Abstract
The release of stress-driven volatiles throughout leaf development has been little studied. Therefore, we subjected poplar leaves during their developmental stage (from two days to two weeks old) to wounding by a single punch hole, and measured online the wound-induced volatile organic compound emissions. Our study shows that the emission of certain volatile compounds fades with increasing leaf age. Among these compounds we found lipoxygenase products (LOX products), acetaldehyde, methyl benzoate, methyl salicylate, and mono- and sesquiterpenes.
In parallel, we studied the fading of constitutive emissions of methanol during leaf maturation, as well as the rise in isoprene constitutive emission during leaf maturation and its relationship to leaf photosynthetic capacity. We found highly significant relationships between leaf chlorophyll content, photosynthetic capacity, and leaf size during leaf ageing.
As the level of constitutive defences increases with increasing leaf age, the strength of the volatile signal is expected to be gradually reduced. The higher elicitation of volatile organic compound emissions (especially LOX products) in younger leaves could be an evolutionary defence against herbivory, given that younger leaves are usually more subjected to infestation and herbivory.
Keywords: isoprene, lipoxygenase products, methanol, leaf age, wounding
1. Introduction
Plants release a part of their assimilated carbon into constitutive (not related to periods of stress) volatile organic compound (VOC) emissions (Grote et al. 2013b; Niinemets et al. 2013). Isoprene is the major compound emitted in mature leaves of many tree species, including Populus tremula. Isoprene is constitutively synthesized as a product of the methylerythritol phosphate (MEP) pathway in isoprene-emitting plants (Sharkey et al. 2008), which is directly related to photosynthetic activity through the Calvin cycle and DMADP (dimethylallyl diphosphate) pool. Based on the close relationship between isoprene synthesis, isoprene emission, and photosynthesis (Loreto and Sharkey 1990; Monson and Fall 1989), leaf isoprene emissions may then vary within the same shoot together with leaf age because of the increasing photosynthetic capacity (Centritto et al. 2004) and chlorophyll content during leaf growth (Rasulov et al. 2014). By extension, the constitutive emission of other volatiles may also vary during leaf development.
Populus genus has acropetally development, which means that the youngest leaves are found at the tip of the shoot, and the leaf age and size increase as we move downwards. Therefore, another important factor for leaf constitutive emissions would be the fact that cell elongation and division occurs more intensively in younger and expanding leaves than in more mature and fully grown leaves. Pectin demethylation during cell wall expansion is a likely source of methanol emission (Fall and Benson 1996; Galbally and Kirstine 2002), and therefore we may find differences in constitutive methanol emission levels in neighbouring leaves within the same shoot.
Plants also emit VOCs when subjected to biotic and abiotic stress, so-called stress-driven emissions (Grote et al. 2013b). For example, as a consequence of mechanical damage, poplar leaves emit a rapid burst of volatiles lasting ca. 5 min (Portillo-Estrada et al. 2015b). The volatile blend is mainly composed of green leaf volatiles, methanol and acetaldehyde. The damage leads one to assume a loss of carbon to the plant as well as a decrease in the photosynthetic activity and a loss of water balance in the leaf. There are studies on the quantitative effect of the degree of damage to wound-induced volatile emissions in P. tremula mature leaves (Brilli et al. 2011; Fall et al. 1999; Portillo-Estrada et al. 2015b), but up to present, there is little experimental data on leaf constitutive volatile emissions linked to the leaf ontogenetic level, and no studies yet on the potentially different wound-induced volatile emissions throughout young leaf development.
We used P. tremula shoots with leaves of increasing age during leaf expansion. We measured both online leaf photosynthetic capacity and constitutive volatile emissions in leaves of increasing age under optimal growth conditions. This was followed by mechanical wounding using a hole puncher to measure the wound-induced responses as a function of leaf age.
We hypothesize that: (1) constitutive isoprene emission is positively related to photosynthetic activity, which also means that it increases with leaf growth; (2) constitutive methanol emission is maximum in younger and smaller leaves; and (3) wound-induced volatile emissions will be linked to leaf age during expansion presumably because of the different physiological activity during leaf development.
Material and methods
Plant material
We used root suckers of 15-20 leaves from a naturally established Populus tremula population at the campus of the Estonian University of Life Sciences (58.39° N, 26.70° E, elevation 41 m). All of the shoots used for this experiment are clones from the same tree, thus minimizing genetic variation effect among replicates. The shoots, grown in the field in natural conditions and natural soil, were cut under water and always transferred to the laboratory in the morning around 9:00. The shoots underwent a period of adaptation to the measurement conditions by keeping them at room temperature beneath a 500 W halogen lamp providing a quantum flux density of ca. 350 μmol m-2 s-1 at leaf level. Measurements were performed in leaves that had an area ranging between 3.2 to 65.2 cm2 and corresponded to the first (ca. 1-2 days old) to the ninth (ca. 14 days old) leaf position from the tip of the shoot, respectively (Fig. 1b).
