Abstract
Background
How coffee consumption relates to mortality in diverse European populations, with variable coffee preparation methods and customs, is unclear.
Objectives
To examine whether coffee consumption is associated with all-cause and cause-specific mortality in men and women.
Design
Prospective cohort study.
Setting
Ten European countries.
Participants
A total of 521,330 men and women enrolled in the European Prospective Investigation into Cancer and Nutrition (EPIC).
Main outcome measure
Multivariable hazard ratios (HRs) and 95% confidence intervals(CIs) estimated using multivariable Cox proportional hazards models. The association of coffee with serum biomarkers of liver function, inflammation, and metabolic health was evaluated in the EPIC Biomarkers sub-cohort (n=14,800).
Results
During a mean follow-up of 16.4 years, 41,693 deaths occurred. Compared with non-consumers, participants in the highest quartile of coffee consumption experienced statistically significant lower all-cause mortality (Men: HR=0.88, 95%CI: 0.82–0.95; P-trend<0.001; Women: HR=0.93, 95%CI: 0.87–0.98; P-trend=0.009). These findings did not vary significantly by country. Inverse associations were observed for digestive disease mortality for men (HR=0.41, 95%CI: 0.32–0.54; P-trend<0.0001) and women (HR=0.60, 95%CI: 0.46–0.78; P-trend<0.0001). Among women only, there was a statistically significant inverse association between coffee and circulatory disease mortality, (HR=0.78, 95%CI: 0.68–0.90; P-trend<0.001), cerebrovascular disease mortality (HR=0.70, 95%CI: 0.55–0.90; P-trend=0.002), and a positive association between coffee and ovarian cancer mortality (HR 1.12, 95% CI: 1.02–1.23 P-trend 0.001). In the EPIC-biomarkers sub-cohort, higher coffee consumption was associated with lower serum alkaline phosphatase, alanine transaminase, aspartate transaminase, and C-reactive protein.
Limitation
Reverse causality may have led to spurious findings; however, results did not differ following exclusion of participants who died within 8-years of baseline. The study is also limited by a single assessment of coffee drinking habits at baseline.
Conclusions
These results confirm prior findings on the reduced risk of mortality associated with coffee drinking but additionally show that this relationship does not vary by country where coffee preparation and drinking habits may differ. The study also reports novel inverse relationships between coffee drinking and digestive disease mortality.
Introduction
Coffee is one of the most commonly consumed beverages with an estimated 2.25 billion cups consumed per day worldwide. Coffee drinking provides exposure to a range of biologically-active compounds, including many with antioxidant activity (1), and higher coffee consumption has also been linked with lower levels of inflammation (2, 3), insulin resistance, and reduced risk of diabetes (4–6). Initial studies investigating the relationship between coffee consumption and all-cause mortality risk were of limited size and reported inconsistent results (7–9). However, recent U.S. based studies have reported that higher coffee consumption was related to lower all-cause mortality risk (10–12). In the NIH-AARP study, 10% and 15% lower all-cause mortality risks were observed in men and women, respectively, when individuals consuming more than 6 cups per day were compared with non-consumers (10). Further, a prospective investigation in Japan also reported inverse associations between coffee drinking and mortality risk (13). To date, a large European based analysis of coffee and mortality risk has not been undertaken.
For cause-specific mortality, findings on coffee drinking and cardiovascular disease mortality have been somewhat mixed (14–17), though recently, the aforementioned NIH-AARP study, and a meta-analysis, reported a lower risk of cardiovascular disease mortality for high-consumers of coffee compared with non-consumers (10, 18). Coffee drinking has not generally been associated with mortality from cancer (8, 10, 16, 18), while for other chronic diseases, such as digestive and respiratory disease mortality, limited data are available.
We investigated the association of coffee consumption with risks of all-cause and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition (EPIC) – a large prospective cohort comprising more than 520,000 participants from ten countries. Our multi-center study design meant that inter-country coffee preparation methods and customs are captured unlike any study to date. In addition, to gain insight into potential biological pathways that may be influenced by coffee drinking; we investigated the association of coffee with serum biomarkers of liver function, inflammation, and metabolic health in a sub-cohort of EPIC participants and their association with all-cause mortality.
Methods
Study population
EPIC is an on-going multicenter prospective cohort study of 521,330 participants, mostly aged 35 years or above, who were recruited in 1992–2000. A detailed description of the methods employed has previously been described (19, 20). This current study includes data from participants recruited, predominantly from the general population, in 10 European countries (Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden and United Kingdom; Supplemental Materials). Written informed consent was provided by all study participants. Ethical approval for the EPIC study was provided from the review boards of the International Agency for Research on Cancer and local participating centers. Exclusions prior to the onset of the analyses included: participants who reported, at baseline, cancer (n=22,537), heart disease (n=12,619), stroke (n=3,683), or diabetes (n=12,461); participants in the highest and lowest 1% of the distribution for the ratio between energy intake to estimated energy requirement (n=8,828); and participants with missing coffee consumption and follow-up information (n=9,459). This analysis, therefore, included 451,743 participants (130,662 men, 321,081 women).
