Abstract
Acute ozone exposure triggers major emissions of volatile organic compounds (VOC), but quantitatively, it is unclear how different ozone doses alter the start and the total amount of these emissions, and the induction rate of different stress volatiles. It is also unclear whether priming (i.e., pre-exposure to lower O3 concentrations) can modify the magnitude and kinetics of volatile emissions. We investigated photosynthetic characteristics and VOC emissions in Phaseolus vulgaris following acute ozone exposure (600 nmol mol-1 for 30 min) under illumination and in darkness and after priming with 200 nmol mol-1 O3 for 30 min. Methanol and lipoxygenase (LOX) pathway product emissions were induced rapidly, followed by moderate emissions of methyl salicylate (MeSA). Stomatal conductance prior to acute exposure was lower in darkness and after low O3 priming than in light and without priming. After low O3 priming, no MeSA and lower LOX emissions were detected under acute exposure. Overall, maximum emission rates and the total amount of emitted LOX products and methanol were quantitatively correlated with total stomatal ozone uptake. These results indicate that different stress volatiles scale differently with ozone dose and highlight the key role of stomatal conductance in controlling ozone uptake, leaf injury and volatile release.
Keywords: volatile organic compounds (VOC), ozone stress, methanol, LOX products, stomatal conductance, proton transfer reaction-time of flight-mass spectrometer (PTR-TOF-MS)
Introduction
Tropospheric ozone (O3) is a major global air pollutant and an important greenhouse gas that seriously impacts human health and natural ecosystems worldwide from tropics to tundra (Fowler et al. 2008). Both plant growth and production as well as plant species biodiversity are significantly disturbed under elevated O3 conditions, which portends a significant threat to global food security (e.g. Schimidhuber & Tubiello 2007; Feng et al. 2008; Furlan et al. 2008; Wittig et al. 2009; Ainsworth et al. 2012; Wilkinson et al. 2012; Agathokleous et al. 2015; Cassimiro & Moraes 2016; Freire et al. 2017). It is estimated that the average current background concentration of O3 in the northern hemisphere is around 20-50 nmol mol-1 and O3 concentrations are predicted to increase due to expected rise in O3 precursor emissions in the future, implying even greater effects of tropospheric O3 on global climate change, and on atmospheric oxidative status by the end of this century (Vingarzan 2004; Fowler et al. 2008).
As a strong polar oxidant, O3 enters plants mainly through the stomata. Thus, stomatal closure is a direct and highly effective way to limit O3 damage, but it also limits CO2 uptake, causing the reduction in net assimilation. Previous studies have observed that elevated O3 concentrations induce both rapid stomatal closure (Kollist et al. 2007; Vahisalu et al. 2008; Vahisalu et al. 2010) and sluggish stomatal responses to changes in environmental conditions (Paoletti & Grulke 2010; Hoshika et al. 2013), which may cause significant decline in photosynthesis and increase leaf water loss relative to net assimilation (leaf water use efficiency). Apart from stomata, semi-volatile and volatile organic compounds exuded by the glandular trichomes at leaf surface and volatiles emitted de novo from mesophyll cells constitute an efficient O3 sink that can reduce O3 concentrations on the leaf surface and in the gas-phase surrounding the leaf, thereby reducing O3 entry through stomata (Loreto et al. 2001; Loreto et al. 2004; Fares et al. 2008; Fares et al. 2010a, 2010b; Jud et al. 2016; Li et al. unpublished). Therefore, O3-induced leaf damage may not be well correlated with the surrounding O3 concentration, but is related to the O3 flux density into the plants through the stomata (Beauchamp et al. 2005; Loreto & Fares 2007; Fares et al. 2010a).
Once O3 has entered the plant, it has to pass the cell wall aqueous phase where it can be partly quenched by water-soluble antioxidants such as ascorbate (e.g. Ranieri et al. 1999; Bechele et al. 2010). Once having passed the cell wall, O3 can directly react with the plasmalemma lipids, resulting in direct damage, but it typically also elicits an endogenous generation of other reactive oxygen species (ROS), including hydrogen peroxide (H2O2), which collectively lead to a disruption and profound reprogramming of cellular metabolism, ultimately leading to major reduction of foliar photosynthetic activity, accelerated senescence, and elicitation of programmed cell death (Long & Naidu 2002; Wohlgemuth et al. 2002; Calatayud et al. 2003; Pasqualini et al. 2003; Beauchamp et al. 2005; Fiscus et al. 2005). Foliage photosynthetic apparatus can be impaired by O3 due to direct inactivation of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) as well as reduction of expression of Rubisco synthase activity (Long & Naidu 2002; Calatayud et al. 2003; Fiscus et al. 2005). O3 has also been shown to alter photosynthetic electron transport in some plants via reduction in efficiency of excitation energy capture by PSII (Fiscus et al. 2005; Flowers et al. 2007; Guidi et al. 2009). Visible symptoms of toxic O3 exposure characteristically include chlorotic or necrotic lesions on the plant leaf surface, which can coalesce to form larger injured areas, ultimately resulting in whole leaf senesce and early abscission (Loreto et al. 2001; Long & Naidu 2002; Wohlgemuth et al. 2002; Pasqualini et al. 2003; Beauchamp et al. 2005; Vickers et al. 2009; Wilkinson et al. 2012). Although the basic principles of O3 impacts on photosynthetic machinery have been uncovered, at quantitative level, O3 dose vs. plant response relationships are still poorly understood. In particular, in natural conditions, O3 concentrations strongly fluctuate and studies have tried to use a certain threshold O3 dose, e.g. AOT40, to characterize the impact of cumulative O3 exposure on plant responses (e.g. Inclan et al. 1999; Bortier et al. 2000; Oksanen & Holopainen 2001). However, due to various O3 quenching mechanisms operative across plant species, differences in stomatal reactions to fluctuating O3 concentrations and differences in endogenous elicitation of ROS production, the plant dose responses can be very complex (Loreto & Fares 2007; Fares et al. 2010). Furthermore, mild stress itself can elicit priming responses, enhancing or reducing the sensitivity to subsequent more severe stress episodes (Conrath et al. 2006; Heil & Kost 2006), but there is only limited body of information of possible modification of acute O3 resistance by mild O3 exposures.
In parallel with primary cellular damage due to O3 reactions with plasmalemma unsaturated lipids, and/or secondary damage due to elicitation of endogenous ROS production by O3, emissions of volatile organic compounds (VOC) such as methanol, lipoxygenase pathway products (e.g. LOX products comprising various C6 aldehydes) and methyl salicylate (MeSA) are typically observed after O3 fumigation (Heiden et al. 1999; Beauchamp et al. 2005). As discussed above, studies on volatile emissions have indicated that stomatal O3 uptake is the primary driver for elicitation of volatile emissions (Beauchamp et al. 2005; Wieser et al. 2013; Jud et al. 2016). Detailed kinetic analysis of volatile emissions during fumigation and through recovery period allows for making inferences of engagement of different stress response pathways and evaluate the relationships between O3 exposure dose and stress response development and strength of plant response. In fact, because immediate photosynthetic responses during and after stress are primarily driven by modifications in stomatal conductance and the reductions due to direct damage occur later (Kollist et al. 2007; Vahisalu et al. 2008; Vahisalu et al. 2010), we argue that monitoring stress volatile fluxes through stress periods can be much more informative for gaining insight into the kinetics of stress development and propagation of lesions than monitoring changes in net assimilation rate (Beauchamp et al. 2005). However, volatile release during and after O3 exposure is characterized by a complex kinetics with immediate emissions during or shortly after O3 exposure followed by secondary emissions in hours after the stress event (Beauchamp et al. 2005). Due to the complex kinetics, quantitative relationships between different O3 treatments and the timing and magnitude of induced volatile responses are still poorly understood. In particular, it is unclear whether the secondary emissions occurring during the recovery phase, often in many hours after the stress, are quantitatively linked to ozone dose. Especially limited is the information about secondary methanol emissions that might result both from the constitutive activity of cell wall pectin methylesterases, but also from de novo induction of stress-dependent pectin methylesterases (Pelloux et al. 2007).
In the present study, we used common bean (Phaseolus vulgaris) known for its sensitivity to O3 (Wenzel & Mehlhorn 1995) to investigate the effects of acute O3 exposure on net assimilation rate, stomatal conductance and biogenic VOC emissions combining high resolution measurements with a proton transfer reaction-time of flight-mass spectrometer (PTR-TOF-MS) and quantitative analysis of emission kinetics during exposure and through a 22 h recovery period (Table 1 and Fig. 1). Such long-term continuous VOC measurements after ozone exposure are rare in the literature, and to our knowledge, PTR-TOF-MS measurements have not been conducted during ozone exposure. The amount of O3 uptake during acute O3 exposure was altered by changing the light conditions and by pre-exposure to lower O3 concentrations, i.e. using treatments known to lead to stomatal closure. We hypothesized that the lower rate of uptake of O3 during acute exposure periods reduces O3-induced damage and that this is associated with slower induction kinetics, with lower maximum rate of volatile emissions and lower total emissions. Because the leaves of P. vulgaris possess capitate glandular trichomes (Li et al. unpublished observations), which might contribute to total O3 loss, total whole leaf O3 absorption was separated among stomatal and non-stomatal components.
Table 1.
List of symbols used with definitions and units.
| Symbol | Definition | Units |
|---|---|---|
| ϕX | emission rates of given VOC | nmol m-2 s-1 |
| ϕM1, X | maximum emission rate at the first emission peak | nmol m-2 s-1 |
| ϕM2, X | maximum emission rate at the second emission peak | nmol m-2 s-1 |
| ФX | the total amount of given VOC emitted over a certain time | µmol m-2 |
| tP1S | start of the first emission burst since the start of the O3 exposure | h |
| tP1E | end of the first emission burst since the start of the O3 exposure | h |
| tP2S | start of the second emission burst since the start of the O3 exposure | h |
| tP2E | end of the second emission burst since the start of the O3 exposure | h |
| DP1 | duration of the first induced emission peak | h |
| DP2 | duration of the second induced emission peak | h |
| tE1 | time from the onset of O3 exposure to the first emission elicitation | h |
| tE2 | time from the onset of O3 exposure to the second emission elicitation | h |
| tM1 | time from elicitation to the first emission maximum | h |
| tM2 | time from elicitation to the second emission maximum | h |
| τI1 | doubling-time for the increase of emissions during the first emission burst | h |
| τD1 | half-time for the decrease of emissions during the second emission burst | h |
| τI2 | doubling-time for the increase of emissions during the second emission burst | h |
| τD2 | half-time for the decrease of emissions during the second emission burst | h |
| kI1 | rate constant for the initial increase of emissions during the first emission burst | h-1 |
| kD1 | rate constant for the decrease of emissions during the first emission burst | h-1 |
| kI2 | rate constant for the initial increase of emissions during the second emission burst | h-1 |
| kD2 | rate constant for the decrease of emissions during the second emission burst | h-1 |
| ϕO3 | the rate of ozone uptake by stomata | nmol m-2 s-1 |
| ФO3 | the total amount of ozone uptake by stomata | µmol m-2 |
| ϕLO3 | the rate of ozone uptake by whole leaf | nmol m-2 s-1 |
| ФLO3 | the total amount of ozone uptake by whole leaf | µmol m-2 |
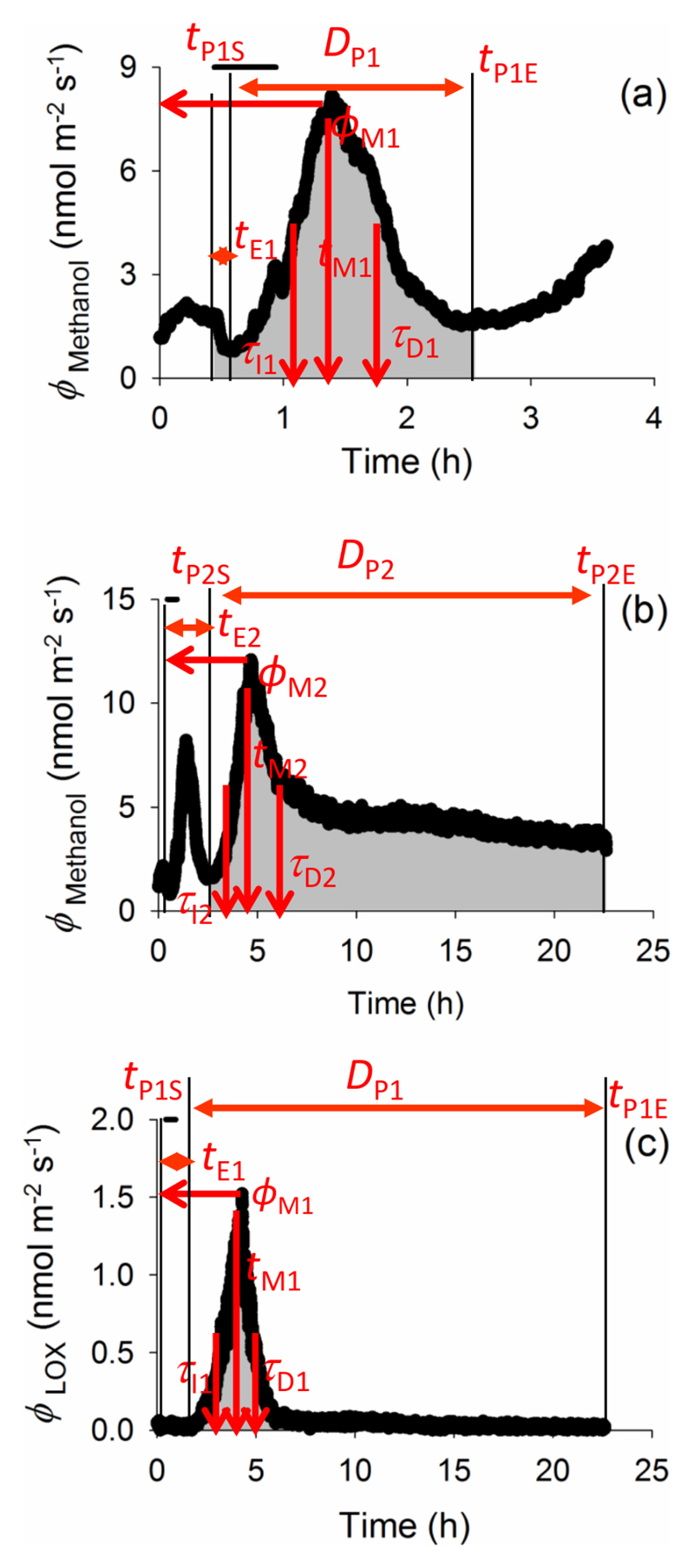
Fig. 1 demonstrates representative time courses of emissions and definition of symbols.