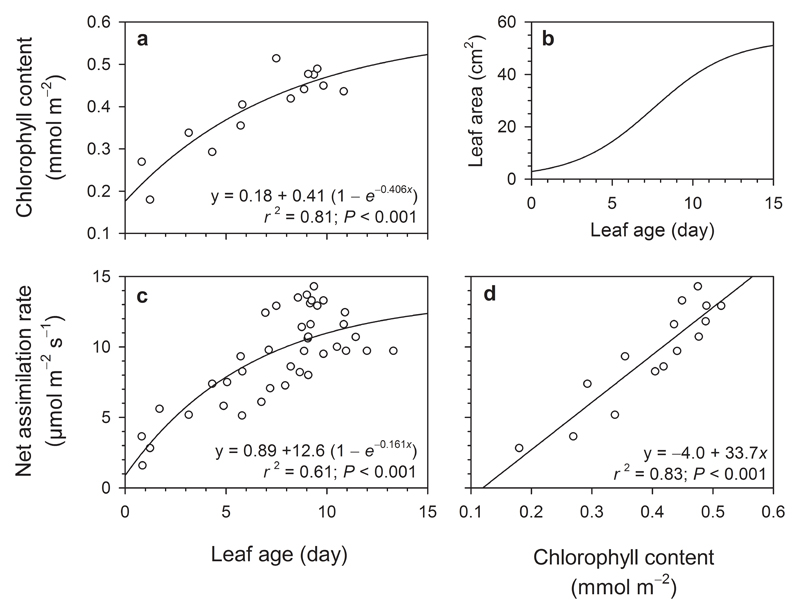
Figure 1.
Ontogenetic changes in (a) the chlorophyll a+b content, (b) leaf area, and (c) photosynthetic capacity in leaves of temperate deciduous broad-leaved poplar (Populus tremula). Panel (d) shows the linear relationship between leaf photosynthetic capacity and chlorophyll content. In (b), leaf size increases with increasing leaf age according to a logistic (or sigmoidal) relationship (see Sun et al. (2013) and Rasulov et al. (2015) for characteristic growth curves of poplar leaves with 5 cm2 leaves being ca. 2 days old, and 50 cm2 leaves 14 days old.
Experimental setup
The experimental setup and procedure were similar to the experiment in Portillo-Estrada et al. (2015b). We measured leaf net CO2 assimilation rate by enclosing the leaves in a standard 8 cm2 leaf cuvette (3010-S of Walz GFs-114 3000) of a GFS-3000 gas-exchange system (Walz GmbH, Effeltrich, Germany). A LED array/PAM-fluorimeter 3055-FL (Walz GmbH) was used for leaf illumination with a saturating quantum flux density of 500 μmol m-2 s-1. Leaf temperature was kept constant at 25 °C. The cuvette was flushed with ambient air at a flow rate of 750 μmol s-1. Air humidity was maintained at a constant level (16000 ppm H2O, approx. 60% relative humidity) and CO2 concentration was 400 μmol mol-1.
Simultaneously with photosynthesis, constitutive leaf volatile emissions were measured online by a PTR-TOF-MS (proton-transfer-reaction time-of-flight mass spectrometer) model 8000 (Ionicon Analytik GmbH, Innsbruck, Austria). The details of the measurement principle, parameters and calibration are described in Portillo-Estrada et al. (2015b). We measured 26 relevant volatile molecules emitted by poplar leaves constitutively and after mechanical wounding (see the detailed list in Table 1 in Portillo-Estrada et al. (2015b)). Among these, the group of lipoxygenase products (LOX products) included C5 and C6 compounds derived from the oxidation of linoleic acid, present in the cell membranes (see biosynthetic pathways in Fall et al. (1999) and Fall et al. (2001)).
Once the leaves had reached steady levels of CO2 exchange, water vapour, and isoprene emission at saturating light level, we averaged three consecutive measurements of CO2 exchange as an estimate of photosynthetic capacity (Amax). Isoprene, methanol, and acetaldehyde emission levels at maximum photosynthetic capacity were estimated by averaging a series of data of ca. 2-3 minutes during the steady state. A total of 45 leaves of different age were measured for constitutive VOC emissions.
The dataset of constitutive isoprene emission through leaf expansion was fitted to an “exponential rise to maximum” equation where the theoretical values would achieve a maximum during leaf maturity (Eller et al. 2012; Sun et al. 2013). Following the same principle (Rasulov et al. 2014; Sun et al. 2013), photosynthetic capacity was fitted to the same function type. As for constitutive methanol emission, we used a negative exponential equation knowing that during leaf maturity there is also a basal level of methanol emission (Eller et al. 2012) and that other authors found a higher emission at the top of poplar shoots (Nemecek-Marshall et al. 1995).
Leaf wounding
Volatile emissions induced by wounding with a hole puncher were tested in leaves of increasing age: first nine leaves from the shoot top. The punch hole area was 19.07 ± 0.15 mm2, and its perimeter 15.48 ± 0.06 mm, which was used to express the volatile emission rates per unit wound length (mm). We used the punch hole procedure to perform the mechanical damage because of the rapidness of the wounding treatment and its high replicability in producing wound edges of given length (Portillo-Estrada et al. 2015b).
The volatile emissions induced during the few minutes following the wounding were integrated and expressed by wound length. Seven minutes of emission data were enough to record the first emission burst of volatiles related to the wounding, after which the emission levels came back to pre-wounding values.