Diet, lifestyle, and anthropometric information collection
Dietary intake was assessed by a number of different instruments that had been developed and validated in a series of studies within the various source populations participating in EPIC (19, 20). The dietary assessment methods selected for individual centers reflected the results of these methodological studies and took into consideration the local context. Self-administered questionnaires were used in all centers, except in Greece, Spain, and Ragusa (Italy), where data were collected at a personal interview. In Malmo (Sweden), a method combining a short non-quantitative food frequency questionnaire with a 7-day dietary diary was used. The information on type of coffee consumed (caffeinated and decaffeinated) was only collected for participants from Germany, Greece, Italy (excluding Naples and Ragusa), the Netherlands and the UK. For other centers, information on caffeine content of coffee was not collected. Participants recorded the number of coffee cups per month, week, or day; the structure of the questions varied somewhat by country and questionnaire. Coffee consumption (in mL/day) was calculated using the typical sizes of coffee cups for each center. Lifestyle questionnaires were used to obtain information on education, smoking habits, alcohol, physical activity, oral contraceptives and menopausal hormone therapy, menopausal status and, in five centers, nonsteroidal anti-inflammatory drug (NSAID) use.
Liver function, circulatory disease, and metabolic biomarker measurement
Baseline data on serum albumin, alkaline phosphatase(ALP), alanine transaminase(ALT), aspartate transaminase(AST), gamma-glutamyltransferase(GGT), hs-C-reactive protein (CRP), glycated hemoglobin(HbA1c), high density lipoprotein cholesterol(HDL-C), and lipoprotein(a) were available for the EPIC Biomarkers’ sub-cohort of 16,775 randomly-selected participants (See Table S1 for measurement method details). After applying the same exclusion criteria used in the main coffee-mortality analyses, 14,800 participants remained.
Assessment of mortality
Data on vital status and the cause and date of death were collected at the EPIC study centers using record linkages with cancer registries, boards of health and death indices in Denmark, Italy, Netherlands, Norway, Spain, Sweden and the UK or through active follow-up (inquiries by mail/telephone, municipal registries, regional health departments, physicians/hospitals) in Germany, Greece and France. Data on causes of deaths were coded in accordance with the International Classification of Diseases, 10th Revision (ICD-10). The following causes of death were investigated: cancer (ICD-10:C00-D48), circulatory (I00-I99), ischemic heart (I20-I25), cerebrovascular (I60-I69), respiratory (J30-J98), digestive diseases (K00-K93), external causes (S00-Y98), and suicides (X60-X84).
Statistical analysis
Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models with age as the primary time metric. Time at study entry was age at recruitment and exit time was age at death or the last date at which follow-up was considered complete in each center. Models were also stratified by age at recruitment in 1-year categories and center to minimize departure from proportionality, and to control for differing follow-up procedures, questionnaire design, and other differences across centers.
To account for between-country variability in volume and concentration of the type of coffee locally consumed, total, caffeinated and decaffeinated coffee were modelled using country-specific quartiles among coffee consumers, and then compared against non-consumers. Analyses using cup-size categories (non-consumers, <1, 1–<2, 2–<3, and 3+ cups/day) were also undertaken. Trend tests across exposure groups were calculated by entering the category variables into the Cox models as a continuous term. Continuous models (HR expressed per cup/day; 1 cup=237 mL) were also used. The multivariable models were adjusted for a set of a priori-determined covariates that included body mass index (BMI: <22, 22–24.9, 25–29.9, 30–34.9, 35+ kg/m2); physical activity (inactive, moderately inactive, moderately active, active); smoking status and intensity (never; current, 1–15 cigarettes/day; current, 16–25 cigarettes/day; current, 25+ cigarettes/day; former, quit ≤10 years; former, quit 11–20 years; former, quit 20+ years; current, pipe/cigar/occasional; current/former); smoking duration (<10, 10–<20, 20–<30, 30–<40, 40+ years); education (none/primary school completed, technical/professional school, secondary school, longer education - including university); menopausal status (premenopausal, postmenopausal, perimenopausal); ever use of oral contraceptives; ever use of menopausal hormone therapy; alcoholic drinks (non-consumers, <5, 5–14.9, 15–29.9, or 30+ g of ethanol/day), total energy (kcal/day) red and processed meats, and fruit and vegetables (all g/day). Further adjustment for dietary intakes of fiber, calcium, fish, soft drinks, and NSAID use resulted in virtually unchanged risk estimates, so these variables were not included in the final multivariable models. The coffee-mortality associations were further assessed across subgroups of smoking status, BMI, physical activity, alcohol, red/processed meat, and fruit/vegetable consumption. Interaction terms (multiplicative scale) between these variables and coffee intake were included in separate models; the statistical significance of the cross-product terms was evaluated using the likelihood ratio test. Similar analyses examined associations according to follow-up time categories (<5 years, 5–<10 years, and ≥10 years). Heterogeneity across countries was explored by a meta-analytic approach (21). To evaluate possible reverse causality, sensitivity analyses were conducted by excluding deaths within the first 5 and 8 years of follow-up, and limiting analyses to participants who self-reported being in ‘excellent’ or ‘good’ health at baseline recruitment. In a supplementary analysis, flexible parametric survival models (22) were used to allow direct estimation of the conditional cumulative incidence and thus absolute risks of death by sex and coffee consumption categories, adjusted for other covariates. Within these models, we employed restricted cubic splines with three internal knots to model the baseline hazard using attained age as the time-scale. Model-based survival functions and their confidence intervals were obtained from fitted models by coffee consumption category and sex, with other categorical covariates set to the most common category, and continuous variables set to their sex-specific means.