Figure 1.
A typical time-course of biogenic volatile organic compound (VOC) emissions from ozone-exposed Phaseolus vulgaris leaves, and definition of key quantitative emission characteristics: (a) first emission burst of methanol, (b) second emission burst of methanol and (c) emission burst of lipoxygenase pathway (LOX) volatile products (mainly various C6 aldehydes and alcohols). The emission pattern of methyl salicylate (MeSA) resembles LOX emissions in (c). In all cases, tE1 and tE2 are the times from the onset of O3 exposure to the elicitation of the first and (if present) the second burst of the given VOC species, respectively. ϕM1 and ϕM2 correspond to the emission maxima for the two bursts and tM1 and tM2 denote the corresponding times from the start of the elicitation to reaching the maxima. τI and τD indicate the initial doubling- and decay-times for the bursts. Integrated emissions for different emission bursts correspond to the shaded area for the duration of induced-emission bursts, DP, i.e. from the start of the given VOC emission elicitation (tPS) until the end of the given compound emission release (tPE; Eq. 3). As the experiment continued for 22 h after the O3 exposure, methanol emissions might not have always reached the initial level (b), and thus, DP and integrated emissions are somewhat underestimated in these cases. The vertical bars indicate periods for exposure to 600 nmol mol-1 O3.
Materials and Methods
Plant material and growth conditions
Phaseolus vulgaris L. cv. Saxa plants were grown from seed (seed source: DALEMA UAB, Vilnius, Lithuania). After germination, seedlings were replanted in 2 L plastic pots filled with commercial potting soil with NPK (N of 100 mg L-1, P of 30 mg L-1, and K of 200 mg L-1) fertilizer (Biolan Oy, Kekkilä Group, Vantaa, Finland). The plants were grown in a plant room with light intensity at plant level of 400 μmol m-2 s-1 (HPI-T Plus 400 W metal halide lamps, Philips) during a 12 h photoperiod. The day/night temperatures were maintained at 24/20°C and daytime humidity at 60%. During growth, supply of nutrients and water was maintained at close to optimal levels.
The plants were grown under these conditions for 4 weeks before starting the experiments. In all experiments, we used similar-sized plants (average height of 30 cm) with similar number of leaves. All measurements were conducted with fully mature non-senescent trifoliate leaves.
Gas-exchange system for photosynthesis, stomatal conductance and volatile organic compound measurements and for ozone fumigation
A custom-built laboratory gas-exchange system described in detail by Copolovici & Niinemets (2010) was used to measure leaf gas exchange characteristics (photosynthetic rate and stomatal conductance) and volatile organic compound (VOC) emissions and for O3 fumigation. Briefly, ambient air was pumped from outside and passed through a 10 L buffer volume to minimize CO2 fluctuations, purified by passing through a custom-made O3 trap and charcoal filter and humidified to standard water vapor concentration. The conditioned air entered to a water-jacketed, temperature-controlled 1.2 L glass chamber. Chamber flow rate was fixed at 1.6 L min-1 and a fan inside the chamber minimized leaf boundary layer resistance (Rasulov et al. 2009), and thus, for the first order decay kinetics, the chamber response half-time was estimated to be 31 s (Niinemets 2012). The system had two identical gas lines, one passing to the leaf chamber (measurement) and the other passing through an equivalent flow resistance (reference). An electronic valve was used to switch between the air streams going to the gas analyzer. In all measurements, leaf temperature was measured with thermocouple and maintained at 25 °C, ambient CO2 concentration was 380-400 µmol mol-1 and light intensity at the leaf surface was set to 500 µmol m2 s-1 when light was on.
O3 was generated by an O3 generator (Stable Ozone Generator, Ultra-Violet Products Ltd, Cambridge, UK) that has a quartz glass reaction chamber illuminated with UV light (λ=185 nm) for O3 production. Ambient air was passed through the reaction chamber and the air enriched with O3 was mixed with the air stream entering the leaf chamber. The O3 flux into the chamber was controlled by a custom-made flow restrictor. As the O3 concentration in the chamber also depends on plant uptake that can vary through the experiment, the O3-enriched air flow was adjusted during the treatment to compensate for changes in O3 uptake such that the stability of O3 concentration achieved was ± 5-10% of the target concentration.
An infra-red gas analyzer (CIRAS II, PP-Systems, Amesbury, MA, USA) operated in an absolute mode was used to measure CO2 and H2O concentrations. A proton transfer reaction-time of flight-mass spectrometer (PTR-TOF-MS, Ionicon Analytik GmbH, Innsbruck, Austria) was employed to determine volatile concentrations, and a chemiluminescence O3 sensor (Model 49i, Thermo Scientific, Massachusetts, USA) was used to measure O3 concentrations. All instruments were interchangeably connected to the chamber inlets and outlets (Copolovici & Niinemets 2010) and operated continuously. Average CO2 and H2O concentrations and all PTR-TOF-MS signals were recorded every 10 s, and the range and average O3 concentrations were recorded for every 15 min period. Reference measurements (ingoing air) were conducted frequently, typically every 10-15 min.
O3 fumigation treatments
Three different experimental treatments with individual leaves were carried out using O3 concentrations that constituted a moderately severe stress and led to visible leaf damage. In contrast to some other studies (e.g. Beauchamp et al. 2005) these exposures did not result in leaf death, at least during 24 h following the experimental treatment.
After leaf enclosure, continuous measurements of photosynthetic characteristics and trace gas exchange were begun immediately. When photosynthesis and VOC emission rates had stabilized, typically 20-30 min after leaf enclosure, O3 fumigation was started. In Treatment 1, the illuminated leaf was fumigated with 600 ± 33 nmol mol-1 O3 for 30 min. In Treatment 2, the leaf acclimated to chamber light conditions was darkened by turning off the light and covering the chamber with an aluminum foil. Following stomatal closure (after 20-30 min in darkness), the leaf was exposed to 600 ± 30 nmol mol-1 O3 for 30 min in darkness. After the O3 exposure, the light was turned on again. Finally, in Treatment 3, the illuminated leaf was exposed first to 200 ± 11 nmol mol-1 O3 for 30 min, after which the O3 concentration was raised to 600 ± 32 nmol mol-1 for an additional 30 min. After each of the three treatments, foliage gas-exchange characteristics and trace gas-exchange were continuously monitored for 21 h under illumination as detailed below. The treated leaves were then removed from the chamber and their area and the quantitative degree of damage were estimated using an image analysis software (Image J, National Institutes of Health, Bethesda, MD, USA). For each treatment, we chose three mature and healthy plant individuals with the same age and same number of leaves, and selected from each plant individual a representative fully mature same-aged and similar-sized leaf for the measurements.
Operation of PTR-TOF-MS and stress volatile estimation
PTR-TOF-MS allows for real-time monitoring of key plant stress-elicited volatiles, and its main advantages over the traditional quadrupole PTR (PTR-QMS) are higher mass resolution and a simultaneous rather than sequential detection of all VOC masses (Jordan et al. 2009; Graus et al. 2010). Operation of the PTR-TOF-MS followed the method described in detail in Graus et al. (2010), Brilli et al. (2011) and Portillo-Estrada et al. (2015). In short, the conditions for PTR-TOF-MS operation were: 60 °C drift tube temperature, 600 V drift voltage and 2.3 mbar drift pressure, corresponding to an E/N ≈ 130 Td in H3O+ reagent ion mode. PTR-TOF-MS was calibrated with a commercial volatile standard containing representatives of key plant volatiles (Ionimed GmbH, Innsbruck, Austria) in the beginning and at the end of the experiment and no changes in sensitivity were observed between these calibration events. In addition, measurements with pure standards, simultaneously with PTR-TOF-MS and GC-MS were further used to check for stability of the compound concentration in the commercial standard and obtain calibration factors for individual volatiles missing in the calibration mixture. The raw data were acquired by the TofDaq software (Tofwerk AG, Switzerland) and post-processed by PTR-MS Viewer software (PTR-MS Viewer v3.1, Tofwerk AG, Switzerland) (Jordan et al. 2009; Portillo-Estrada et al. 2016).
Methanol and methyl salicylate (MeSA) were detected as the protonated parent ions at m/z of 33.034 and 153.088, respectively (Brilli et al. 2011; Tasin et al. 2012; Maja et al. 2014; Brilli et al. 2016; Giacomuzzi et al. 2016; Portillo-Estrada et al. 2016; Yener et al. 2016; Portillo-Estrada et al. 2017). To identify the signals of volatile products of the lipoxygenase pathway (LOX) in the blend of parent and fragment masses detected by PTR-TOF-MS, pure chemicals (Sigma-Aldrich, St. Louis, MO, USA) were individually analyzed and mass signals consistent with previous studies were found (e.g. Brilli et al. 2011, Portillo-Estrada et al. 2016). The total LOX product emission presented in this study is the sum of the dominant compounds with m/z of 81.070, 83.085, 85.101, 99.080, 101.096 and 143.107, representing 2-hexenal and 3-hexenal (C6H10O)H+, 3-hexenol (C6H12O)H+, 1-hexanol (C6H14O)H+, 3-hexenol (C6H12O)H+ and hexenyl acetate (C8H14O2)H+, respectively (Beauchamp et al. 2005; Giacomuzzi et al. 2016; Portillo-Estrada et al. 2016; Portillo-Estrada et al. 2017). The identity of O3-induced LOX compounds and MeSA from P. vulgaris leaves was also confirmed in pilot experiments by separate GC-MS analyses carried out according to the protocol of Copolovici et al. (2012) and Kännaste et al. (2014). We estimated that for MeSA and LOX volatiles, the detection limit with our setup was better than 0.05 nmol m-2 s-1 and for methanol better than 0.2 nmol m-2 s-1. Given that the key volatiles elicited upon ozone exposure are relatively non-reactive with ozone (Arneth & Niinemets 2010; Holopainen et al. 2013 for the reaction rate constants for ozone), and that the emissions were elicited mostly after the ozone exposure (Fig. 1), we did not consider possible volatile losses during ozone exposure.
Chlorophyll fluorescence measurements
Photosystem II (PS II) activity was assessed for each individual leaf before and after the O3 treatment with a portable Imaging PAM chlorophyll fluorimeter and ImagingWin software (IMAG-MIN/B, Heinz Walz GmbH, Effeltrich, Germany). The MINI version of the Imaging PAM M-series has a measurement window area of 24×32 mm. A CCD camera (640×480 pixels) serves for fluorescence imaging and 12 high-power LED lamps provide actinic light and high-intensity light flashes. After the leaf was fixed in the leaf holder of the Imaging PAM and dark adapted for 30 min, the minimum (Fo) fluorescence yield was measured. The leaf was further illuminated with a 500 ms pulse of saturating irradiance (2700 µmol quanta m-2 s-1) to measure the maximum (Fm) dark-adapted fluorescence yield. The spatial-average maximum dark-adapted quantum yield of PSII, Fv/Fm was calculated as (Fm-Fo)/Fm.
Empty chamber corrections
Before inserting the leaves in the gas-exchange chamber, the background VOC emission rates from the empty chamber were measured to correct for possible release of volatiles adsorbed previously on the gas-exchange system components. In general, such corrections were minor (1%), but are included in the calculations for consistency (Niinemets et al. 2011). In addition, O3 destruction due to surface reactions (“uptake”) by an empty chamber ( nmol mol-1) was measured before inserting the leaves in the chamber and calculated as:
| (1) |
where is the O3 concentration at the chamber inlet (either 200 or 600 nmol mol-1)and that at the chamber outlet. The obtained values were subsequently used to correct all measurements of leaf O3 uptake. Overall, this correction was small, less than 5% of total O3 uptake when leaves were enclosed in the chamber.
Calculations of photosynthetic characteristics, trace gas emission rates and O3 uptake
The net assimilation rate (A) and stomatal conductance for water vapor (gs) were calculated per unit enclosed leaf area according to the equations of von Caemmerer & Farquhar (1981). We estimated that with our measurement system, the accuracy was better than 0.3 μmol m-2 s-1 for net assimilation rate and better than 0.15 mmol m-2 s-1 for the transpiration rate.
Volatile emissions rates (ϕX, nmol m-2 s-1) were calculated as:
| (2) |
where Co(X) is the concentration (nmol mol-1) of the target VOC (compound X) measured at the chamber outlet and Ci(X) that measured at the chamber inlet, Cc(X) is the correction to account for possible release of the given compound from system components as explained above (generally negligible in the current study), F is the molar flow rate through the chamber (1.19 * 10-3 mol s-1), and S is the leaf area enclosed in the chamber (m2) (Beauchamp et al. 2005).