The datasets of wound-induced volatile emission though leaf expansion were fitted to a negative exponential equation assuming that the emission of the volatiles of study was positive or close to zero during leaf maturity, as shown by some studies (Brilli et al. 2011; Portillo-Estrada et al. 2015b).
Leaf area and leaf age
Once leaf cuvette measurements were finished, the leaf petiole was removed and the leaf area measured by scanning the leaf blade. Leaf area picture processing occurred as in Portillo-Estrada et al. (2015a) to minimize errors in the leaf area estimation due to shadows in the leaf picture.
Leaf age was estimated from leaf area after modelling the data published by Rasulov et al. (2015) on the evolution of the leaf area during leaf expansion of clonal P. tremula individuals used for this experiment. The data was fitted to a sigmoidal function (r2 = 0.998, P < 0.001):
| (Eqn 1) |
and then inversed to a logit function to estimate the leaf age (Lage, in days) based on the leaf area (Larea, in cm2):
| (Eqn 2) |
Leaf chlorophyll content
Circular leaf discs of 1 cm diameter were taken by a cork borer and stored at -80 ºC for further analysis of leaf chlorophyll content. Leaf discs were ground with Precellys 24 tissue homogenizer (Bertin Technologies, France) in Precellys lysing kits for soft tissue CK 14 (2 mL plastic tubes with 1 mm diameter plastic balls inside) at 0 ºC in 100 % acetone with added calcium carbonate. The extracts were then centrifuged and filtered through a 0.45 μm PTFE membrane filter. Leaf chlorophyll content was determined with an Agilent Technologies 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) using a linear gradient of acetone concentration in water as in Opris et al. (2013).
Leaf chlorophyll content (mmol m-2) increase through leaf age was fitted with the same equation type than photosynthetic capacity, knowing that leaves will achieve a maximum of chlorophyll content at maturity (Rasulov et al. 2014).
Results
Photosynthesis and constitutive volatile emissions throughout leaf expansion
We confirmed a highly significant (r2 = 0.88 and P < 0.001; multiple linear regression analysis) three-way interaction between leaf age (Lage, in days), leaf chlorophyll content (LChl, in mmol m-2) and net photosynthetic capacity (Amax, μmol m-2 s-1) at a constant saturating photosynthetic photon flux density:
| (Eqn 3) |
The equation was also significant (r2 = 0.84 and P < 0.001) when accounting for leaf area (Larea, in cm2) instead of leaf age:
| (Eqn 4) |
As leaves expanded, leaf chlorophyll content raised to a level of 0.4 to 0.5 mmol m-2 (ca. 40 to 50 μg cm-2) (Fig. 1a) and leaf photosynthetic capacity attained a level of 10-15 μmol m-2 s-1 (Fig. 1c) following the trend of an “exponential rise to maximum” function in both cases. Photosynthetic capacity linearly increased as leaf chlorophyll content increased during leaf development (Fig. 1d).
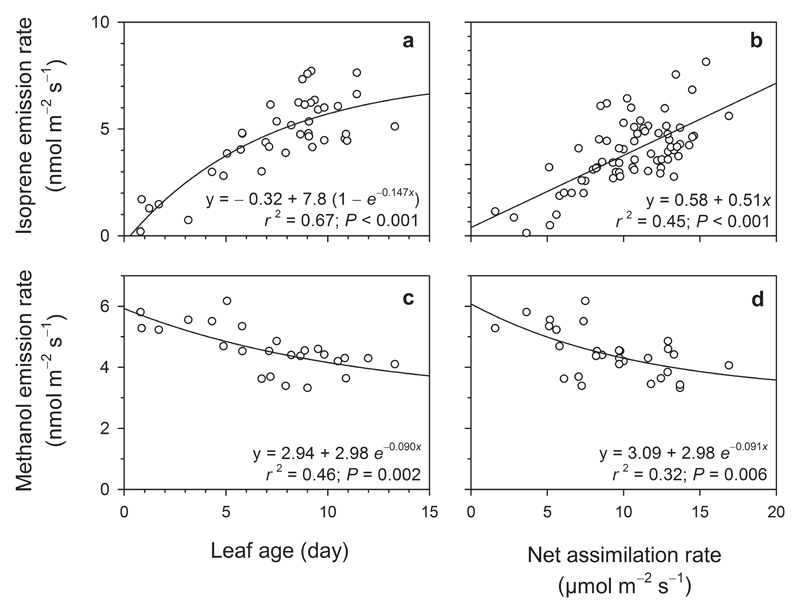
When applying the same light and temperature conditions to P. tremula leaves of increasing age, the constitutive volatile emissions of isoprene increased exponentially to a maximum, reached after 10-15 days of development (Fig. 2a). Leaf isoprene emission positively correlated with the net CO2 assimilation rate (Fig. 2b). Methanol emission rates were higher as leaves were younger and smaller (Fig. 2c). We also found a vague relationship (r2 = 0.31; P = 0.001) between leaf methanol emission rate and leaf photosynthesis (Fig. 2d).
Figure 2.
Leaf isoprene and methanol emission rates in relation to (a, c) the leaf age and (b, d) leaf photosynthetic capacity in leaves of Populus tremula.