Liver function, inflammation, and metabolic biomarker measurement
In the EPIC biomarkers sub-cohort, mean levels of serum liver function, inflammatory, and metabolic biomarkers were calculated for coffee consumption categories. For biomarker values that were non-normally distributed, data were log-transformed and geometric means were calculated for each coffee consumption category (see Figure 3A footnote for multivariable adjustments). Also in the sub-cohort, Cox proportional hazards models, using the same criteria as the coffee-mortality analyses, were used to assess the relationships between serum levels (sex-specific quartiles) of albumin, ALP, ALT, AST, GGT, CRP, HbA1c, HDL-C, and lipoprotein(a) with all-cause mortality (see Figure S2 footnote for multivariable adjustments).
All statistical tests were two-sided and a P-value of <0.05 was considered statistically significant.
Role of the Funding Source
The various funders of the EPIC study had no role in study design, conduct, or reporting of the results.
Results
After a mean follow-up of 16.4 years, 18,302 and 23,391 all-cause deaths were recorded among men and women respectively. Of the total 41,693 all-cause deaths: 18,003 were from cancer; 9,106 from circulatory diseases; 2,380 from cerebrovascular diseases; 3,536 from ischemic heart disease; 1,213 from digestive diseases; 1,589 from respiratory diseases; 1,571 from external causes; and 418 from suicide. The mortality rates, age-adjusted to European standard populations (23), were 118 and 78 deaths per 10,000 person-years in men and women, respectively. Intakes of coffee by daily volume consumed were highest in Denmark (median 900 mL/day for men and women) and the Netherlands (median 625 mL/day for men and 500 mL/day for women) and were lowest in Italy (median 91 mL/day for men and 93 m/L/day for women) and Spain (median 100 mL/day for men and 118 m/L/day for women; Table S2). Compared to non-consumers, men and women with higher reported coffee intakes were more likely to be younger and current smokers, and reported higher intakes of red and processed meats and alcohol; with lower consumption of fruit and vegetables (Table 1).
Table 1.
Baseline characteristics of study participants by categories (non-consumers plus country specific quartiles) of daily coffee consumption.
| Characteristic | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Category of total coffee consumption | Category of total coffee consumption | |||||||||||
| Non-consumers | Q2 | Q4 | Non-consumers | Q2 | Q4 | |||||||
| Total coffee consumption median (mL/day) | 0 | 300 | 855 | 0 | 253 | 684 | ||||||
| N | 6,477 | 29,809 | 28,535 | 25,384 | 66,279 | 62,773 | ||||||
| N all-cause deaths | 1,039 | 4,440 | 3,601 | 1,817 | 5,236 | 4,162 | ||||||
| Age at recruitment (years)† | 52.7 | (45.3–59.6) | 53.3 | (47.3–59.9) | 50.1 | (42–7.56.2) | 50.8 | (45.4–57.2) | 51.8 | (45.2–58.9) | 49.2 | (44.1–54.6) |
| Body mass index (kg/m2) † | 26.3 | (24.1–28.7) | 26.1 | (24.0–28.4) | 26.2 | (24.1–28.5) | 23.6 | (21.3–26.8) | 24.1 | (21.9–27.1) | 24.3 | (22.0–27.3) |
| Education | ||||||||||||
| Longer education including University (%) | 23.2 | 26.1 | 26.9 | 23.0 | 23.2 | 23.0 | ||||||
| Smoking status | ||||||||||||
| Current (%) | 18.1 | 26.3 | 42.8 | 11.2 | 16.3 | 31.1 | ||||||
| Physical activity | ||||||||||||
| Active (%) § | 25.5 | 24.6 | 23.7 | 11.9 | 16.4 | 14.3 | ||||||
| Total energy intake (kcal/day) † | 2300 | (1893–2773) | 2312 | (1914–2756) | 2469 | (2049–2960) | 1906 | (1547–2312) | 1867 | (1551–2240) | 1947 | (1604–2356) |
| Red and processed meat consumption (g/day) † | 82.4 | (49.0–123.2) | 86.9 | (51.6–128.7) | 95.1 | (58.8–137.6) | 59.7 | (35.4–88.0) | 59.5 | (34.6–88.5) | 65.3 | (38.9–95.5) |
| Fruit & vegetable consumption (g/day) † | 380.8 | (229.3–615.6) | 325.9 | (200.8–512.2) | 315.5 | (192.8–516.4) | 461.2 | (305.7–645.5) | 416.3 | (278.4–588.7) | 419.4 | (268.9–605.5) |
| Alcohol intake (g/day) † | 7.4 | (0.6–24.0) | 12.9 | (4.4–30.2) | 12.5 | (4.1–28.5) | 1.2 | (0–6.7) | 4.0 | (0.8–11.5) | 3.7 | (0.6–11.3) |
| Ever use of contraceptive pill | ||||||||||||
| Yes (%) | 52.0 | 55.9 | 61.4 | |||||||||
| Ever use of menopausal hormone therapy | ||||||||||||
| Yes (%) | 23.8 | 23.9 | 22.7 | |||||||||
| Menopausal status | ||||||||||||
| Postmenopausal (%) | 41.5 | 46.2 | 35.4 | |||||||||
Values are medians (interquartile range) unless otherwise stated.