The temporal pattern of VOC emissions showed an exponential increase shortly after initiation of O3 exposure, followed by a non-linear decay after the emission had reached a maximum value. Various aspects of the kinetics of the rise and decay of VOC emission rates were analyzed, and the key quantitative emission characteristics estimated using first order exponential rise and decay models for different parts of the time kinetics of emissions (Table 1 for acronyms and definitions of all quantitative characteristics). The exponentially increasing part of the response was quantitatively analyzed according to:
| (3) |
where ϕ(t) is the emission rate at time t and ϕ(tPS) is the VOC emission rate measured at the start of the emission burst following the start of O3 exposure. The parameter kI is the rate constant for the initial increase of VOC emission rate, and the corresponding doubling-time (τI) for the emission burst is calculated as ln(2)/kI.
The decreasing part of the emission burst was analyzed quantitatively by:
| (4) |
where ϕM is the maximum VOC emission rate. Analogously, the parameter kD is the rate constant for the decrease of VOC emission rate, and the corresponding half-time (τD) for the emission burst is calculated as ln(2)/kD. Figure 1 shows a typical example of VOC emission release patterns of this experiment, as well as the definition of key quantitative emission characteristics used above. When a second emission burst was present, decay and rise characteristics for this second rise of emissions were also calculated (Fig. 1, Table 1).
The total amount of given VOC emitted over a certain time (ФX) (nmol m-2) was calculated as:
| (5) |
where tPS and tPE are the start and end times of the release of given VOC species, respectively, Δt is the measurement time interval (10 s), and ϕX is the emission rate of the given compound measured over this time interval (Beauchamp et al. 2005). The total emission values presented here correspond to 21 h after starting of elicitation of given compound (Dp) (Table 1 and Fig.1).
The rate of O3 uptake by whole leaf (ϕLO3, nmol m-2 s-1) was calculated as:
| (6) |
where Cin and Cout are the O3 concentrations of the air entering and exiting the leaf chamber (nmol mol-1) and is the amount of O3 removed by the empty chamber (nmol mol-1). In this study we calculated Cin and Cout as the average values over a certain time due to the fluctuations of concentration produced by O3 generator and manual adjustment of Cin to account for changes in O3 uptake during the exposure.
The rate of O3 uptake by stomata (ϕO3, nmol m-2 s-1) was determined by:
| (7) |
where Cout is the O3 concentrations of the air exiting the leaf chamber (nmol mol-1) and is intercellular O3 concentration (nmol mol-1), and 2.03 is the ratio of water vapor to O3 diffusivities. This estimate was derived from O3 diffusion coefficient in air of 1.267 * 10-5 m2 s-1 at 22.84 °C (Ivanov et al. 2007) and water vapor diffusion coefficient in air of 2.569 * 10-5 m2 s-1 at the same temperature calculated according to Chapman and Enskog (Niinemets & Reichstein 2003a). Analysis of possible temperature effects on the collision integral for both water vapor and O3 (Tucker & Nelken 1982) suggested that temperature effects on this ratio were minor (the ratio differed less than 0.1% for 25 °C used for leaf measurements and 22.84 °C used to estimate O3 diffusivity).
Although it has for long been considered that the internal O3 concentration is close to zero because of its high reactivity once taken up (Laisk et al. 1989), Moldau & Bichele (2002) showed that very high ambient concentrations of O3 led to low, but not zero, internal O3 concentrations , biasing O3-uptake flux calculations up to 5%. Since the internal O3 concentration was unknown, this correction was not considered in our study. Given that an average value had to be used for Cout, the corresponding average value of stomatal conductance (gs) was used in these calculations. The rate of non-stomatal O3 uptake was calculated as the difference between total (Eqn 6) and stomatal uptake rates (Eqn 7).
The total amount of O3 uptake (ϕY) over the given exposure period (O3 dose) was calculated as:
| (8) |
where t is the time after starting O3 exposure, Δt is the time interval duration of the O3 exposure (30 min), and ϕY is the mean rate of O3 uptake by the whole leaf through stomata measured during this time interval (Beauchamp et al. 2005). Total and non-stomatal O3 uptakes were calculated analogously.
Statistical analyses
All treatments were conducted in three replications with different plants. The statistical significance of the effects of O3 exposure treatment were tested with one-way ANOVA followed by Tukey’s post hoc test using SPSS 16.0 (SPSS, Chicago, Illinois, USA). In addition, linear- and non-linear regressions were fitted to the data based on the shape of the response. All statistical tests were considered significant at P < 0.05.
Results
Leaf damage, ozone uptake, chlorophyll fluorescence and photosynthesis
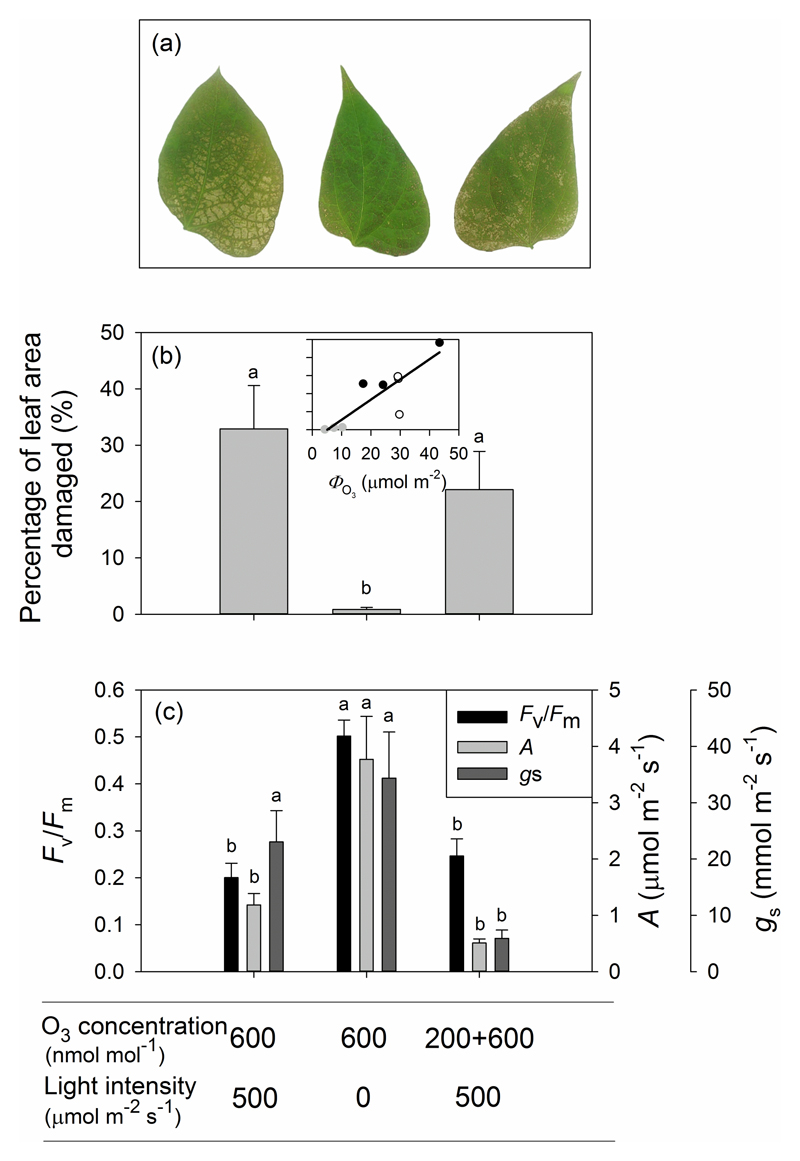
Although necrotic spots on leaves were visible in all treatments after O3 exposure, the area of necrotic lesions in Treatment 2 (exposure to 600 nmol mol-1 O3 in the darkness) was much less than in Treatments 1 (exposure to 600 nmol mol-1 O3 in the light) and 3 (exposure first to 200 nmol mol-1 and then to 600 nmol mol-1 O3 in the light; Fig. 2a,b). Photosynthetic rates (A) and the maximum quantum efficiency of PSII (Fv/Fm) obtained from chlorophyll fluorescence measurements were significantly reduced in P. vulgaris leaves after O3 exposure in both Treatments 1 and 3 compared with Treatment 2 (Fig. 2c). However, there was no statistical difference in average stomatal conductance (gs) after treatment between Treatments 1 and 2 (Fig. 2c; P > 0.10). Relative to the values prior to O3 fumigation, Fv/Fm decreased on average (±SE) by 74 ± 3.9 % in Treatment 1, 31 ± 6.6 % in Treatment 2 and 67 ± 4.8 % in Treatments 3. The declines were also observed in photosynthetic rate (A) under O3 exposure in all treatments, 87 ± 3.6 % for Treatment 1, 59 ± 8.3 % for Treatment 2 and 93 ± 1.7 % for Treatment 3.
Figure 2.
Illustration of visible leaf injury in representative O3-fumigated leaves (a), percentage of leaf area damaged (b) and physiological data collected from P. vulgaris leaves before O3 fumigation and 21 hours after O3 fumigation (c). The relationship between the percentage of leaf area damaged and total amount of O3 uptake (ΦO3) in (b) were fitted by a linear regression (y=1.12x-5.69; r2=0.74, P<0.005). Error bars denote ± standard error (SE). The means were compared by ANOVA and the means separated by Tukey’s tests. Significant differences (P < 0.05) between means are indicated by different letters.
Correlations between O3 uptake and stomatal conductance
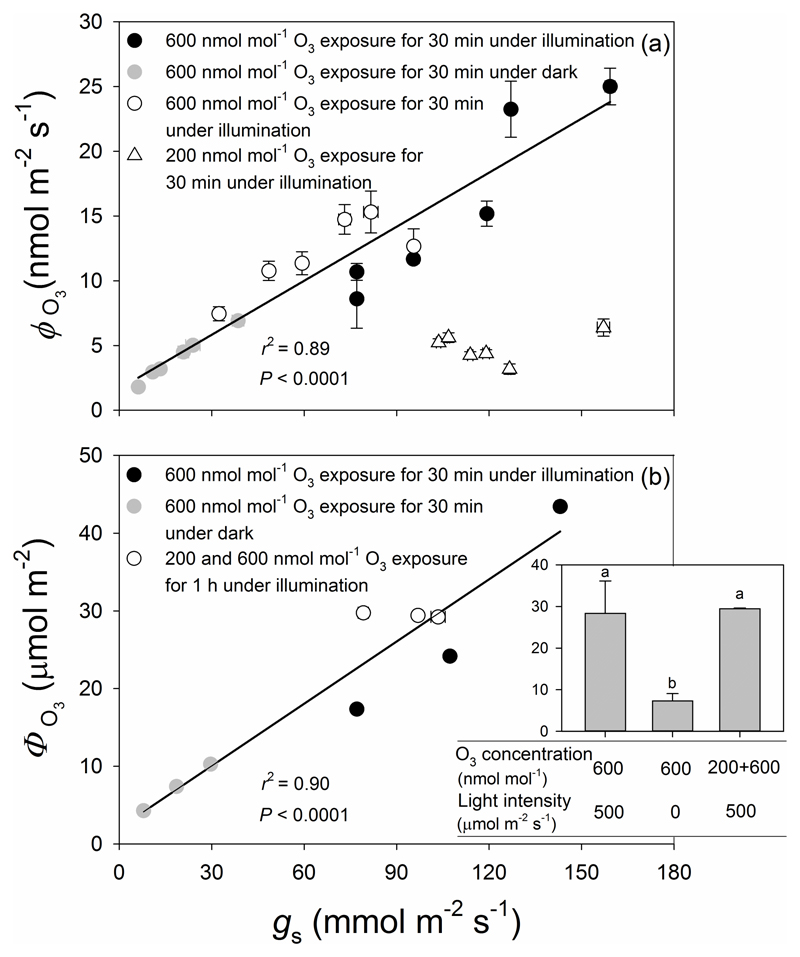
A strong linear relationship was found between O3 uptake flux and stomatal conductance when leaves were fumigated under a constant O3 concentration of 600 nmol mol-1 (P < 0.0001; circles in Fig. 3a). Although the range of stomatal conductance for fumigations with 200 nmol mol-1 O3 was small (triangles, Fig. 3a), the rate of O3 uptake at a given conductance of about 110 mmol m-2 s-1 was approximately 30 % of that obtained at 600 nmol mol-1 O3, i.e., the difference in uptake between these treatments scaled was strongly driven by the ambient O3 concentration. In addition, the total rate of O3 uptake (non-stomatal + stomatal) (ϕLO3) was similarly correlated with stomatal uptake (data not show), which indicates that the O3 uptake rate was highly dependent on the O3 concentration during fumigation (Table 2).
Figure 3.
Stomatal conductance (gs) in relation to O3 uptake rate per leaf area (ϕO3) (a) and total amount of O3 uptake (ФO3) over the given exposure period (b and inset) in O3-exposed leaves of P. vulgaris. Black circles correspond to illuminated leaves fumigated with 600 ± 33 nmol mol-1 of O3 for 30 min. Grey circles correspond to leaves exposed to 600 ± 30 nmol mol-1 of O3 for 30 min in darkness. White triangles and circles correspond to the same treatment, for illuminated leaves exposed first to 200 ± 11 nmol mol-1 of O3 for 30 min (white triangles) and then to 600 ± 32 nmol mol-1 of O3 for an additional 30 min (white circles). Data for 600 nmol mol-1 O3 fumigation for 30 min were fitted by linear regressions (for (a): y=0.139x+1.67; r2=0.89, P<0.0001; for (b): y=0.267x+2.07; r2=0.90, P<0.0001). Error bars indicate ± SE.