We could visually identify a threshold at one week of leaf development (typically a leaf blade of 5.5 cm in length at the midrib level, maximum 5 cm in width, leaf area of ca. 20 cm2, and 7 days of development) where the exponential character of the relationships (Fig. 1a and c) turned to an almost steady response against the x-axis (leaf area) also showing larger variability in leaf chlorophyll content and photosynthetic capacity. In accordance with that, leaf chlorophyll a+b content of the youngest leaves (smaller than 20 cm2) was smaller, 0.307 ± 0.032 mmol m-2 (or 27.4 ± 2.9 μg cm-2, average ± SE), than for the more mature leaves (larger than 20 cm2), 0.466 ± 0.010 mmol m-2 (or 41.6 ± 0.9 μg cm-2) (P < 0.0001, two-tailed Student’s t-test) (Fig. 1a). Leaf net CO2 assimilation was also significantly (P < 0.0001) smaller in younger leaves, 5.7 ± 0.7 μmol m-2 s-1, than in larger and more mature leaves, 10.88 ± 0.39 μmol m-2 s-1 (Fig. 1c).
Concerning the constitutive volatile emissions, isoprene emission rates were lower (P < 0.0001) in younger leaves, 2.60 ± 0.49 nmol m-2 s-1, than in older leaves, 6.24 ± 0.43 nmol m-2 s-1 (Fig. 2a). Contrarily, leaf methanol emission rate during the first week of leaf expansion (leaf area smaller than 20 cm2) was significantly (P < 0.0001) higher, 5.43 ± 0.17 nmol m-2 s-1, than in more mature leaves, 4.09 ± 0.13 nmol m-2 s-1 (Fig. 2c).
The changes of leaf chlorophyll content, photosynthetic capacity, and constitutive emissions of isoprene and methanol with increasing leaf area (cm2) can be found in Figure S1, Suppl. 1.
Wound-induced volatile emissions throughout leaf expansion
Mechanical damage (leaf punch) was performed to leaves of increasing leaf age (i.e. increasing leaf size), and the subsequent induced volatile emissions measured.
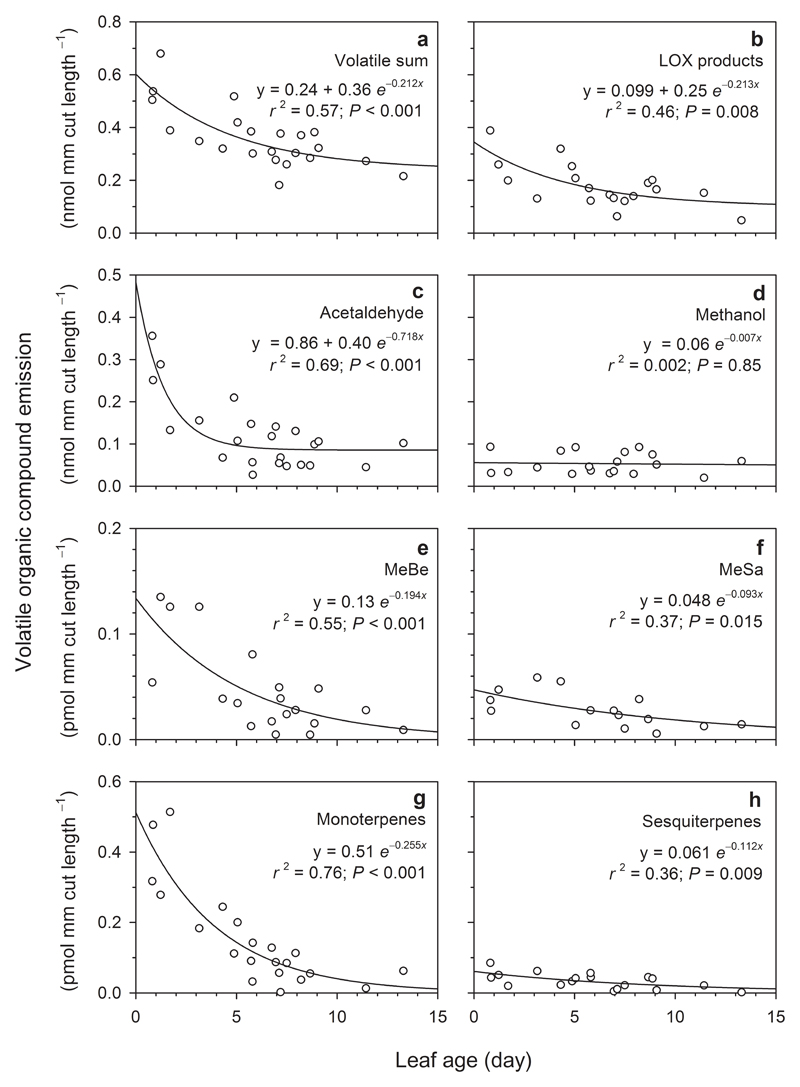
The sum of all volatiles emitted during the post-wounding period was larger in younger leaves than in further developed leaves (Fig. 3a), and similarly occurred in LOX products (Fig. 3b) and acetaldehyde (Fig. 3c) emissions. Contrarily, leaf age did not have an effect on methanol wound-induced emission (Fig. 3d). At a much smaller level (lower than 1 pmol mm-1 cut length, i.e. three orders of magnitude smaller than LOX products), the wound-induced emissions of methyl benzoate (Fig. 3e), methyl salicylate (Fig. 3f), monoterpenes (Fig. 3g), and sesquiterpenes (Fig. 3h) presented significant relationships with the leaf age, following an exponential decay function in all cases.