Physically active participants are those who either had a sedentary job with >1 hour recreational activity per day, or a standing job with >0.5 hour recreational activity per day, or a physical job with at least some recreational activity, or a heavy manual job.
Coffee consumption and all-cause mortality
High coffee consumers had lower all-cause mortality risks compared to non-consumers after adjustments for smoking and other covariates in the multivariable models (Men: HR comparing highest quartile of coffee consumption with non-consumers=0.88; 95%CI: 0.82–0.95; P-trend<0.001; Women: HR=0.93; 95% CI: 0.87–0.98; P-trend=0.009; Table 2). When cup-size categories were used, similar inverse associations were observed for men (≥3 cups/day vs. non-consumers, HR=0.82, 95%CI: 0.76–0.89; P-trend<0.001) and women (≥3 cups/day vs. non-consumers, HR=0.92, 95%CI: 0.87–0.98; P-trend<0.001) (data not tabulated). There was no evidence of heterogeneity by country for the association between coffee consumption and all-cause mortality (P-heterogeneity 0.71 for men and 0.37 for women). Overall, similar inverse associations and linear trends were observed for consumption of caffeinated and decaffeinated coffee, albeit in men, the association of caffeinated coffee with all-cause mortality was less pronounced than for decaffeinated coffee, with a statistically significant lower risk not observed in the highest quartile of consumption (Tables S3 and S4).
Table 2.
Associations of daily coffee consumption and all-cause and cause-specific mortality among men and women.
| Category of coffee consumption (mL/day) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Non-consumers | Q1 Low consumption | Q2 Medium-low consumption | Q3 Medium-high consumption | Q4 High consumption | P-trend | Per cup | |
| All-cause | |||||||
| Men | |||||||
| N deaths | 1039 | 4,972 | 4,440 | 4,250 | 3,601 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.89 (0.83–0.95) | 0.89 (0.83–0.95) | 0.90 (0.84–0.96) | 1.07 (0.99–1.15) | <0.001 | |
| Basic model plus smoking variables - HR (95% CI) † | 1.00 | 0.88 (0.82–0.94) | 0.83 (0.77–0.89) | 0.78 (0.73–0.84) | 0.83 (0.77–0.89) | <0.001 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.94 (0.87–1.00) | 0.88 (0.82–0.95) | 0.84 (0.78–0.90) | 0.88 (0.82–0.95) | <0.001 | 0.97 (0.96–0.98) |
| Women | |||||||
| N deaths | 1,817 | 6,882 | 5,236 | 5,294 | 4,162 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.90 (0.85–0.95) | 0.90 (0.85–0.95) | 0.95 (0.90–1.01) | 1.10 (1.04–1.16) | <0.001 | |
| Basic model plus smoking variables - HR (95% CI) † | 1.00 | 0.91 (0.86–0.96) | 0.87 (0.82–0.91) | 0.87 (0.82–0.92) | 0.90 (0.85–0.96) | 0.004 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.94 (0.89–0.99) | 0.90 (0.85–0.95) | 0.90 (0.85–0.95) | 0.93 (0.87–0.98) | 0.009 | 0.99 (0.98–1.00) |
| Cancer (C00-D48) | |||||||
| Men | |||||||
| N deaths | 386 | 1,923 | 1,861 | 1,816 | 1628 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.90 (0.81–1.01) | 1.01 (0.90–1.13) | 1.04 (0.93–1.16) | 1.27 (1.13–1.42) | <0.001 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.92 (0.82–1.03) | 0.97 (0.86–1.08) | 0.92 (0.82–1.03) | 0.99 (0.88–1.11) | 0.45 | 1.00 (0.99–1.02) |
| Women | |||||||
| N deaths | 645 | 2,917 | 2,305 | 2,417 | 2,105 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.98 (0.90–1.07) | 1.07 (0.98–1.17) | 1.12 (1.03–1.23) | 1.33 (1.22–1.46) | <0.001 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 1.00 (0.92–1.10) | 1.05 (0.96–1.15) | 1.04 (0.95–1.14) | 1.12 (1.02–1.23) | 0.001 | 1.03 (1.01–1.04) |
| Circulatory diseases (I00-I99) | |||||||
| Men | |||||||
| N deaths | 257 | 1352 | 1148 | 1091 | 922 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.96 (0.84–1.10) | 0.91 (0.80–1.05) | 0.92 (0.80–1.05) | 1.12 (0.97–1.29) | 0.03 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 1.02 (0.89–1.17) | 0.93 (0.80–1.07) | 0.87 (0.76–1.00) | 0.93 (0.80–1.08) | 0.004 | 0.97 (0.95–0.99) |
| Women | |||||||
| N deaths | 334 | 1399 | 968 | 959 | 676 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.85 (0.75–0.96) | 0.75 (0.66–0.85) | 0.82 (0.72–0.93) | 0.94 (0.82–1.08) | 0.95 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.89 (0.78–1.01) | 0.74 (0.65–0.85) | 0.77 (0.67–0.88) | 0.78 (0.68–0.90) | <0.001 | 0.96 (0.94–0.99) |