Table 2.
Average ±SE O3 uptake by the entire leaf (ΦLO3, μmol m-2) and via the stomata (ΦO3, μmol m-2) and percentage of non-stomatal O3 uptake (%) from leaves of Phaseolus vulgaris in response to different O3 treatments under illumination and in darkness.
| O3 uptake | O3 concentration (nmol mol-1) | ||
|---|---|---|---|
| 600 | 600 | 200 + 600 | |
| Light intensity (μmol m-2 s-1) | |||
| 500 | 0 | 500 | |
| O3 uptake by the entire leaf (ΦLO3, μmol m-2) | 66.2 ± 9.6 a* | 24.3 ± 4.9 b | 64.9 ± 5.7 a |
| O3 uptake via the stomata (ΦO3, μmol m-2) | 28.3 ± 7.8 a | 7.3 ± 1.7 b | 29.5 ± 0.15 a |
| Percent of Non-stomatal O3 uptake (%) | 58.4 ± 6.5 a | 70.3 ± 1.4 a | 53.9 ± 4.0 a |
The O3 treatment 200 + 600 nmol mol-1 denotes a 30 min pre-exposure to 200 nmol mol-1 O3 (priming) followed by a further 30 min exposure to 600 nmol mol-1 O3.
different letters indicate statistical significance at P < 0.05.
The amount of O3 taken up by leaves in the three treatments shown in Fig. 3b reflects differences in stomatal conductance over the course of the O3 fumigation period. Thus, total O3 uptake in Treatment 1, in which fumigation took place under illuminated conditions, was substantially greater than the uptake in Treatment 2, in which fumigation occurred in darkness and stomatal conductance was substantially less (84.7 ± 3.1% less) than in Treatment 1. Although the leaves in Treatment 3 were exposed to 200 nmol mol-1 O3 for 30 min prior to further 30 min exposure to 600 nmol mol-1, the total amount of O3 taken up was not statistically different from that taken up by the leaves in Treatment 1 because of more rapid stomatal closure during 600 nmol mol-1 fumigation (Table 2). In addition, a strong non-linear relationship (P < 0.0001) was found between the total amount of O3 taken up (ΦO3) and the percentage of leaf area damaged (Fig. 2b). More than half of total O3 uptake (ΦLO3) was due to leaf surface deposition and there were no significant differences in the percentage of non-stomatal O3 uptake among the treatments (Table 2).
Time-courses of gas-exchange during and after O3 exposure
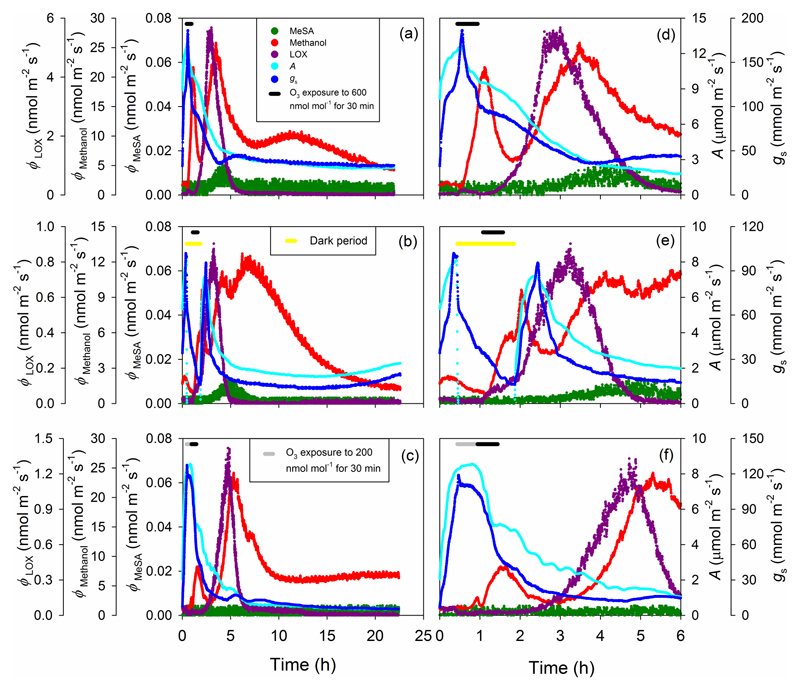
Both photosynthetic rate (A) and stomatal conductance (gs) rapidly decreased in all Phaseolus vulgaris leaves after O3 exposure, and representative kinetics in each of the three Treatments used to estimate the key kinetic characteristics (Fig. 1, Table 1) are analyzed in the following (Fig. 4). For the representative leaf in Treatment 1, the photosynthetic rate (A) was approximately 9.6±1.3 µmol m-2 s-1 prior to O3 fumigation, but began to decline shortly after the start of fumigation, declining by ~20% over the 30 min fumigation period (Fig. 4a,d). Following fumigation, photosynthetic rate continued to decline almost linearly for approximately 3 hours, reaching a value of 5.2 ± 0.2 µmol m-2 s-1. Photosynthetic rate continued to decline slowly before leveling off at ~1.4 µmol m-2 s-1 after about 12 hours. Stomatal conductance (gs) evinced a sudden transient increase at the beginning of O3 fumigation and then declined in parallel with photosynthetic rate (A) for the first 3 hours following fumigation (Fig. 4a,d). Stomatal conductance then recovered slightly, and remained stable for the remaining 18 hours of the experiment at a value approximately 30% of the pre-fumigation value.
Figure 4.
Representative time-courses of photosynthetic rates, stomatal conductance and induced volatile emissions in O3-exposed P. vulgaris leaves for three different treatments. In the first treatment (a and d), the leaf was exposed to 600 nmol mol-1 O3 under continuous illumination for 30 min, in the second treatment (b and e), the leaf was exposed to 600 nmol mol-1 O3 in darkness for 30 min, and in the third treatment (c and f), the leaf was exposed to 200 nmol mol-1 O3 for 30 min and to 600 nmol mol-1 O3 for 30 min under continuous illumination. In (a)-(c), the whole measurement period including the period for leaf adaptation within the leaf chamber prior to fumigation, fumigation period and recovery period after fumigation until 22 hour is shown. In (d)-(f), the same data are zoomed out for the period from the onset of measurement until 6 hours. In all cases, black bars on the top left indicate the period of 600 nmol mol-1 O3 fumigation, the grey bar denotes the period of 200 nmol mol-1 O3 fumigation and the yellow bar denotes the leaf darkening period. LOX stands for volatile products of the lipoxygenase pathway and MeSA for methyl salicylate.
For the representative leaf in Treatment 2 (Fig. 4b,e), the pre-fumigation rate of photosynthesis in the light was 7.97 ± 0.08 µmol m-2 s-1. When the leaf was darkened, including the 30 min O3 fumigation period, A became negative, and gs declined by about 86% from its previous value in the light. During the first 30 min after the light was turned back on, both the photosynthetic rate (A) and stomatal conductance recovered rapidly, returning to nearly the same levels as before darkening, but both then declined sharply over the next 60 minutes to only ~50% of their initial values. Both A and gs then continued to decline slowly over the next 11 hours, and then both started to recover at about 12 hours after the fumigation. Although the photosynthetic rate in Treatment 2 declined significantly from pre-fumigation values, A was less affected than in Treatment 1, never falling below about 30% of the pre-fumigation value.
In treatment 3, neither photosynthetic rate (A) nor stomatal conductance (gs) was affected by the 30-min exposure to 200 nmol mol-1 O3, but when O3 concentration was raised to 600 nmol mol-1 for 30 min, changes in photosynthetic characteristics were similar to those in the Treatment 1 (Fig. 4c,f). Both A and gs fell by 41% during the 600 nmol mol-1 fumigation period, then continued to decline more slowly over the following 4 hours before leveling off at low values about 11 hours after stopping the fumigation (Fig. 4c,f). Although gs exhibited two slight transient periods of recovery, between ca. 4-6 h and 7-9 h (Fig. 4c), gs was more strongly affected by O3 fumigation in Treatment 3 than in either Treatments 1 or 2. The effects of fumigation on A were very similar in Treatments 1 and 3, and more severe than in Treatment 2. In addition, during the 21 hours following the end of O3 exposure, a significant recovery of photosynthetic rate (A) and stomatal conductance (gs) was observed only in the Treatment 2 (Fig. 4b).
Time-courses of emissions of methanol, LOX products and MeSA
Prior to ozone exposure, methanol emission rates did not differ significantly among plants used in different treatments (1.77 ± 0.158 nmol m-2 s-1, 1.76 ± 0.368 nmol m-2 s-1 and 1.66 ± 0.247 nmol m-2 s-1 for Treatments 1, 2 and 3, respectively), and the emissions of volatile LOX products and MeSA were not detectable. During and following the exposure to O3, characteristic emissions bursts of methanol, LOX volatiles and MeSA were observed (Fig. 4 for the temporal patterns emissions for representative leaves in each of the three treatments). All elicited VOC emissions exhibited a sigmoidal rise to a peak value and then decayed to near pre-fumigation values (Fig. 4), and the emissions of these key volatile classes followed the same general pattern in all leaves, but there were specific compound and treatment differences as discussed in the following.
Methanol emissions exhibited a bi- or tri-phasic response pattern. A few minutes after the onset of fumigation with high O3 of 600 nmol mol-1, methanol emissions began to rise, peaking shortly after the fumigation ended and then declined rapidly (methanol burst 1; Fig. 4). A second, much larger burst of methanol emissions was observed in all experiments beginning 1-2 hours after the end of the fumigation and peaking several hours later (methanol burst 2; Fig. 4). In most cases, a third rise of methanol emission was also observed (Fig. 4a,b).
For the first methanol emission burst, in Treatments 1 and 3 where O3 fumigation was carried out in the light (Fig. 4a,c,d,f), methanol emissions increased within 10 min since the start of the fumigation and began to fall rapidly within 10 min after cessation of the fumigation. In Treatment 2 where O3 fumigation occurred in darkness (Fig. 4b,e), the elicitation response was similar, but in contrast to illuminated leaves, when the fumigation period ended, methanol emissions remained constant in the dark for an additional 15 minutes. When the leaf was then illuminated, a second short burst of methanol emissions was observed, perhaps associated with rapid stomatal opening (Fig. 4e). In Treatment 3 where the leaves were exposed first to a lower O3 dose of 200 nmol mol-1, the induction of methanol emissions was very slight (Fig. 4f). When the exposure concentration was raised to 600 nmol mol-1, methanol emissions increased rapidly, exhibiting dynamics similar to Treatment 1.
The second burst of methanol emission was similar in all treatments (Fig. 4a,b,c). However, in Treatment 1, a broad, but a smaller late rise of methanol emission occurred at about 12 h since the start of fumigation, while in Treatment 2, this tertiary rise occurred earlier, at ca. 6 h and partly run into the second emission burst (Fig. 4b). In Treatment 3, this tertiary peak was almost absent, and overall, the long-term methanol emissions decreased earlier than in the two other treatments (Fig. 4c,f).
No enhancement of LOX product emissions was observed during and immediately after O3 exposure, but in all cases, exposure to O3 resulted in a single large burst of LOX product emissions beginning 1-2 h after the exposure, and peaking just before the second methanol emission burst. A burst of MeSA emissions was observed in all leaves of Treatments 1 and 2 (Fig. 4a,b,d,e), but not in any of the leaves in Treatment 3 (Fig. 4c,f). The onset, duration and magnitude of LOX product and MeSA emissions varied with treatment as discussed below.
Maximum and total integrated O3-elicited volatile emissions
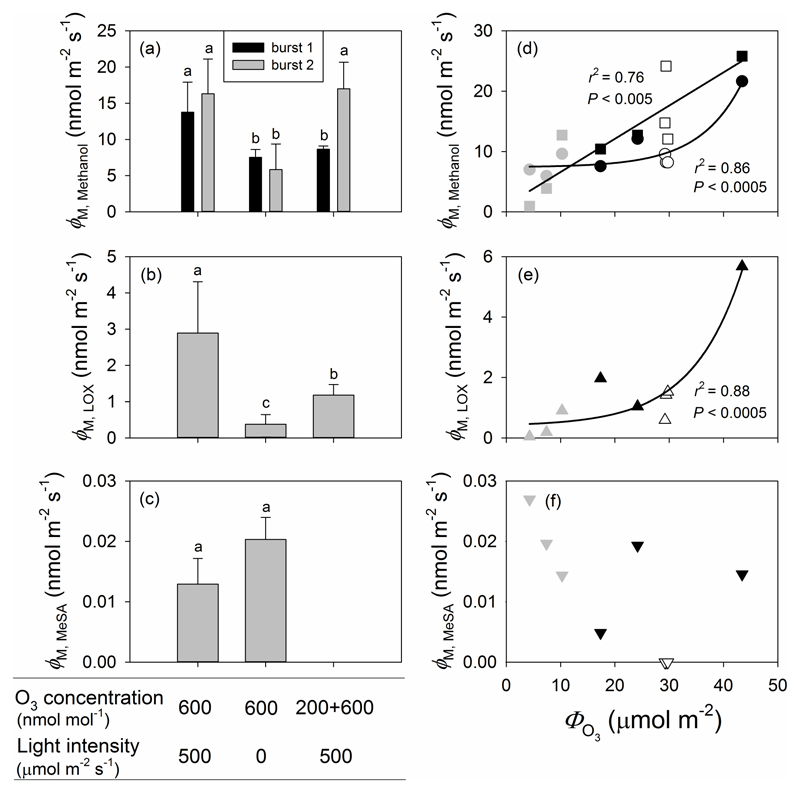
Despite qualitatively similar emission dynamics discussed above, quantitatively, the size of the VOC emission bursts and the total amount of methanol and LOX products emitted over the 21 h recovery period following O3 exposure differed between the three treatments. The maximum emission rates observed during the initial burst of methanol (burst 1) were significantly greater in Treatment 1 than in either Treatments 2 or 3, which did not differ significantly from each other (Fig. 5a). In addition, a strong non-linear relationship (P < 0.0005) was found between the total amount of O3 taken up (ΦO3) and the maximum emission rate of the first methanol burst (Fig. 5d). In contrast, the maximum methanol emission rates observed during the second burst were significantly higher in both Treatments 1 and 3 than in Treatment 2, and the correlation between total amount of O3 taken up (ФO3) and the maximum emission rate of the second methanol emission burst was linear (P < 0.005; Fig. 5d).