Figure 3.
Correlations of the integrated volatile emissions released by leaf wounds to leaf age Populus tremula leaves. The wounds were produced by a single punch hole (15.48 mm edge length). In (a), the sum of volatiles includes 26 relevant compounds and in (b), LOX products include C5 (pentenyl family) and C6 (hexenal and hexanal families). For full list of compounds we refer to Portillo-Estrada et al. (2015b). MeBe (e) stands for methyl benzoate, and MeSa (f) for methyl salicylate.
The relationships of leaf wound-induced emissions with leaf area can be found in Figure S2, Suppl. 1.
Discussion
Leaf chlorophyll content, photosynthesis, and isoprene emission throughout leaf ontogeny
In previous studies on hybrid poplar, leaf chlorophyll content and photosynthetic capacity were observed to increase rapidly during early leaf development (Rasulov et al. 2014; Reich 1983), both parameters being positively interrelated (Reich 1983). Similarly to Reich (1983), we found that linear fitting was the best option to model the increase of the light-saturated photosynthesis over rising chlorophyll content (Fig. 1d).
Leaf net photosynthesis raised exponentially during leaf ageing and reached a maximum value at ca. 10 and 15 μmol m-2 s-1 in mature leaves. These CO2 assimilation levels as well as the concomitant isoprene emissions during leaf ontogeny (Fig. 2a) are in accordance with the findings of Sun et al. (2013) during poplar canopy development, confirming Hypothesis 1. Moreover, the observed isoprene emission levels (Fig. 2a) were comparable to those reported by Loreto et al. (2007) at different leaf positions from the apex (also studying the effects of leaf ontogeny) at ambient CO2 concentration and at a temperature of 25 °C.
The correlation between isoprene emission and photosynthesis rates (Fig. 2b) is well known throughout scientific literature, especially when one considers the instantaneous responses of isoprene emission and photosynthesis to changes in light intensity (e.g., Grote et al. 2013a; Monson 2013). Contrarily to light manipulation experiments where isoprene emission is related to photosynthesis, different relationships can be observed across long-term environmental gradients (Rasulov et al. 2015) and throughout leaf ontogeny (Niinemets et al. 2015; Rasulov et al. 2014). In fact, throughout leaf lifespan, isoprene emission is typically induced somewhat later than positive net photosynthesis rates are observed (Harley et al. 1994; Rasulov et al. 2014). In our case, we measured the maximum photosynthetic capacity at saturating light level and associated maximum isoprene emissions at a given leaf age (Fig. 2a, b). Clearly, isoprene emission and photosynthesis rate were positively correlated, but we nevertheless want to emphasize that using leaves of different age and shoots integrates random effects that make the observed relationship somewhat scattered (Fig. 2b).
Despite the precise knowledge on the isoprene biosynthetic pathways, the evolutionary and ecological reasons of leaf isoprene emission are still unclear, but different plausible hypotheses have been proposed (Sharkey et al. 2008; Vickers et al. 2009). Isoprene emission is responsive to leaf temperature and sensitive to light input, but ultimately related to isoprene synthase activity (Kuzma and Fall 1993) and dimethylallyl diphosphate pool size (Niinemets and Sun 2015), that we hypothesize smaller in younger leaves, as observed by Rasulov et al. (2014).
Constitutive methanol emissions during leaf development
Methanol emission has been shown as plant waste product that, due to its solubility in water and high volatility, is unavoidably emitted (Peñuelas and Llusià 2004). Despite that, Peñuelas et al. (2005) suggested a potential ecological role in mediating plant-insect interactions after measuring large emission amounts caused by caterpillar feeding (Peñuelas et al. 2005). In addition, a controlled mechanical wounding experiment on mature poplar leaves showed that methanol emission can constitute 15% of wound-induced emissions and is highly correlated with the degree of damage (Portillo-Estrada et al. 2015b).
As proposed by several authors, constitutive methanol emission peak is closely related to leaf expansion. This is due to pectin demethylation during cell wall expansion (Harley et al. 2007; Karl et al. 2003). In our study, constitutive methanol emission consequently peaked at the earliest developmental stage (Fig. 2c), when the growth rate was presumably at its highest (Sun et al. 2013). This has been also found by other studies (Fares et al. 2010) and confirms Hypothesis 2. There was also a weak relationship between constitutive methanol emission and photosynthesis (Fig. 2d), most probably driven by the simultaneous leaf expansion and net assimilation rate (Fig. 1c).
Wound-induced volatile emissions related to leaf age
Total volatile emissions after wounding were constituted mainly by LOX products, acetaldehyde and methanol emissions (Fig. 3). The level of wound-induced emissions was dependent on leaf ontogenetic level in many cases, which confirms Hypothesis 3.