| Cerebrovascular diseases (I60-I69) | |||||||
| Men | |||||||
| N deaths | 58 | 284 | 231 | 195 | 150 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.89 (0.66–1.19) | 0.80 (0.60–1.08) | 0.77 (0.57–1.04) | 0.92 (0.67–1.25) | 0.42 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.94 (0.70–1.27) | 0.83 (0.61–1.12) | 0.76 (0.56–1.04) | 0.83 (0.60–1.14) | 0.04 | 0.94 (0.89–0.99) |
| Women | |||||||
| N deaths | 114 | 472 | 358 | 317 | 201 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.83 (0.67–1.03) | 0.78 (0.63–0.98) | 0.77 (0.62–0.97) | 0.82 (0.64–1.04) | 0.15 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.85 (0.68–1.05) | 0.77 (0.62–0.96) | 0.74 (0.59–0.92) | 0.70 (0.55–0.90) | 0.002 | 0.94 (0.90–0.99) |
| Ischemic heart disease (I20-I25) | |||||||
| Men | |||||||
| N deaths | 112 | 597 | 533 | 534 | 474 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.94 (0.77–1.15) | 0.92 (0.75–1.13) | 0.94 (0.77–1.15) | 1.15 (0.94–1.42) | 0.015 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 1.03 (0.84–1.26) | 0.96 (0.78–1.18) | 0.92 (0.75–1.13) | 0.97 (0.79–1.20) | 0.24 | 0.99 (0.96–1.02) |
| Women | |||||||
| N deaths | 83 | 415 | 296 | 266 | 216 | ||
| Basic model - HR (95% CI) † | 1.00 | 0.96 (0.75–1.23) | 0.84 (0.65–1.09) | 0.81 (0.63–1.05) | 1.07 (0.83–1.40) | 0.96 | |
| Multivariable model - HR (95% CI) ‡ | 1.00 | 1.03 (0.80–1.32) | 0.83 (0.64–1.08) | 0.74 (0.57–0.96) | 0.82 (0.62–1.07) | <0.001 | 0.94 (0.90–0.98) |
| Digestive diseases (K00-K93) § | |||||||
| Men | |||||||
| N deaths | 274 | 144 | 105 | 82 | |||
| Basic model - HR (95% CI) † | 1.00 | 0.72 (0.59–0.89) | 0.53 (0.42–0.67) | 0.55 (0.42–0.70) | <0.0001 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.69 (0.56–0.85) | 0.46 (0.37–0.59) | 0.41 (0.32–0.54) | <0.0001 | 0.77 (0.72–0.81) | |
| Women | |||||||
| N deaths | 273 | 134 | 122 | 79 | |||
| Basic model - HR (95% CI) † | 1.00 | 0.75 (0.60–0.92) | 0.76 (0.61–0.94) | 0.77 (0.60–1.00) | 0.004 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.70 (0.56–0.86) | 0.67 (0.54–0.84) | 0.60 (0.46–0.78) | <0.0001 | 0.86 (0.81–0.92) | |
| Respiratory diseases (J30-J98) § | |||||||
| Men | |||||||
| N deaths | 240 | 162 | 161 | 151 | |||
| Basic model - HR (95% CI) † | 1.00 | 0.89 (0.73–1.09) | 1.03 (0.84–1.27) | 1.55 (1.25–1.91) | 0.004 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.81 (0.66–0.99) | 0.84 (0.69–1.04) | 1.05 (0.84–1.30) | 0.62 | 1.01 (0.96–1.06) | |
| Women | |||||||
| N deaths | 316 | 212 | 185 | 162 | |||
| Basic model - HR (95% CI) † | 1.00 | 1.08 (0.91–1.29) | 1.16 (0.96–1.40) | 1.74 (1.43–2.13) | <0.0001 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.95 (0.79–1.14) | 0.83 (0.69–1.01) | 0.91 (0.74–1.12) | 0.14 | 0.98 (0.94–1.03) | |
| External causes (S00-Y98) § | |||||||
| Men | |||||||
| N deaths | 285 | 181 | 187 | 183 | |||
| Basic model - HR (95% CI) † | 1.00 | 0.84 (0.70–1.02) | 0.87 (0.72–1.05) | 1.03 (0.85–1.25) | 0.66 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.83 (0.68–1.00) | 0.82 (0.68–1.00) | 0.90 (0.74–1.10) | 0.10 | 0.96 (0.91–1.01) | |
| Women | |||||||
| N deaths | 284 | 157 | 157 | 137 | |||
| Basic model - HR (95% CI) † | 1.00 | 0.93 (0.76–1.13) | 0.93 (0.76–1.14) | 1.07 (0.86–1.32) | 0.96 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.93 (0.76–1.13) | 0.91 (0.74–1.11) | 0.96 (0.77–1.20) | 0.47 | 0.98 (0.93–1.04) | |
| Suicide (X60-X84) § | |||||||
| Men | |||||||
| N deaths | 91 | 51 | 47 | 53 | |||
| Basic model - HR (95% CI) † | 1.00 | 0.78 (0.55–1.10) | 0.72 (0.50–1.03) | 0.91 (0.64–1.30) | 0.25 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 0.75 (0.52–1.06) | 0.64 (0.44–0.92) | 0.71 (0.50–1.02) | 0.02 | 0.90 (0.83–0.98) | |
| Women | |||||||
| N deaths | 67 | 40 | 31 | 38 | |||
| Basic model - HR (95% CI) † | 1.00 | 1.06 (0.71–1.58) | 0.81 (0.52–1.25) | 1.19 (0.78–1.81) | 0.82 | ||
| Multivariable model - HR (95% CI) ‡ | 1.00 | 1.06 (0.71–1.59) | 0.77 (0.50–1.20) | 0.98 (0.63–1.51) | 0.61 | 0.97 (0.87–1.09) | |
Basic model – Cox regression using total energy intake (kcal/day) and stratified by age (1-year categories) and center.