Figure 5.
Maximum emission rates of methanol (a), LOX products (b) and MeSA (c), and corresponding correlations with the total amount of O3 uptake (ФO3) (d, e, f) in O3-exposed P. vulgaris leaves. The MeSA emission burst was absent in Treatment 3 in (c). In all cases, black symbols denote data from the illuminated leaves fumigated with 600 ± 33 nmol mol-1 O3 for 30 min (Treatment 1), grey symbols correspond to leaves exposed to 600 ± 30 nmol mol-1 O3 for 30 min in darkness (Treatment 2), and white symbols denote the illuminated leaves first exposed to 200 ± 11 nmol mol-1 O3 for 30 min and then to 600 ± 32 nmol mol-1 for additional 30 min (Treatment 3). In (d), the data for the first methanol emission burst (circles) were fitted by an exponential regression (y=0.0569 * 1.14x+7.40; r2=0.86, P<0.0005) and the data for the second methanol emission burst (squares) were fitted by a linear regression (y=0.55x+1.10; r2=0.76, P<0.005). In (e), the date were fitted by an exponential regression (y=0.0453 * 1.11x+0.389; r2=0.88, P<0.0005). Error bars indicate ± SE. Statistical data analysis as in Fig. 2.
The maximum emission rate of LOX products was greater in Treatment 1 than in either Treatment 2 or 3 (Fig. 5b), and it was strongly correlated with the total amount of O3 taken up (ФO3) (P < 0.0005; Fig. 5e). No significant differences in maximum emission rates of MeSA between Treatments 1 and 2 leaves were found, while MeSA emission was undetectable in Treatment 3 (Fig. 5c), and the maximum MeSA emission rate was not correlated with the O3 dose (Fig. 5f).
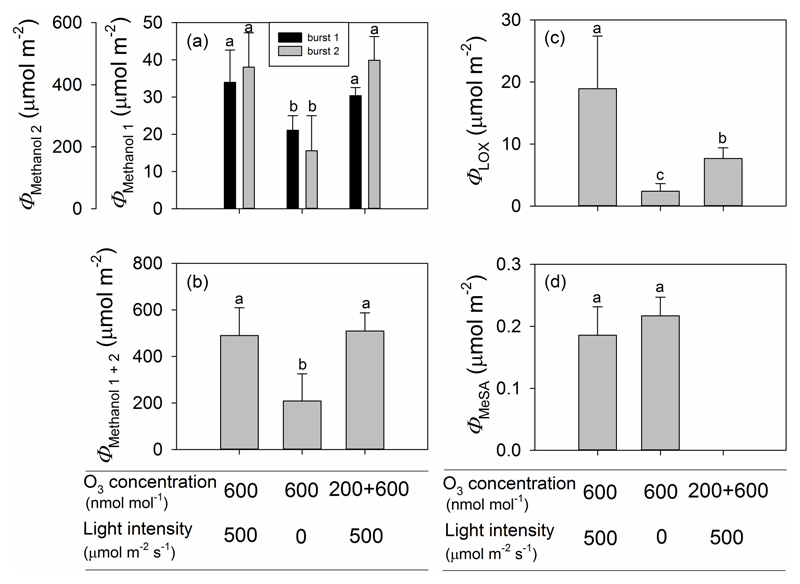
Overall, the amount of methanol released during the second bursts (after the initial methanol burst, Fig. 4a,b,c) was much greater, 10-25-fold greater, than the amount of methanol released during the first burst, reflecting the circumstance that the second burst of methanol emission persisted for over five hours (Fig. 6a). Differently from the maximum methanol emission rate during burst 1 (Fig. 5a), no significant difference was observed in the total amount of methanol released during burst 1 between Treatments 1 and 3 (Fig. 6a). Leaves in both these treatments, however, released significantly more methanol during the first burst than those in Treatment 2 (Fig. 6a). Consistent with observed differences in maximum peak height (Figs. 4a, b, c), the total amount of methanol emissions during methanol burst 2 was significantly lower in Treatment 2 than in either Treatments 1 or 3, which did not differ significantly from each other (Fig. 6a). The total emission of methanol during O3 exposure and through the recovery phase in Treatments 1 and 3 exceeded more than twice that in Treatment 2, because the emissions during the secondary bursts dominated the total emissions (Fig. 6b).
Figure 6.
Total amount of emitted methanol for the first and for the second methanol emission burst (a), the sum two methanol emission bursts (b), total LOX product emission (c) and total MeSA emission (d) for three O3 treatments in P. vulgaris leaves. Error bars stand for ± SE. Treatments as in Fig. 5 and statistics as in Fig. 2.
Total LOX emissions were vastly different among treatments and the treatments ranked according to total emission of LOX products as Treatment 1 > Treatment 3 > Treatment 2 (Fig. 6c). Consistent with the data on maximum emission rates of MeSA (Fig. 5c), total amount of MeSA released did not differ significantly between Treatments 1 and 2 (Fig. 6d).
Start and rate of elicitation and decay of volatile emissions
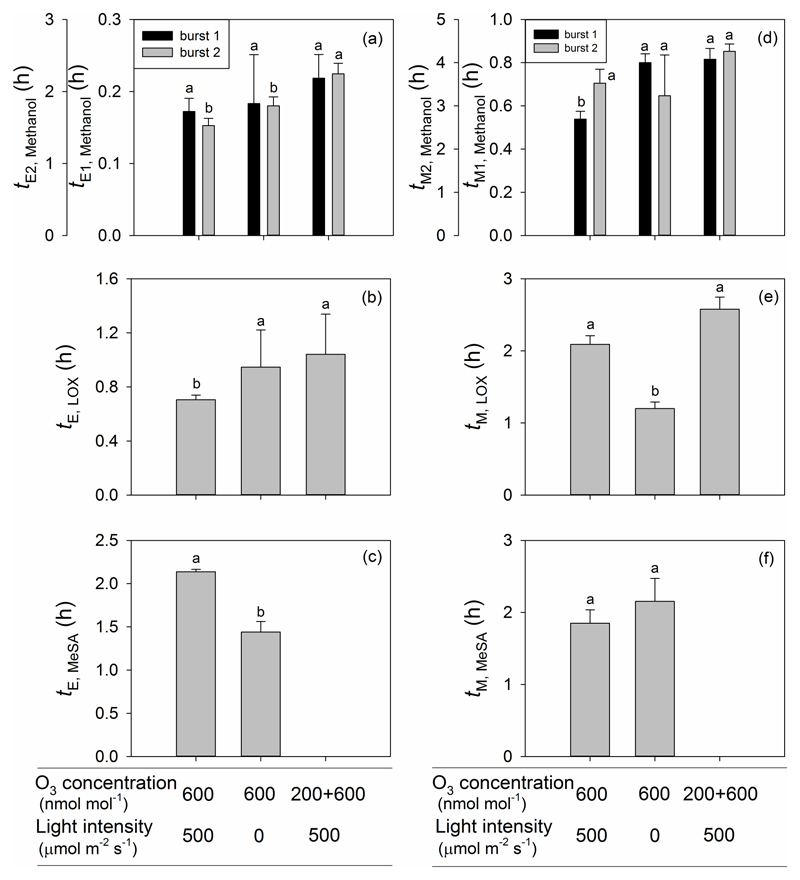
The delay between the onset of O3 exposure and the initiation of the first methanol burst (tE1, Fig. 1 for definitions) was about 10 min and it did not differ significantly between the treatments (Fig. 7a). The time from the onset of elicitation to the maximum of the first methanol burst (tM1) was significantly lower in Treatment 1 than in Treatments 2 and 3 (Fig. 7d), and this was also similar for the duration of the emission burst lasting on average (± SE) 1.28 ± 0.145 h, 1.87 ± 0.196 h and 1.76 ± 0.375 h for Treatments 1, 2 and 3, respectively (the mean for the Treatment 1 is significantly different from the two other treatments at P < 0.05). However, the time until the second burst was initiated (tE2) was significantly less in Treatments 1 and 2 than in Treatment 3 (Fig. 7a). There was no significant difference in the time from the onset of elicitation to the second methanol peak in all cases (Fig. 7d), but the duration of the second methanol emission burst (Fig. 4) was longer in Treatments 1 (19.5 ± 0.164 h) and 3 (19.0 ± 0.125 h) than in Treatment 2 (14.8 ± 2.23 h, means are significantly different at P < 0.02). The burst of LOX products after the onset of O3 fumigation occurred earlier in Treatment 1 than in Treatment 2 and 3 (Fig. 7b), and the time from the onset of elicitation to the peak in emissions of LOX products was significantly greater in Treatments 1 and 3 than in Treatment 2 (Fig. 7e). In contrast, the duration of the LOX burst (Fig. 4) was the greatest in Treatment 3 (9.10 ± 1.77 h), followed by Treatment 1 (5.53 ± 0.206 h) and Treatment 2 (3.36 ± 0.058 h; the means are statistically significant at P < 0.01). The MeSA emission burst occurred earlier in Treatment 2 than in Treatment 1 (Fig. 7c), but the time from the onset of elicitation to the peak of MeSA emission did not differ among Treatments 1 and 2 (Fig. 7f).
Figure 7.
The lag time from the onset of O3 exposure to the elicitation (a-c), and from elicitation to the maximum emission (d-f) for the first and second burst of methanol (a, d), LOX products (b, e) and MeSA (c, f) for three O3 treatments in P. vulgaris leaves. Error bars indicate ± SE. Treatments as in Fig. 5 and statistical analysis as in Fig.2.
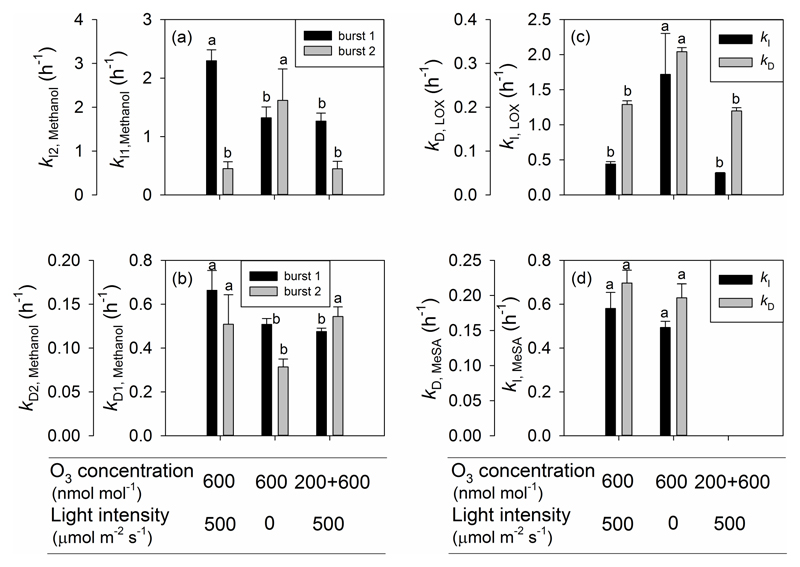
To further characterize O3 effects on the emission kinetics, the rate constants for induction (kI, Eqn 4) and decay (kD, Eqn 5) were determined. For the first burst of methanol emission, both the initial increase and decrease of emissions were faster in Treatment 1 than in either Treatments 2 or 3 (Fig. 8a,b). For the second burst of methanol release, the initial increase of emissions was faster and decrease was slower in Treatment 2 than in either Treatments 1 or 3 (Fig. 8a,b). Both the rise and decline kinetics were much faster for the LOX product emission burst in Treatment 2 than in either Treatments 1 or 3 (Fig. 8c). However, for MeSA emission, there were no significant differences in kI and kD among Treatments 1 and 2 (Fig. 8d).
Figure 8.
The rate constant for the initial increase for the first and the second emission burst of methanol (a), LOX products (c) and MeSA (d), and the rate constant for decrease for the first and the second emission burst of methanol (b), LOX products (c) and MeSA (d) for three treatments in O3-exposed P. vulgaris leaves. Error bars indicate ± SE. Treatments as in Fig. 5 and statistical analysis as in Fig. 2.
