterranean shrubland submitted to a field experimental drought and warming. Physiologia Plantarum. 2006;127:632–649. [Google Scholar]
- Mactavish HS, Menary RC. The Effect of Flower Maturity and Harvest Timing on Floral Extract from Boronia megastigma (Nees) Annals of Botany. 1997;80:299–303. [Google Scholar]
- Memari HR, Pazouki L. In: Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Niinemets Ü, Monson RK, editors. Vol. 5 Springer Netherlands; Dordrecht: 2013. [Google Scholar]
- Nagegowda Da, Gutensohn M, Wilkerson CG, Dudareva N. Two nearly identical terpene synthases catalyze the formation of nerolidol and linalool in snapdragon flowers. The Plant journal. 2008;55:224–39. doi: 10.1111/j.1365-313X.2008.03496.x. [DOI] [PubMed] [Google Scholar]
- Neilson RF, Pitelka LF, Solomon AM, et al. Forecasting Regional to Global Plant Migration in Response to Climate Change. 2005;55:749–759. [Google Scholar]
- Niinemets Ü. Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends in Plant Science. 2010;15:145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Reichstein M. A model analysis of the effects of nonspecific monoterpenoid storage in leaf tissues on emission kinetics and composition in Mediterranean sclerophyllous Quercus species. Global Biogeochemical Cycles. 2002;16:57–1–57–26. [Google Scholar]
- Niinemets U, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in plant science. 2004;9:180–6. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Arneth A, Kuhn U, Monson RK, Penuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010a;7:2203–2223. [Google Scholar]
- Niinemets Ü, Monson RK, Arneth a, et al. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010b;7:1809–1832. [Google Scholar]
- Niinemets U, Kannaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. 2013;4 doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe SM, Ciccioli P, Brancaleoni E, Loreto F, Niinemets Ü. Emissions of monoterpenes linalool and ocimene respond differently to environmental changes due to differences in physico-chemical characteristics. Atmospheric Environment. 2006;40:4649–4662. [Google Scholar]
- Penuelas J, Llusia J. The complexity of factors driving volatile organic compound emissions by plants. Biologia Plantarum. 2001;44:481–487. [Google Scholar]
- Peñuelas J. An increasingly scented world. New Phytologist. 2008;180:735–738. doi: 10.1111/j.1469-8137.2008.02658.x. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J. BVOCs: plant defense against climate warming? Trends in Plant Science. 2003;8:105–109. doi: 10.1016/S1360-1385(03)00008-6. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Staudt M. BVOCs and global change. Trends in Plant Science. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Noel JP, Dudareva N. Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science. 2006;311:808–11. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-113. 2013 [Google Scholar]
- Proffit M, Schatz B, Bessière J-M, Chen C, Soler C, Hossaert-McKey M. Signalling receptivity: comparison of the emission of volatile compounds by figs of Ficus hispida before, during and after the phase of receptivity to pollinators. Vol. 45. Balaban Publishers; 2008. pp. 15–24. [Google Scholar]
- R Foundation for Statistical Computing, editor. R Development Core Team. R: A Language and Environment for Statistical Computing. Vol. 1 Vienna: 2011. [Google Scholar]
- Raguso RA. Wake Up and Smell the Roses: The Ecology and Evolution of Floral Scent. Annual Review of Ecology, Evolution, and Systematics. 2008;39:549–569. [Google Scholar]
- Raguso RA, Levin RA, Foose SE, Holmberg MW, McDade LA. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Riffell JA, Alarcon R, Abrell L. Floral trait associations in hawkmoth-specialized and mixed pollination systems. Communicative & Integrative Biology. 2008;1:6–8. doi: 10.4161/cib.1.1.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell JA, Lei H, Abrell L, Hildebrand JG. Neural Basis of a Pollinator’s Buffet: Olfactory Specialization and Learning in Manduca sexta. Science. 2013;339:200–204. doi: 10.1126/science.1225483. [DOI] [PubMed] [Google Scholar]
- Van Schie CCN, Haring Ma, Schuurink RC. Regulation of terpenoid and benzenoid production in flowers. Current opinion in plant biology. 2006;9:203–8. doi: 10.1016/j.pbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD. Pollinator-mediated evolution of floral signals. Trends in Ecology & Evolution. 2013;28:307–315. doi: 10.1016/j.tree.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia. 1993;95:328–333. doi: 10.1007/BF00320984. [DOI] [PubMed] [Google Scholar]
- Staudt M, Bertin N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak (Quercus ilex L.) leaves. Plant, Cell & Environment. 1998;21:385–395. [Google Scholar]
- Trowbridge AM, Stoy PC. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Vol. 5. Springer Netherlands; Dordrecht: 2013. pp. 21–46. [Google Scholar]
- Wright GA, Choudhary AF, Bentley MA. Reward quality influences the development of learned olfactory biases in honeybees. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2597–2604. doi: 10.1098/rspb.2009.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]