Multivariable model - Cox regression using body mass index (<22; 22–<25; 25–<30; 30–<35; 35+ kg/m2), physical activity index (inactive; moderately inactive; moderately active; active), education status (none; primary school completed; technical/professional school; secondary school; longer education including university; or not specified), alcohol consumption (non-consumers; <5; 5–<15; 15–<30; 30+ g/day), smoking status and intensity (never; current, 1–15 cigarettes per day; current, 16–25 cigarettes per day; current, 16+ cigarettes per day; former, quit ≤10 years; former, quit 11–20 years; former, quit 20+ years; current, pipe/cigar/occasional; current/former, missing; unknown), smoking duration (<10; 10–<20; 20–<30; 30–<40; 40+ years; smoking duration unknown), ever use of contraceptive pill (yes; no; or unknown), menopausal status (premenopausal; postmenopausal; perimenopausal/unknown menopausal status; or surgical postmenopausal), ever use of menopausal hormone therapy (yes; no; or unknown), and intakes of total energy (kcal/day), red and processed meat (g/day), and fruits and vegetables (g/day) (all continuous), and stratified by age (1-year categories), and center.
Categories were based on country-specific quartiles of coffee consumption after exclusion of non-consumers. Quartile cut-offs (in mL) were: Denmark: 500, 900 and 1,300; France: 150, 280 and 450; Germany: 261, 395 and 580; Greece: 70, 140 and 240; Italy: 60, 92 and 138; The Netherlands: 375, 500 and 750; Norway: 300, 420 and 540; Spain: 50, 105 and 196; Sweden: 300, 400 and 601; and the United Kingdom: 83, 380 and 488.
Due to low case numbers among non-consumers, reference category merged with low consumers (Q1).
Adjusted cumulative incidence curves for all-cause mortality by coffee consumption categories are presented in Figure S1. For men, compared to non-consumers of coffee, the cumulative incidence of death until age 80 years was 3.1% (95% CI: 1.74–4.53) and 2.2% (95% CI: 0.80–3.68) lower among those in the third and highest quartile of coffee consumption, respectively. For women, the cumulative incidence of death until age 80 years was 1.4% (95% CI: 0.55–2.28) and 0.8% (95% CI: −0.12–1.69) lower among those in the third and highest quartile of coffee consumption when compared against non-coffee consumers.
Coffee consumption and cause-specific mortality
Strong inverse associations were observed between coffee consumption and risks of digestive disease deaths for men (Q4 vs. non-consumers/Q1, HR=0.41, 95%CI: 0.32–0.54; P-trend<0.0001) and women (Q4 vs. non-consumers/Q1, HR=0.60, 95%CI: 0.46–0.78; P-trend<0.0001) (Table 2). Similar strength inverse associations were observed when cup-size categories were used (data not shown). Just over one-third of digestive disease deaths were due to liver disease. There was a statistically significant inverse association between coffee and liver disease deaths (sexes combined: Q4 vs. non-consumers, HR=0.20, 95%CI: 0.13–0.29), whereas the results of non-liver digestive disease deaths were inconclusive (sexes combined: Q4 vs. non-consumers, HR 0.81, 95% CI: 0.56–1.16). There was a strong, inverse association between deaths from liver cirrhosis and coffee drinking (sexes combined: Q4 vs. non-consumers, HR 0.21 95% CI: 0.13–0.34. Similar inverse associations were observed for alcoholic and non-alcoholic cirrhosis (data not shown).
Consumption of coffee was also inversely associated with circulatory diseases; this association was more pronounced in women and the inverse associations were stronger for deaths by cerebrovascular disease (Q4 vs. non-consumers, HR=0.70, 95%CI: 0.55–0.90; P-trend=0.002) (Table 2). In general, the associations between coffee and cause-specific mortality were weakened when caffeinated and decaffeinated coffee were analyzed separately, albeit associations were in the same direction for both coffee types (Tables S3 and S4). The association of coffee drinking with cancer-related death was not statistically significant in men whereas in women a positive association was found (Q4 vs. non-consumers, HR=1.12, 95%CI: 1.02–1.23; P-trend=0.001). In further analyses by cancer site, we observed a statistically significant positive association between coffee and ovarian cancer-specific mortality (Q4 vs. non-consumers, HR=1.31, 95% CI, 1.07–1.61) in a multivariable model that included smoking and other risk factors (Table S5). There were also suggestive positive associations between coffee and mortality from colorectal and lung cancer in women, whereas in men, there were statistically significant inverse associations between low-medium consumption of coffee and lung cancer mortality (Table S5). Coffee drinking was statistically significantly inversely associated with liver cancer mortality in both men and women. Respiratory disease mortality was not related to coffee consumption in the full models (Table 2). Coffee consumption was not associated with deaths caused by external causes; however, an inverse relationship was observed between suicide and coffee for men, but not women (Table 2).