Methanol, as well as emitted as a product of leaf expansion, it can be emitted due to oxidative stress. Such conditions occur upon leaf wounding (Brilli et al. 2011; Loreto et al. 2006; Portillo-Estrada et al. 2015b). However, we did not find a higher elicitation of methanol emission in younger leaves and it was steady throughout leaf expansion (Fig. 3c). The cause could be related to the previously mentioned methanol high volatility and solubility in water. For that reason, methanol emission is directly related to stomatal conductance, preventing the formation of a stock within the leaf. Therefore methanol pool size in the cytosol and interstitial spaces of leaf mesophyll could be always small regardless of leaf age. This makes inevitably high methanol emission in young leaves certainly de novo emission (not originated from a stock). In conclusion, upon leaf wounding and breakage of leaf tissues, no additional methanol would be released from any stock in younger leaves.
Acetaldehyde is one of the most frequently identified oxygenated compounds emitted from leaves (Monson 2013). It could be related to leaf wounding in the way that the pool of acetyl-CoA may react with C6 aldehydes synthesized upon wounding (in the LOX product blend) to form C6 acetates, and acetaldehyde could leak from the acetyl-CoA pool during this reaction (Graus et al. 2004). The question lies in why younger leaves could hold a bigger acetyl-CoA or pyruvate pool, which are precursors of acetaldehyde emission. Young leaves have been associated with higher growth respiration (Loreto et al. 2007), dark respiration (Rasulov et al. 2014) and mitochondrial activity (Dickmann et al. 1975) than older leaves. Therefore the higher activity could explain a higher acetyl-CoA and pyruvate content in younger poplar leaves, making the response to wounding greater through acetaldehyde emission.
LOX products were emitted in higher amounts by younger leaves. Linoleic acid is the precursor of LOX products (Fall et al. 1999; Fall et al. 2001), and it is found in cell membranes. In principle, young leaves could have smaller content of linoleic acid per unit area in comparison to older leaves, which are thicker and supposedly have more membranes per area unit. However, the content of lipoxygenase isozymes in young leaves is by far higher than in older leaves (Saravitz and Siedow 1995). This suggests that the higher enzymatic activity may therefore drive the higher response in younger leaves, as we observed (Fig. 3b). In ecological terms, a higher LOX product emission is beneficial for younger leaves in keeping herbivores away, because young leaves with thinner cell walls (Tosens et al. 2012) lack constitutive mechanical defences and also contain less non-volatile defensive metabolites such as condensed tannins (Kursar and Coley 1991). In addition, young leaves have been shown to be more heavily consumed by herbivores (Dudt and Shure 1994; Johnson et al. 1984; Southwood et al. 1986). Nevertheless, we must acknowledge that the results of our study cannot be fully extrapolated to a herbivory situation. Bricchi et al. (2010) found significant differences in leaf responses to mechanical wounding in comparison to real herbivory: membrane polarization and volatile blend composition; most probably due to the lack of herbivore-derived oral secretions in mechanical wounding.
At a much lower level, monoterpenes, sesquiterpenes, and methylated benzenoids as MeBe and MeSa were emitted after leaf wounding. Their emission level depended also on leaf age. The emission of LOX products is an ubiquitous response across species and stresses, whereas the emission of volatile mono- and sesquiterpenes and benzenoids such MeSa are stress dependent, reflecting selective activation of genes after the stress event (Copolovici et al. 2014; Possell and Loreto 2013). Monoterpenes and sesquiterpenes were most likely emitted as a result of the exposure of the non-specific pools of these volatiles in leaf lipid and liquid phases (Niinemets and Reichstein 2002) to the ambient upon leaf wounding, and therefore should not reflect de novo emissions. Newly synthesized isoprenoids normally peak in the subsequent hours to days after wounding (Erb et al. 2015). Their accumulation in leaf structures (especially in conifer needles) protects the plant against herbivory by decreasing leaf palatability (Dicke and Baldwin 2010) and may help to seal leaf wounds (Loreto et al. 2008). But poplar leaves do not have specialized structures to store terpenoids. However, a basal synthesis level could happen in poplar leaves, and according to our results, could be greater in younger leaves.
The synthesis and emission of volatile hormones such as MeBe and MeSa have been found to attract natural predators during plant feeding as a defence response to plant herbivory (War et al. 2011; Zhao et al. 2010) as well as repel parasites themselves (Snoeren et al. 2010). However, the peak of synthesis of MeBe and MeSa normally occurs hours to days after the wounding (Niinemets and Monson 2013; Staudt et al. 2010), but our results then suggest that there could be a minimal basal synthesis forming a small pool of these compounds in younger leaves. Our results are in agreement with findings that in general, young leaves of Populus spp. have a greater capacity for biotic stress-dependent induction of monoterpene emission than older leaves (Brilli et al. 2009).
Fares et al. (2010) also found a higher level of stress-induced volatile emission in younger leaves when exposed to oxidative stress (2-week exposure to 80 ppb of ozone). In particular, they found a higher amount of LOX products in younger leaves. In accordance to our results, the level of stress-induced methanol emission did not depend on leaf age during poplar leaf expansion. Our study evidences an effect of leaf age on stress-induced volatile emissions in poplar. More research is needed to characterize this relationship across other species.