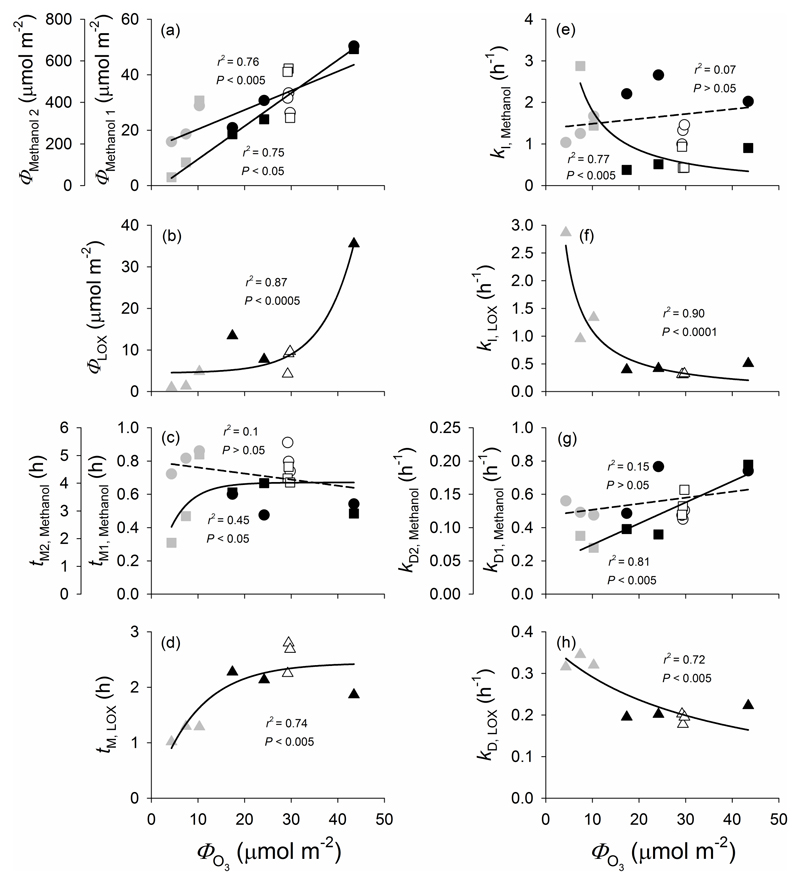
The total emissions of the first and second methanol emission bursts and LOX products were strongly correlated with total amount of O3 taken up (ФO3) (Fig. 9a,b). The time from the onset of elicitation to the peak in emissions of the first methanol burst and LOX emission burst were nonlinearly correlated with total amount of O3 taken up (ФO3) (Fig. 9c,d). The correlations between total amount of O3 taken up (ФO3) and kI and kD broadly reflected differences among the treatments with kI2 for methanol (Fig. 9e), kI (Fig. 9f) and kD (Fig. 9h) for LOX products decreasing and kD values for both the first and subsequent methanol emission burst (Fig. 9g) increasing with increasing the amount of O3 taken up.
Figure 9.
Correlation of total emissions (a and b), the lag time from elicitation to the maximum emission (c and d), the rate constant for the initial increase (e and f) and decrease (g and h) for the two methanol bursts (a, c, e, g) and LOX products (b, d, f, h) with the total amount of O3 taken up (ϕO3) in O3-exposed leaves of P. vulgaris. In all cases, black symbols denote illuminated leaves fumigated with 600 ± 33 nmol mol-1 O3 for 30 min (Treatment 1), grey symbols the leaves exposed to 600 ± 30 nmol mol-1 O3 for 30 min in darkness (Treatment 2), and white symbols the illuminated leaves exposed first to 200 ± 11 nmol mol-1 O3 for 30 min and then to 600 ± 32 nmol mol-1 O3 for additional 30 min (Treatment 3). In (a), (c), (e) and (g), the circles correspond to the first and squares to the second methanol emission burst. In (a), the data for both the first (circles; y=0.695x+13.4; r2=0.76, P<0.005) and second (squares: y=13.9x+72.2; r2=0.75, P<0.005) methanol emission burst were fitted by linear regressions. In (b), the data for LOX products were fitted by a non-linear regression (y=0.0607 * 1.15x+4.46; r2=0.87, P<0.0005). In (c), the data for the first methanol emission burst were fitted by a linear (circles; y=-0.00370x+0.798; r2=0.1, P>0.05) and the data for the second burst by a non-linear regression (squares; y=4.02(1-exp(-0.215x)); r2=0.45, P<0.05). In (d), the LOX product data were fitted by an exponential regression (y=2.44 * (1-0.898x); r2=0.74, P<0.005). In (e), the data for the first methanol emission burst were fitted by a linear (circles; y=0.0117x+1.37; r2=0.07, P>0.05) and the data for the second burst by a non-linear regression (squares; y=18.9/x-0.0904; r2=0.77, P<0.005). In (f), the data for LOX products were fitted by a non-linear regression (y=11.6/x-0.0612; r2=0.90, P<0.0001). In (g), the data for both the first (circles; y=0.471+0.00360x; r2=0.15, P>0.05) and second (squares; y=0.00320x+0.0426; r2=0.81 P<0.005) methanol emission burst were fitted by linear regressions. In (h), the data for LOX product were fitted by a non-linear regression (y=12.6/(33.1+x); r2=0.72, P<0.005).
Discussion
O3 exposure and uptake via stomata
Ambient ozone concentrations significantly vary over the land surface, and large-scale predictions of O3 effects on plant growth, production and biodiversity are commonly based on this variation in surrounding air O3 concentrations (e.g. Feng et al. 2008; Wittig et al. 2009; Ainsworth et al. 2012). However, O3 sensitivity varies among plant species (Flowers et al. 2007; Brauer et al. 2016; Li et al. 2016; Osborne et al. 2016), and plant responses to chronic low to moderate level O3 exposure can quantitatively and qualitatively differ from the responses to acute O3 exposure (Beauchamp et al. 2005; Calfapietra et al. 2013; Heiden et al. 1999). Furthermore, the overall physiological effects can be importantly driven by O3 uptake via stomata (Beauchamp et al. 2005; Jud et al. 2016). Given that stomatal conductance is affected by light level, modifications in light availability as occurring commonly during the day and among the days can importantly alter plant responses to O3. Furthermore, stomatal effects can also be altered by pre-exposure to lower O3 concentrations, e.g. exposure to the morning low O3 concentrations can force stomatal closure, thereby reducing O3 uptake during the rest of the day when ambient concentrations are higher (Nolle et al. 2002; Ribas & Peñuelas 2004; Xu et al. 2008). Thus, quantitative characterization of initial responses of plants to elevated O3 under different light levels and pre-exposure to lower-level O3 can provide important insight into factors affecting plant responses to acute O3 stress.
Stomatal uptake at the given ambient O3 concentration can be significantly affected by surface reactions due to high-reactivity volatile and non-volatile compounds stored in the trichomes on leaf surface or released from the leaf interior. Previously, Jud et al. (2016) found that the glandular trichomes of Nicotiana tabacum are an efficient chemical barrier against stomatal O3 uptake due to amplification of surface reactions that deplete O3 at the leaf surface. Indeed, peltate glandular trichomes have been found to produce and store biogenic volatile organic compounds and alter plant abiotic or biotic stress induced responses (Corsi & Bottega 1999; Gang et al. 2001; Wagner et al. 2004). However, Phaseolus vulgaris has capitate glandular trichomes (Bahafid et al. 2017; Li et al. unpublished observations), the role of which as the storage of biogenic volatiles is less clear (Levin 1973; Corsi & Bottega 1999), but which nevertheless might contribute to depleting O3 at the leaf surface. In this study, we investigated the O3 depletion by Phaseolus vulgaris under dark and light conditions and found that the percentage of non-stomatal O3 uptake in all three treatments was actually higher than the stomatal uptake (Table 2). This is a direct indication for the high O3 depletion capacity of the surface, and indeed such a strong surface deposition capacity can directly reduce stomatal O3 uptake. In our study, the variation in total O3 uptake among treatments was mainly driven by variation in stomatal O3 uptake (Table 2) with Treatment 2, 600 nmol mol-1 O3 exposure in darkness exhibiting the lowest O3 uptake due to stomatal closure in the darkness (Table 2). While the total O3 uptakes did not differ among O3 exposure treatments in the light, exposure to the lower O3 concentration of 200 nmol mol-1 in Treatment 3 reduced stomatal conductance such that during the subsequent exposure to the higher O3 concentration of 600 nmol mol-1, O3 uptake was less than in Treatment 1 where the leaves were immediately exposed to the high O3 concentration (Table 2). This could allow plants to respond faster to oxidative stress caused by O3, preventing O3 entry and minimizing potential damage and preventing the over-accumulation ROS. These data collectively indicate that both the modification of light level and initial exposure to lower O3 concentration significantly reduced the O3 uptake due to acute exposure.
Impact of elevated O3 on photosynthetic characteristics
Generally, O3 exposure results in decreased net photosynthetic carbon assimilation (A), stomatal conductance (gs) and the maximum quantum efficiency of PSII (Fv/Fm) (Long & Naidu 2002; Calatayud et al. 2003; Fiscus et al. 2005; Flowers et al. 2007; Kollist et al. 2007; Vahisalu et al. 2008; Guidi et al. 2009; Vahisalu et al. 2010). A reduction of A in O3 exposed leaves typically results from decreases in gs and losses in Rubisco activity (Fiscus et al. 2005). In our study, the reductions in photosynthetic characteristics and total O3 uptake through stomata (ФO3) were strongly related (Fig. 2), consistent with past observations (e.g. Calatayud et al. 2003). In fact, leaves in Treatment 2 showed a minor degree of foliage visible injury and a moderate reduction in photosynthetic characteristics with some changes in only A and gs (Fig. 2). Because the pre-exposure to the lower concentration of 200 nmol mol-1 led to a significant decline in gs, and thus, O3 uptake during the subsequent exposure to the higher concentration of 600 nmol mol-1 was less in Treatment 3 than in Treatment 1. However, the total O3 uptake through stomata (ФO3) was similar in Treatments 1 and 3, and the degree of final leaf damage and reduction in Fv/Fm and A were statistically not different among these treatments (Fig. 2), suggesting that the cumulative stress resulting from exposures to both 200 and 600 nmol mol-1 in Treatment 3 was equivalent to the stress resulting from 600 nmol mol-1 only in Treatment 1, and further underscoring that O3 effect on foliage photosynthetic characteristics is dose-dependent.
Different application of O3 has varying effects on VOC emissions
The release of plant stress volatiles such as methanol, LOX products and MeSA is highly enhanced by a variety of abiotic stresses including temperature, mechanical damage and O3 exposure (Beauchamp et al. 2005; Loreto & Schnitzler 2010; Niinemets 2010; Brilli et al. 2011; Copolovici et al. 2012; Portillo-Estrada et al. 2015; Pazouki et al. 2016). As the emissions of stress volatiles are often quantitatively related to the severity of stress (Beauchamp et al. 2005; Copolovici et al. 2012; Portillo-Estrada et al. 2015; Pazouki et al. 2016), they can serve as an important tool to monitor development of stress response and recovery and gain insight into overall stress severity under given environmental conditions.
Ozone-induced multiphasic methanol emissions that were quantitatively related to ozone dose constitute one of the most conspicuous findings of this study. In particular, a major rapid burst of methanol was induced in all treatments, followed by a subsequent longer-term burst or multiple overlapping bursts (Fig. 4) consistent with past observations in Nicotiana tabacum (Beauchamp et al. 2005). However, in our study with P. vulgaris, large methanol emissions were induced almost immediately upon fumigation with 600 nmol mol-1 O3, and methanol emissions were also increased during fumigation with the lower O3 concentration of 200 nmol mol-1 (Fig. 4). In contrast, in N. tabacum in the study of Beauchamp et al. (2005), there was a certain delay in the rise of methanol emissions after fumigation, and methanol emissions were elicited upon exposure to O3 concentrations larger than 500 nmol mol-1. These results show that P. vulgaris is more vulnerable to O3 than N. tabacum.
Although the maximum emission rate of the first methanol peak correlated with total O3 uptake through stomata (ФO3) (Fig. 5d), the leaves in Treatments 2 and 3 exhibited a much lower maximum emission rate than the leaves in Treatment 1 (Fig. 5a), suggesting that the magnitude of the first methanol emission peak was driven by the initial O3 uptake that was greater in Treatment 1 than in the two other treatments. Interestingly, we observed that the maximum methanol emission rate was greater for the second than for the first methanol emission burst in Treatment 3, and this second emission peak in this treatment was similar to that in Treatment 1 (Fig. 5a). Furthermore, the total integrated emissions during the second peak were also much greater (Fig. 6a) due to longer duration (Fig. 7d), and scaled with total O3 uptake through the exposure (Fig. 9a). The question of what triggers the second methanol peak during post-O3-exposure has not been explained in previous studies, but O3-driven methanol release likely results from the activation of pectin methylesterases in cell walls and concomitant demethylation of the cell wall pectins (Pelloux et al. 2007; Beauchamp et al. 2005). There is a certain constitutive pectin methylesterase activity that is likely responsible for the initial methanol release, but plants have multiple pectin methylesterases (Pelloux et al. 2007) and the subsequent methanol release might be partly associated with de novo expression of these enzymes. While the first methanol emission burst likely reflects the response to the immediate damage, accumulation of lesions post-O3-exposure and/or elicitation of repair processes or hypersensitive programmed cell death like processes might explain why the post-exposure second methanol peak emission was strongly correlated with the total amount of O3 uptake (ФO3).
Notably, the pronounced decline in both stomatal conductance and methanol emissions observed when the leaves were darkened (Fig. 4e) indicates that stomata can exert short-term control over methanol release as previously reported (Nemecek-Marshall et al. 1995; Niinemets & Reichstein 2003a, b; Niinemets et al. 2004; Hüve et al. 2007; Harley et al. 2007). Stomatal control on methanol emissions is due to high methanol water solubility such that the rise of intercellular gas-phase methanol concentrations upon stomatal closure cannot keep up with stomatal movements (Niinemets & Reichstein 2003a, b; Niinemets et al. 2004; Hüve et al. 2007; Harley et al. 2007). However, when stomata open, methanol stored in the leaf liquid phase is rapidly released, resulting in a burst of methanol emission as was observed upon switching on the light in our study (Fig. 4e). Clearly, stomatal responses can interfere with the kinetics of methanol synthesis in cell walls and might explain the multi-phasic nature of methanol emission during the recovery phase (Fig. 4). Further studies are needed to gain an insight into the physiological and gene expression controls on methanol emissions after O3 exposure.