Subgroup and sensitivity analyses
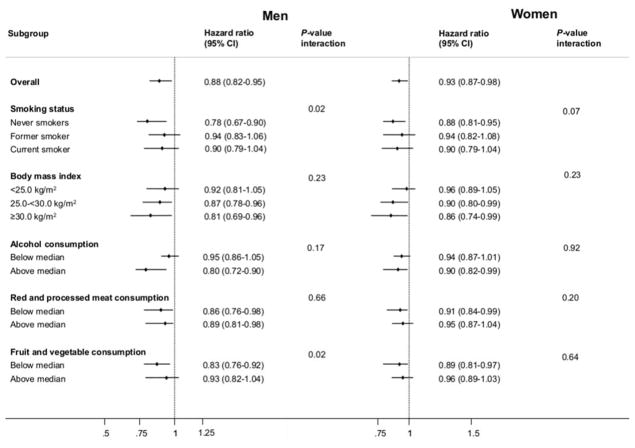
Smoking was the most influential confounder for the all-cause mortality analyses (Table 2); however, because smoking is positively associated with both coffee consumption and risk of death, confounding in this case would obscure a possible reduction in risk associated with coffee. As expected, statistical adjustment for smoking strengthened the association between coffee and reduced risk of death. Inverse all-cause mortality associations with coffee consumption were observed among never smokers, and across subgroups of other mortality risk factors (Figure 1). Similarly, among never smokers, inverse coffee associations were observed for deaths caused by cancer, circulatory diseases, digestive diseases, and respiratory diseases (Table S6).
Figure 1.
Subgroup analysis of the associations of daily coffee consumption and all-cause mortality among men and women.
Hazard ratios for all-cause mortality are for the comparison of men and women in the highest quartile of consumers (high consumption) compared versus non-consumers. Multivariable model only - Cox regression using body mass index (<22; 22–<25; 25–<30; 30–<35; 35+ kg/m2), physical activity index (inactive; moderately inactive; moderately active; active), education status (none; primary school completed; technical/professional school; secondary school; longer education including university; or not specified), alcohol consumption (non-consumers; <5; 5–<15; 15–<30; 30+ g/day), smoking status and intensity (never; current, 1–15 cigarettes per day; current, 16–25 cigarettes per day; current, 16+ cigarettes per day; former, quit ≤10 years; former, quit 11–20 years; former, quit 20+ years; current, pipe/cigar/occasional; current/former, missing; unknown), smoking duration (<10; 10–<20; 20–<30; 30–<40; 40+ years; smoking duration unknown), ever use of contraceptive pill (yes; no; or unknown), menopausal status (premenopausal; postmenopausal; perimenopausal/unknown menopausal status; or surgical postmenopausal), ever use of menopausal hormone therapy (yes; no; or unknown), and intakes of total energy (kcal/day), red and processed meat (g/day), and fruits and vegetables (g/day) (all continuous), and stratified by age (1-year categories) and center. Categories were based on country-specific quartiles of coffee consumption after exclusion of non-consumers. Quartile cut-offs (in mL) were: Denmark: 500, 900 and 1,300; France: 150, 280 and 450; Germany: 261, 395 and 580; Greece: 70, 140 and 240; Italy: 60, 92 and 138; The Netherlands: 375, 500 and 750; Norway: 300, 420 and 540; Spain: 50, 105 and 196; Sweden: 300, 400 and 601; and the United Kingdom: 83, 380 and 488. Median consumption: alcohol = 12.6 g/day (men) and 3.4 g/day (women); red and processed meat = 90.2 g/day (men) and 60.3 g/day (women); fruit and vegetables = 324 g/day (men) and 413 g/day (women).
No heterogeneity of the all-cause mortality associations was observed according to follow-up time categories (Table S7). The all-cause and cause-specific mortality associations were virtually unchanged when deaths which occurred during the first 5 and 8 years of follow-up were excluded (Tables S8 and S9). Similar associations were also observed when analyses were limited to individuals who reported being in ‘excellent’ or ‘good’ health at baseline (n=119,609 participants; Table S10), and when analyses were limited to sole consumers of caffeinated and decaffeinated coffee only (data not shown).
Serum levels of liver, inflammation and metabolic biomarkers by coffee consumption
In the EPIC-Biomarkers sub-cohort, compared to non-coffee and/or low consumers, higher coffee consumers had statistically significantly lower mean levels of the liver enzymes ALP, ALT, AST, and GGT, lower levels of CRP, and higher serum albumin (all P-trends<0.05; Table 3). For women only, higher coffee consumption was correlated with lower serum HbA1c, lipoprotein (a), and higher HDL-C. A total of 891 all-cause deaths were recorded in the EPIC-Biomarkers sub-cohort. Serum levels of ALP, AST, GGT, and CRP were associated with all-cause mortality when the highest and lowest quartiles were compared (Figure S2). Higher serum levels of albumin and ALT were associated with lower all-cause mortality.
Table 3.