Conclusion
This study provides evidence of major variation in wound-induced volatile release through leaf expansion. However, the key implication of the study is that even for the same species and tree shoot, volatiles released in response to the elicitor of given strength can vastly vary in dependence on leaf ontogeny. As the level of constitutive defences increases with leaf age, the strength of the volatile signal is expected to be gradually reduced. The higher elicitation of BVOC emissions (especially LOX products) in younger leaves could be an evolutionary defence against herbivory, given that younger leaves are usually more subject to infestation and herbivory. Further studies are needed to test whether herbivory also causes similar BVOC emission level changes through leaf development.
Supplementary Material
Acknowledgements
We thank Peter Harley, Eero Talts, and Tiina Tosens for constructive discussions during this study. This work was supported by the Estonian Ministry of Science and Education [institutional grant IUT-8-3], Estonian Science Foundation [grant 9253], the European Commission through the European Regional Fund [Centre of Excellence in Environmental Adaptation] and Marie Curie [grant ERMOS73] and through the Transnational Access to Research Infrastructures activity [ExpeER], the European Research Council [advanced grant 322603, SIP-VOL+] and the European Social fund ESF [MJD 438].
References
- Bricchi I, Leitner M, Foti M, Mithofer A, Boland W, Maffei ME. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: early signaling and volatile emission in Lima bean (Phaseolus lunatus L.) Planta. 2010;232:719–729. doi: 10.1007/s00425-010-1203-0. [DOI] [PubMed] [Google Scholar]
- Brilli F, Ciccioli P, Frattoni M, Prestininzi M, Spanedda AF, Loreto F. Constitutive and herbivore-induced monoterpenes emitted by Populus x euramericana leaves are key volatiles that orient Chrysomela populi beetles. Plant Cell Environ. 2009;32:542–552. doi: 10.1111/j.1365-3040.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction "time-of-flight" mass spectrometry (PTR-TOF) Plos One. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centritto M, Nascetti P, Petrilli L, Raschi A, Loreto F. Profiles of isoprene emission and photosynthetic parameters in hybrid poplars exposed to free-air CO2 enrichment. Plant Cell Environ. 2004;27:403–412. [Google Scholar]
- Copolovici L, Väärtnõu F, Portillo Estrada M, Niinemets Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014;34:1399–1410. doi: 10.1093/treephys/tpu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the 'cry for help'. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dickmann DI, Gjerstad DH, Gordon JC. Developmental patterns of CO2 exchange, diffusion resistance and protein synthesis in leaves of Populus x euramericana. In: Marcelle R, editor. Environmental and Biological Control of Photosynthesis. Springer; Netherlands: 1975. pp. 171–181. [Google Scholar]
- Dudt JF, Shure DJ. The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology. 1994;75:86–98. [Google Scholar]
- Eller ASD, de Gouw J, Graus M, Monson RK. Variation among different genotypes of hybrid poplar with regard to leaf volatile organic compound emissions. Ecol Appl. 2012;22:1865–1875. doi: 10.1890/11-2273.1. [DOI] [PubMed] [Google Scholar]
- Erb M, Veyrat N, Robert CAM, Xu H, Frey M, Ton J, Turlings TCJ. Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun. 2015;6 doi: 10.1038/ncomms7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R, Benson AA. Leaf methanol - The simplest natural product from plants. Trends Plant Sci. 1996;1:296–301. [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. J Geophys Res-Atmos. 1999:104, 15963–15974. [Google Scholar]
- Fall R, Karl T, Jordon A, Lindinger W. Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmos Environ. 2001;35:3905–3916. [Google Scholar]
- Fares S, Oksanen E, Lannenpaa M, Julkunen-Tiitto R, Loreto F. Volatile emissions and phenolic compound concentrations along a vertical profile of Populus nigra leaves exposed to realistic ozone concentrations. Photosynthesis Res. 2010;104:61–74. doi: 10.1007/s11120-010-9549-5. [DOI] [PubMed] [Google Scholar]
- Galbally IE, Kirstine W. The production of methanol by flowering plants and the global cycle of methanol. J Atmos Chem. 2002;43:195–229. [Google Scholar]
- Graus M, Schnitzler JP, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J. Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiol. 2004;135:1967–1975. doi: 10.1104/pp.104.043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013a. pp. 315–355. [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Vol. 5. Springer; Netherlands: 2013b. pp. 315–355. [Google Scholar]
- Harley P, Greenberg J, Niinemets Ü, Guenther A. Environmental controls over methanol emission from leaves. Biogeosciences. 2007;4:1083–1099. [Google Scholar]
- Harley PC, Litvak ME, Sharkey TD, Monson RK. Isoprene emission from velvet bean leaves. Interactions among nitrogen availability, growth photon flux density, and leaf development. Plant Physiol. 1994;105:279–285. doi: 10.1104/pp.105.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ND, Chu CC, Ehrlich PR, Mooney HA. The seasonal dynamics of leaf resin, nitrogen, and herbivore damage in Eriodictyon californicum and their parallels in Diplacus aurantiacus. Oecologia. 1984;61:398–402. doi: 10.1007/BF00379642. [DOI] [PubMed] [Google Scholar]
- Karl T, Guenther A, Spirig C, Hansel A, Fall R. Seasonal variation of biogenic VOC emissions above a mixed hardwood forest in northern Michigan. Geophys Res Lett. 2003;30 [Google Scholar]
- Kursar TA, Coley PD. Nitrogen content and expansion rate of young leaves of rain forest species: implications for herbivory. Biotropica. 1991;23:141–150. [Google Scholar]