Large O3-induced post-exposure bursts of LOX product emissions were found in this study (Fig. 4), and both maximum LOX emission rate (Fig. 5e) and total emissions (Fig. 9b) depended on total O3 uptake (ФO3) as has been observed in N. tabacum, albeit at higher O3 exposures (Beauchamp et al. 2005). Lower emissions of LOX products in Treatment 2, imply that stomatal closure under darkness limited both O3 uptake and acute responses. Despite the similar O3 uptake in both Treatments 1 and 3, Treatment 3 displayed lower values of maximum emission rate and total emissions of LOX products than expected based on total O3 uptake, suggesting that formation of reactive oxygen species (ROS) was less in this treatment. In our study, the initial methanol and LOX product emissions behaved similarly across the treatments (Fig. 4), suggesting that it is the initial level of ROS formation that is responsible for the magnitude of these emissions.
MeSA is a volatile plant stress hormone, release of which has been observed in several cases upon severe O3 exposure (Heiden et al. 1999; Vuorinen et al. 2004; Beauchamp et al. 2005). In P. vulgaris in our study, MeSA release was observed following exposures to high O3 concentrations of 600 nmol mol-1 in Treatments 1 and 2. However, no MeSA emission was detected in Treatment 3, where the leaves were first exposed to the lower-level O3 of 200 nmol mol-1 prior to the exposure to high concentration of O3 of 600 nmol mol-1. Apparently, prior low-level O3 exposure can inhibit MeSA formation both due to de novo synthesis as well as release from glycosidically bonded form during subsequent high-level O3 fumigation. In Treatments 1 and 2, MeSA maximum emission rate and total emissions were not correlated with total O3 uptake (ФO3). In light of the dose response of LOX products and methanol, the non-dose response for MeSA is puzzling, but it could be indicative of the circumstance that elicitation of MeSA emissions is associated with exceeding a certain threshold for damage rather than with damage per se. This non-dose response resembles effects of herbivory stress where across diverse volatiles elicited, some are strongly correlated with the severity of damage, while the others are not and seem to serve as infochemicals of the presence of stress (Copolovici et al. 2011).
Kinetics of O3-elicited volatile release upon different O3 stresses
On the basis of experiments in N. tabacum, it has been suggested that the temporal shapes of O3-induced volatile emissions for different compounds are similar and characterized by an initial exponential or sigmoidal increase to a maximum level, followed by a decrease until the baseline emissions have been reached (Beauchamp et al. 2005). We have studied the kinetics of O3-elicited volatile release in P. vulgaris by fitting the temporal shapes of increase and decrease by first order exponential relationships. We observed that both the rate of increase and decrease of methanol and LOX products were strongly enhanced by O3 exposure (Fig. 8). Albeit the emission kinetics was similar for MeSA (Fig. 4), timing of its release was not dependent on the O3 dose, again suggesting that once a certain threshold O3 concentration was reached, the emission was initiated and the subsequent fading of these emissions were unrelated to accumulated O3 dose.
In the present study, a similar induction time for the initiation of the methanol burst among the three treatments (Fig. 7a) might indicate that activation of demethylation of pectins is primarily driven by the presence of stress elicitor. However, the faster increase of the first burst of methanol emission in Treatment 1 (Fig. 8) indicates that the degree of activation is much greater upon higher initial O3 uptake. However, it is less clear why the rate of decrease of emissions was greater in the Treatments 1 and 3 than in Treatment 2 (Fig. 8b). It might indicate a faster quenching of the initial rise of ROS, but further studies are needed to gain an insight into the downregulation of methanol emissions during the initial impact response. In the case of the secondary methanol emission bursts, the onset of these emissions was similar, but the rise occurred faster and decline more slowly in Treatment 2 (Fig. 8a,b) than in the two other treatments. As discussed above, activation of this late-induced response was likely linked to the plant-internal long-term processes, indicating activation of a gene expression response, and it is plausible that pre-exposure to lower O3 concentration of 200 nmol mol-1 already primed the leaf for these later-occurring responses.
The faster emission burst of LOX products in Treatment 1 than in the other treatments (Fig. 7b), indicates that in this treatment the processes downstream of the activation of pectin methylesterases were induced earlier, again suggesting that this was the most severe treatment. Provided that LOX emissions are typically associated with an oxidative burst (e.g. Beauchamp et al. 2005), this evidence might indicate that reactive oxygen species (ROS) were triggered more quickly in Treatment 1 than in Treatments 2 and 3. However, once induced, LOX emissions raised and decreased faster in Treatment 2 than in the two other treatments (Fig. 8c). This faster increase and decrease kinetics of LOX products in this treatment is unclear. However, several LOX compounds have low volatility (e.g. Henry’s law constant for 2-hexenol of 0.90 Pa m3 mol-1 at 25 °C (Vempati 2014) is only moderately greater than that for methanol of 0.46 Pa m3 mol-1 (Niinemets & Reichstein 2003a) and it is also relatively low, 5.1 Pa m3 mol-1 for 2-hexenal according to webbook: nist.gov/chemistry/), and thus, such response might indicate an interference of low stomatal conductance with LOX release similarly to methanol (Niinemets & Reichstein 2003a for detailed analyses of stomatal controls on emissions of compounds with different values of Henry’s law constant). When light was switched on, LOX products synthesized in darkness and temporarily stored in leaf liquid phase might have contributed to the total emission flux, resulting in a larger emission flux than the rate of LOX product synthesis. However, previous studies have also suggested that darkness itself can induce a temporary burst of LOX products which could be triggered by changes of foliar pH due to transitions from light to dark conditions (Hauser et al. 1995; Graus et al. 2004; Brilli et al. 2011). However, in our study, such a LOX burst upon switch-off the light was only observed in one leaf from Treatment 2 (data not shown). On the other hand, switching on light can also contribute to modified leaf redox status and thereby alter leaf ROS concentrations in Treatment 2.
Despite similar total amount of O3 uptake in Treatments 1 and 3, leaves in Treatment 3 had significantly lower total emission and maximum emission rate of LOX products and needed a longer time to reach the maximum value (Figs. 4, 5b, 6c), suggesting that priming by low-level O3 played an important role in coping with higher-level O3.
Taken together, analyses of the volatile elicitation kinetics are partly consistent with the hypothesis that more severe O3 stress resulted in earlier and faster induction of stress volatiles. However, there were several surprising differences among the treatments which might be partly related to stomatal effects on water-soluble volatile emission, but might also be associated with modified timing of ROS burst. Further studies should examine O3-driven volatile emissions in relation to the kinetics of ROS development
Conclusions
Our results demonstrated that short-term (30 minute) exposure to relatively high O3 concentrations (~600 nmol mol-1) can lead to severe visible leaf injury and reductions in leaf physiological activity in Phaseolus vulgaris. These changes are accompanied by a characteristic release of stress volatiles methanol, LOX products and MeSA during the exposure and through recovery in dose-dependent manner for methanol and LOX products and non-dose-dependent manner for MeSA. Overall, the emission responses after stress impact were long-lasting, and emission bursts were observed many hours after the stress impact, especially for methanol, indicating a major elicitation of the stress response pathways. Photosynthetic data and emissions of LOX products and methanol during the early emission burst suggested that stomatal closure due to darkness and pre-exposure to low-level O3 protect leaves against high-level O3-induced injury in Phaseolus vulgaris. Reduced stomatal conductance is an important factor preventing O3 entry into plant leaves, thus reducing plant damage and the amount of methanol and LOX products released into the atmosphere. These observations have important implications for understanding plant responses to O3 in natural environments where both light and O3 concentrations strongly vary during the day and among the days, and could drive ecological success of species with different sensitivity to O3 and stomatal responses to environmental changes. Surprisingly, MeSA emission was inhibited by pre-exposure of leaves to lower O3 concentration of 200 nmol mol-1, but longer-term methanol emissions, albeit being multiphasic, scaled with total O3 uptake. This suggests that different pathways are differently regulated during exposure and through recovery, resulting in different dose responses. Further work is needed to gain insight into the kinetics and dose responses of stress volatile release as determined by plant-internal processes and application of O3. Our study also demonstrates that there is evidence of both quantitative dose-dependent relationships between O3 dose and the kinetics and magnitude of elicitation of volatiles, but also provide evidence of non-dose emission responses, especially during the recovery phase. These non-dose responses suggest that O3 responses are more complicated than generally thought.
Summary Statement.
Acute ozone exposure resulted in dose-dependent multiphasic methanol and monophasic lipoxygenase pathway volatile emissions, and non-dose-dependent methyl salicylate emissions in Phaseolus vulgaris. Stomatal closure due to darkness and pre-exposure to low-level O3 (priming) protected leaves against high-level O3-induced injury.
Acknowledgments
This work was supported by grants from the European Research Council (advanced grant 322603, SIP-VOL+), and the European Regional Development Fund (Centre of Excellence EcolChange) and the Estonian Ministry of Science and Education (institutional grant IUT-8-3). We thank two anonymous referees for their helpful comments.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Agathokleous E, Saitanis CJ, Koike T. Tropospheric O3, the nightmare of wild plants: a review study. Journal of Agricultural Meteorology. 2015;71(2):142–152. [Google Scholar]
- Ainsworth EA, Yendrek AR, Sitch A, Collins WJ, Emberson LD. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annual Reviews of Plant Biology. 2012;63:637–661. doi: 10.1146/annurev-arplant-042110-103829. [DOI] [PubMed] [Google Scholar]
- Arneth A, Niinemets Ü. Induced BVOCs: how to bug our models? Trends in Plant Science. 2010;15:118–125. doi: 10.1016/j.tplants.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Bahafid W, Joutey NT, Sayel H, Asri M, Laachari F, Ghachtouli NEL. Soil bioaugmentation with Cyberlindnera fabianii diminish phytotoxic effects of chromium (VI) on Phaseolus vulgaris L. Journal of Materials and Environmental Sciences. 2017;8:438–443. [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, et al. Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relationships between ozone uptake and emission of LOX products. Plant, Cell and Environment. 2005;28:1334–1343. [Google Scholar]
- Bichele I, Moldau H, Padu E. Estimation of plasmalemma conductivity to ascorbic acid in intact leaves exposed to ozone. Physiologia Plantarum. 2000;108:405–412. [Google Scholar]
- Bortier K, Ceulemans R, De Temmerman L. Effects of ozone exposure on growth and photosynthesis of beach seedlings (Fagus sylvatica) New Phytologist. 2000;146:271–280. doi: 10.1046/j.1469-8137.2000.00633.x. [DOI] [PubMed] [Google Scholar]
- Brauer M, Freedman G, Frostad J, van Donkelaar A, Martin RV, Dentener F, et al. Cohen A. Ambient air pollution exposure estimation for the global burden of disease 2013. Environmental Science & Technology. 2016;50:79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- Brilli F, Gioli B, Fares S, Terenzio Z, Zona D, Gielen B, et al. Ceulemans R. Rapid leaf development drives the seasonal pattern of volatile organic compound (VOC) fluxes in a ‘coppiced’ bioenergy poplar plantation. Plant, Cell and Environment. 2016;39:539–555. doi: 10.1111/pce.12638. [DOI] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, et al. Hansel A. Detection of plant volatiles after leaf wounding and darkening by Proton Transfer Reaction “Time-of-Flight” Mass Spectrometry (PTR-TOF) PLoS ONE. 2011;6(5):e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calatayud A, Iglesias DJ, Talón M, Barreno E. Effects of 2-month ozone exposure in spinach leaves on photosynthesis, antioxidant system and lipid peroxidation. Plant Physiology and Biochemistry. 2003;41:839–845. [Google Scholar]
- Calfapietra C, Pallozzi E, Lusini I, Velikova V. Modification of BVOC emissions by changes in atmospheric [CO2] and air pollution. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 253–284. [Google Scholar]
- Cassimiro JC, Moraes RM. Response of a tropical tress species to ozone: visible leaf injury, growth, and lipid peroxidation. Environmental Sciences and Pollution Research. 2016;23:8085–8090. doi: 10.1007/s11356-015-5961-x. [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, et al. Mauch-Mani B. Priming: getting ready for battle. Molecular Plant-Microbe Interactions. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. Journal of Plant Physiology. 2012;169:664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signaling compounds in three tree species with differing waterlogging tolerance. Plant, Cell and Environment. 2010;33:1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Corsi G, Bottega S. Glandular hairs of Salvia officinalis: new data on morphology, localization and histochemistry in relation to function. Annals of Botany. 1999;84:657–664. [Google Scholar]
- Fares S, Goldstein AH, Loreto F. Determinants of ozone fluxes and metrics for ozone risk assessment in plants. Journal of Experimental Botany. 2010a;61:629–633. doi: 10.1093/jxb/erp336. [DOI] [PubMed] [Google Scholar]
- Fares S, Loreto F, Kleist E, Wildt J. Stomatal uptake and stomatal deposition of ozone in isoprene and monoterpene emitting plants. Plant Biology. 2008;10:44–54. doi: 10.1055/s-2007-965257. [DOI] [PubMed] [Google Scholar]
- Fares S, McKay M, Holzinger R, Goldstein AH. Ozone fluxes in a Pinus ponderosa ecosystem are dominated by non-stomatal processes: evidence from long-term continuous measurements. Agricultural and Forest Meteorology. 2010b;150:420–431. [Google Scholar]
- Feng Z, Kobayashi K, Ainsworth EA. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): a meta-analysis. Global Change Biology. 2008;14:2696–2708. [Google Scholar]
- Fiscus EL, Booker FL, Burkey KO. Crop responses to ozone: uptake, models of action, carbon assimilation and partitioning. Plant, Cell and Environment. 2005;28:997–1011. [Google Scholar]
- Flowers MD, Fiscus EL, Burkey KO, Booker FL, Dubois J-JB. Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris L.) genotypes differing in sensitivity to ozone. Environmental and Experimental Botany. 2007;61:190–198. [Google Scholar]
- Fowler D, Amann M, Anderson R, Ashmore M, Cox P, Depledge M, et al. Stefenson D. Ground-level ozone in the 21st century: future trends, impacts and policy implications. The Royal Society; London, UK: 2008. [Google Scholar]
- Freire LS, Gerken T, Ruiz-Plancarte J, Wei D, Fuentes JD, Katul GG, et al. Chamercki M. Turbulent mixing and removal of ozone within an Amazon rainforest canopy. Journal of Geophysical Research – Atmospheres. 2017;122:2791–2811. [Google Scholar]
- Furlan CM, Moraes RM, Bulbovas P, Sanz MJ, Domingos M, Satatino A. Tibouchina pulchra (Cham.) Cogn., a native Atlantic Forest species, as a bio-indicator of ozone: visible injury. Environmental Pollution. 2008;152:361–365. doi: 10.1016/j.envpol.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiology. 2001;125:539–555. doi: 10.1104/pp.125.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomuzzi V, Cappellin L, Khomenko I, Biasioli F, Schütz S, Tasin M, et al. Angeli S. Emission of volatile compounds from apple plants infested with Pandemis heparana larvae, antennal response of conspecific adults, and preliminary field trial. Journal of Chemical Ecology. 2016;42:1265–1280. doi: 10.1007/s10886-016-0794-8. [DOI] [PubMed] [Google Scholar]
- Graus M, Müller M, Hansel A. High resolution PTR-TOF: quantification and formula confirmation of VOC in real time. Journal of the American Society for Mass Spectrometry. 2010;21:1037–1044. doi: 10.1016/j.jasms.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Graus M, Schnitzler J-P, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser L. Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiology. 2004;135:1967–1975. doi: 10.1104/pp.104.043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi L, Degl’Innocenti E, Martinelli F, Piras M. Ozone effects on carbon metabolism in sensitive and insensitive Phaseolus cultivars. Environmental and Experimental Botany. 2009;66:117–125. [Google Scholar]
- Harley P, Greenberg J, Niinemets Ü, Guenther A. Environmental controls over methanol emission from leaves. Biogeosciences. 2007;4:1083–1099. [Google Scholar]
- Hauser M, Eichelmann H, Oja V, Heber U, Laisk A. Stimulation by light of rapid pH regulation in the chloroplast stroma in vivo as indicated by CO2 solubilization in leaves. Plant Physiology. 1995;108:1059–1066. doi: 10.1104/pp.108.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiden AC, Hoffmann T, Kahl J, Kley D, Klockow D, Langebartels C, et al. Wildt J. Emission of volatile organic compounds from ozone-exposed plants. Ecological Applications. 1999;9:1160–1167. [Google Scholar]
- Heil M, Kost C. Priming of indirect defences. Ecology Letters. 2006;9:813–817. doi: 10.1111/j.1461-0248.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- Holopainen JK, Nerg A-M, Blande JD. Multitrophic signalling in polluted atmospheres. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tress volatile organic compound emissions. Springer; Berlin: 2013. pp. 285–314. [Google Scholar]
- Hoshika Y, Omasa K, Paoletti E. Both ozone exposure and soil water stress are able to induce stomatal sluggishness. Environmental and Experimental Botany. 2013;88:19–23. [Google Scholar]
- Hüve K, Christ MM, Kleist E, Uerlings R, Niinemets Ü, Walter A, Wildt J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. Journal of Experimental Botany. 2007;58:1783–1793. doi: 10.1093/jxb/erm038. [DOI] [PubMed] [Google Scholar]
- Inclan R, Ribas A, Peñuelas J, Gimeno BS. The relative sensitivity of different Mediterranean plant species to ozone exposure. Water, Air, and Soil Pollution. 1999;116:273–277. [Google Scholar]
- Ivanov AV, Trakhtenberg S, Bertram AK, Gershenzon YM, Molina MJ. OH, HO2, and ozone gaseous diffusion coefficients. Journal of Physical Chemistry A. 2007;111:1632–1637. doi: 10.1021/jp066558w. [DOI] [PubMed] [Google Scholar]
- Jordan A, Haidacher S, Hanel G, Hartungen E, Märk L, Seehauser H, et al. Märk TD. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) International Journal of Mass Spectrometry. 2009;286:122–128. [Google Scholar]
- Jud W, Fischer L, Canaval E, Wohlfahrt G, Tissier A, Hansel A. Plant surface reactions: an ozone defence mechanism impacting atmospheric chemistry. Atmospheric Chemistry and Physics. 2016;16:277–292. [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant isoprenoids: methods and protocols. Humana Press; New York: 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K, et al. Kollist H. A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiologia Plantarum. 2007;129:796–803. [Google Scholar]
- Laisk A, Kull O, Moldau H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiology. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA. The role of trichomes in plant defense. Quarterly Review of Biology. 1973;48:3–15. [Google Scholar]
- Li P, Calatayud V, Gao F, Uddling J, Feng Z. Differences in ozone sensitivity among woody species are related to leaf morphology and antioxidant levels. Tree Physiology. 2016;36:1105–1116. doi: 10.1093/treephys/tpw042. [DOI] [PubMed] [Google Scholar]
- Long SP, Naidu SL. Effect of oxidants at the biochemical, cell and physiological levels, with particular reference to ozone. In: Bell JNB, Treshow M, editors. Air Pollution and Plant Life. John Wiley & Sons, Ltd.; West Sussex: 2002. pp. 69–88. [Google Scholar]
- Loreto F, Fares S. Is ozone flux inside leaves only a damage indicator? Clues from volatile isoprenoid studies. Plant Physiology. 2007;143:1096–1100. doi: 10.1104/pp.106.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiology. 2001;126:993–1000. doi: 10.1104/pp.126.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Manes F, Kollist H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiology. 2004;24:361–367. doi: 10.1093/treephys/24.4.361. [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Maja MM, Kasurinen A, Yli-Pirilä P, Joutsensaari J, Klemola T, Holopainen T, Holopainen J. Contrasting responses of silver birch VOC emissions to short- and long-term herbivory. Tree Physiology. 2014;34:241–252. doi: 10.1093/treephys/tpt127. [DOI] [PubMed] [Google Scholar]
- Moldau H, Bichele I. Plasmalemma protection by the apoplast as assessed from above-zero ozone concentrations in leaf intercellular air spaces. Planta. 2002;214:484–487. doi: 10.1007/s00425-001-0678-0. [DOI] [PubMed] [Google Scholar]
- Nemecek-Marshall M, MacDonald RC, Franzen JF, Wojciechowski CL, Fall R. Methanol emission from leaves: Enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development. Plant Physiology. 1995;108:1359–1368. doi: 10.1104/pp.108.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü. Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends in Plant Science. 2010;15:145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Whole plant photosynthesis. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 399–423. [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, et al. Peñuelas J. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: Differential sensitivity of the emission rates to stomatal closure explained. Journal of Geophysical Research - Atmospheres. 2003a;108:4208. doi: 10.1029/2002JD002620. [DOI] [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: A sensitivity analysis. Journal of Geophysical Research - Atmospheres. 2003b;108:4211. doi: 10.1029/2002JD002626. [DOI] [Google Scholar]
- Nolle M, Ellul R, Heinrich G, Güsten H. A long-term study of background ozone concentrations in the central Mediterranean-diurnal and seasonal variations on the island of Gozo. Atmospheric Environment. 2002;36:1391–1402. [Google Scholar]
- Oksanen E, Holopainen T. Responses of two birch (Betula pendula Roth) clones to different ozone profiles with similar AOT40 exposure. Atmospheric Environment. 2001;35:5245–5254. [Google Scholar]
- Osborne SA, Mills G, Hayes F, Ainsworth EA, Büker P, Emberson L. Has the sensitivity of soybean cultivars to ozone pollution increased with time? An analysis of published dose-response data. Global Change Biology. 2016;22:3097–3111. doi: 10.1111/gcb.13318. [DOI] [PubMed] [Google Scholar]
- Paoletti E, Grulke NE. Ozone exposure and stomatal sluggishness in different plant physiognomic classes. Environmental Pollution. 2010;158:2664–2671. doi: 10.1016/j.envpol.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Pasqualini S, Piccioni C, Reale L, Ederli L, Della Torr G, Ferranti F. Ozone-induced cell death in tobacco cultivar Bel W3 plant. The role of programmed cell death in lesion formation. Plant Physiology. 2003;133:1122–1134. doi: 10.1104/pp.103.026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazouki L, Kanagendran A, Li S, Kännaste A, Memari HR, Bichele R, Niinemets Ü. Mono- and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: From gene expression to emission responses. Environmental and Experimental Botany. 2016;132:1–15. doi: 10.1016/j.envexpbot.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Niinemets Ü. Fading of wound-induced volatile release during Populus tremula leaf expansion. Journal of Plant Research. 2017;130:157–165. doi: 10.1007/s10265-016-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula) Journal of Chemical Ecology. 2015;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri A, Castagna A, Padu E, Moldau H, Rahi M, Soldatini GF. The decay of O3 through direct reaction with cell wall ascorbate is not sufficient to explain the different degrees of O3-sensitivity in two poplar clones. Journal of Plant Physiology. 1999;154:250–255. [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthesis kinetics in aspen leaves. Plant Physiology. 2009;149:1609–1618. doi: 10.1104/pp.108.133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas À, Peñuelas J. Temporal patterns of surface ozone levels in different habitats of the North Western Mediterranean basin. Atmospheric Environment. 2004;38:985–992. [Google Scholar]
- Schmidhuber J, Tubiello FN. Global food security under climate change. Proceedings of the National Academy of Sciences USA. 2007;104:19703–19708. doi: 10.1073/pnas.0701976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasin M, Cappellin L, Biasioli F. Fast direct injection mass-spectrometric characterization of stimuli for insect electrophysiology by proton transfer reaction-time of flight mass-spectrometry (PTR-ToF-MS) Sensors. 2012;12:4091–4104. doi: 10.3390/s120404091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker WA, Nelken LH. Diffusion coefficients in air and water. In: Lyman WJ, Reehl WF, Rosenblatt DH, editors. Handbook of chemical property estimation methods: Environmental behavior of organic compounds. McGraw-Hill; New York: 1982. pp. 17/11–17/25. [Google Scholar]
- Vahisalu T, Kollist H, Wang Y-F, Nishimura N, Chan W-Y, Valerio G, et al. Kangasjärvi J. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, et al. Kollist H. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. The Plant Journal. 2010;62:442–453. doi: 10.1111/j.1365-313X.2010.04159.x. [DOI] [PubMed] [Google Scholar]
- Vempati HS. Physico-chemical properties of green leaf volatiles. MSc. Thesis; Graduate Faculty of the Louisiana State University and Agriculture and Mechanical College: 2014. pp. 20–24. [Google Scholar]
- Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Hewitt CN. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant, Cell and Environment. 2009;32:520–531. doi: 10.1111/j.1365-3040.2009.01946.x. [DOI] [PubMed] [Google Scholar]
- Vingarzan R. A review of surface ozone background levels and trends. Atmospheric Environment. 2004;38:3431–3442. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vuorinen T, Nerg A-M, Holopainen JK. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environmental Pollution. 2004;131:305–311. doi: 10.1016/j.envpol.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichomes. Annals of Botany. 2004;93:3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser G, Hecke K, Tausz M, Matyssek R. Foliage type specific susceptibility to ozone in Picea abies, Pinus cembra and Larix decidua at treeline: A synthesis. Environmental and Experimental Botany. 2013;90:4–11. [Google Scholar]
- Wenzel AA, Mehlhorn H. Zinc deficiency enhances ozone toxicity in bush beans (Phaseolus vulgaris L. cv. Saxa) Journal of Experimental Botany. 1995;46:867–872. [Google Scholar]
- Wilkinson S, Mills G, Illidge R, Davies WJ. How is ozone pollution reducing our food supply? Journal of Experimental Botany. 2012;63:527–536. doi: 10.1093/jxb/err317. [DOI] [PubMed] [Google Scholar]
- Wittig VE, Ainsworth EA, Naidu AL, Karnosky DF, Long SP. Quantifying the impact of current and future tropospheric ozone on the tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biology. 2009;15:396–424. [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell and Environment. 2002;25:717–726. [Google Scholar]
- Xu X, Lin W, Wang T, Yan P, Tang J, Meng Z, Wang Y. Long-term trend of surface ozone at a regional background station in eastern China 1991-2006: enhanced variability. Atmospheric Chemistry and Physics. 2008;8:2595–2607. [Google Scholar]
- Yener S, Sánchez-López JA, Granitto PM, Cappellin L, Märk TD, Zimmermann R, et al. Biasioli F. Rapid and direct volatile compound profiling of black and green teas (Camellia sinensis) from different countries with PTR-ToF-MS. Talanta. 2016;152:45–53. doi: 10.1016/j.talanta.2016.01.050. [DOI] [PubMed] [Google Scholar]