- Kuzma J, Fall R. Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol. 1993;101:435–440. doi: 10.1104/pp.101.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK. The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ. 2007;30:662–669. doi: 10.1111/j.1365-3040.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Kesselmeier J, Schnitzler JP. Volatile organic compounds in the biosphere-atmosphere system: a preface. Plant Biol. 2008;10:2–7. doi: 10.1111/j.1438-8677.2007.00021.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- Monson RK. Metabolic and gene expression controls on the production of biogenic volatile organic compounds. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer; Berlin: 2013. pp. 153–179. [Google Scholar]
- Monson RK, Fall R. Isoprene emission from aspen leaves - Influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 1989;90:267–274. doi: 10.1104/pp.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecek-Marshall M, MacDonald RC, Franzen FJ, Wojciechowski CL, Fall R. Methanol emission from leaves - Enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development. Plant Physiol. 1995;108:1359–1368. doi: 10.1104/pp.108.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Monson RK. State-of-the-art of BVOC research: what do we have and what have we missed? A synthesis. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Vol. 5. Springer; Berlin: 2013. pp. 509–528. [Google Scholar]
- Niinemets Ü, Reichstein M. A model analysis of the effects of nonspecific monoterpenoid storage in leaf tissues on emission kinetics and composition in Mediterranean sclerophyllous Quercus species. Glob Biogeochem Cycle. 2002;16:1110. [Google Scholar]
- Niinemets Ü, Sun Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J Exp Bot. 2015;66:841–851. doi: 10.1093/jxb/eru443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Sun ZH, Talts E. Controls of the quantum yield and saturation light of isoprene emission in different-aged aspen leaves. Plant Cell and Environment. 2015;38:2707–2720. doi: 10.1111/pce.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris O, Copaciu F, Soran ML, Ristoiu D, Niinemets Ü, Copolovici L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol Environ Saf. 2013;87:70–79. doi: 10.1016/j.ecoenv.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Stefanescu C, Llusià J. Caterpillars of Euphydryas aurinia (Lepidoptera: Nymphalidae) feeding on Succisa pratensis leaves induce large foliar emissions of methanol. New Phytol. 2005;167:851–857. doi: 10.1111/j.1469-8137.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J. Plant VOC emissions: making use of the unavoidable. Trends Ecol Evol. 2004;19:402–404. doi: 10.1016/j.tree.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M, Copolovici L, Niinemets Ü. Bias in leaf dry mass estimation after oven-drying isoprenoid-storing leaves. Trees. 2015a;29:1805–1816. [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula) J Chem Ecol. 2015b;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possell M, Loreto F. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 209–235. [Google Scholar]
- Rasulov B, Bichele I, Hüve K, Vislap V, Niinemets Ü. Acclimation of isoprene emission and photosynthesis to growth temperature in hybrid aspen: resolving structural and physiological controls. Plant, Cell & Environment. 2015;38:751–766. doi: 10.1111/pce.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB. Effects of low concentrations of O3 on net photosynthesis, dark respiration, and chlorophyll contents in aging hybrid poplar leaves. Plant Physiol. 1983;73:291–296. doi: 10.1104/pp.73.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravitz DM, Siedow JN. The lipoxygenase isozymes in soybean [Glycine max (L.) Merr.] leaves (changes during leaf development, after wounding, and following reproductive sink removal) Plant Physiol. 1995;107:535–543. doi: 10.1104/pp.107.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Wiberley AE, Donohue AR. Isoprene emission from plants: Why and how. Ann Bot. 2008;101:5–18. doi: 10.1093/aob/mcm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeren TAL, Mumm R, Poelman EH, Yang Y, Pichersky E, Dicke M. The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum. J Chem Ecol. 2010;36:479–489. doi: 10.1007/s10886-010-9787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood TRE, Brown VK, Reader PM. Leaf palatability, life expectancy and herbivore damage. Oecologia. 1986;70:544–548. doi: 10.1007/BF00379901. [DOI] [PubMed] [Google Scholar]
- Staudt M, Jackson B, El-Aouni H, Buatois B, Lacroze JP, Poessel JL, Sauge MH. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol. 2010;30:1320–1334. doi: 10.1093/treephys/tpq072. [DOI] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Rasulov B, Noe SM. Elevated atmospheric CO2 concentration leads to increased whole-plant isoprene emission in hybrid aspen (Populus tremula x Populus tremuloides) New Phytol. 2013;198:788–800. doi: 10.1111/nph.12200. [DOI] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro-Díez P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 2012;35:839–856. doi: 10.1111/j.1365-3040.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- War AR, Sharma HC, Paulraj MG, War MY, Ignacimuthu S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signaling & Behavior. 2011;6:1973–1978. doi: 10.4161/psb.6.12.18053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Guan J, Ferrer JL, Engle N, Chern M, Ronald P, Tschaplinski TJ, Chen F. Biosynthesis and emission of insect-induced methyl salicylate and methyl benzoate from rice. Plant Physiol Biochem. 2010;48:279–287. doi: 10.1016/j.plaphy.2010.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.