Multivariable-adjusted mean serum levels of liver function, circulatory disease, and metabolic biomarkers across coffee consumption categories among men and women (n=14,800).
| Non-consumers | Categories of coffee consumption | P-trend | ||||
|---|---|---|---|---|---|---|
| Q1 Low consumption | Q2 Medium-low consumption | Q3 Medium-high consumption | Q4 High consumption | |||
| Albumin (g/L) ‡ | ||||||
| Men | 46.70 | 46.18 | 46.49 | 46.61 | 46.93 | 0.005 |
| Women | 45.95 | 45.56 | 45.80 | 46.03 | 46.04 | 0.006 |
| Alkaline phosphatase (ALP) (μkat/L)** | ||||||
| Men | 1.15 | 1.10 | 1.10 | 1.07 | 1.09 | <0.0001 |
| Women | 1.08 | 1.07 | 1.04 | 1.04 | 1.01 | <0.0001 |
| Alanine transaminase (ALT) (u/L)** | ||||||
| Men | 25.37 | 24.49 | 24.60 | 24.23 | 23.77 | <0.0001 |
| Women | 17.32 | 17.53 | 17.30 | 17.09 | 16.81 | 0.028 |
| Aspartate transaminase (AST) (u/L) | ||||||
| Men | 32.18 | 30.92 | 29.96 | 29.57 | 29.31 | <0.0001 |
| Women | 26.80 | 26.51 | 26.02 | 25.82 | 25.53 | 0.001 |
| Gamma-glutamyltransferase (GGT) (μkat/L)** | ||||||
| Men | 0.53 | 0.56 | 0.56 | 0.53 | 0.51 | <0.0001 |
| Women | 0.29 | 0.33 | 0.31 | 0.32 | 0.29 | <0.0001 |
| hs-CRP (nmol/L)** | ||||||
| Men | 10.93 | 11.05 | 11.26 | 11.50 | 12.76 | 0.068 |
| Women | 13.56 | 13.20 | 12.22 | 12.00 | 11.01 | <0.0001 |
| Glycated hemoglobin (HbA1c; %) ‡ | ||||||
| Men | 5.50 | 5.50 | 5.50 | 5.50 | 5.50 | 0.028 |
| Women | 5.50 | 5.40 | 5.40 | 5.40 | 5.40 | 0.007 |
| HDL-cholesterol (mmol/L (mg/dL)) ‡ | ||||||
| Men | 1.31 (50.65) | 1.32 (50.81) | 1.30 (50.37) | 1.29 (49.81) | 1.28 (49.50) | 0.250 |
| Women | 1.60 (61.64) | 1.62 (62.71) | 1.62 (62.62) | 1.61 (62.24) | 1.62 (62.47) | 0.001 |
| Lipoprotein (a) (μmol/L)** | ||||||
| Men | 14.28 | 14.49 | 14.62 | 14.06 | 13.89 | 0.240 |
| Women | 12.93 | 12.39 | 12.06 | 12.25 | 11.14 | 0.002 |
Multivariable means adjusted for country, smoking status (never, former, current, or missing), age (continuous), body mass index (<22; 22–<25; 25–<30; 30–<35; 35+ kg/m2), alcohol consumption (g/day; continuous), total energy intake (kcal/day; continuous). Categories were based on country-specific quartiles of coffee consumption after exclusion of non-consumers. Quartile cut-offs (in mL) were: Denmark: 500, 900 and 1,300; France: 151, 277 and 437; Germany: 262, 404 and 580; Italy: 60, 90 and 130; The Netherlands: 447, 536 and 768; Spain: 50, 110 and 200; Sweden: 321, 455 and 611; and the United Kingdom: 192, 477 and 855.
Arithmetic mean.
Geometric mean.
Trend tests across exposure groups were calculated by entering the category variables into the models as continuous terms.
Discussion
In this large-scale analysis of a multi-country European population, higher consumption of coffee was associated with lower risks of death, and in particular, mortality due to digestive and circulatory diseases. The inverse association between all-cause mortality and coffee was generally apparent for both caffeinated and decaffeinated coffee consumption. Coffee drinking was also associated with variation in serum biomarkers of liver function, inflammation, insulin sensitivity and blood lipids; additionally, these same biomarkers were also associated with all-cause mortality, adding some degree of biological plausibility to the potential protective effects of coffee on common health outcomes.
The relation between coffee drinking and mortality has been investigated in numerous smaller studies, with mixed results reported (7–9). Consistent with the current investigation, prospective studies in Japan and the U.S. have published inverse associations between coffee consumption and all-cause mortality (10–13, 16, 17). The current investigation was the largest worldwide study to date to examine the coffee and mortality relationship, and the first comprehensive European based investigation. Previous European studies were of much smaller size and based within individual countries, where coffee intakes and preparation methods are relatively homogenous. In contrast, our analysis of EPIC data from 10 European countries with ~42,000 documented deaths would have captured the inter-country coffee preparation methods and customs unlike any other study to date. Similar to the findings from the NIH-AARP analysis, our observed inverse association between coffee and all-cause mortality was consistent across subgroups of other lifestyle, anthropometric and dietary variables and was apparent for both caffeinated and decaffeinated coffee. The caffeinated and decaffeinated data should, however, be interpreted cautiously as decaffeinated coffee consumption were very low in several of the populations included in EPIC and separate information for decaffeinated coffee was not collected in all EPIC centers. Further, the analyses may be contaminated by participants habitually consuming both types of coffee. Nevertheless, in sensitivity analyses, where only sole-consumers of caffeinated or decaffeinated coffee were analyzed, the associations remained essentially unaltered.
Our results revealed that coffee consumption was strongly inversely associated with liver disease mortality. Previous studies have reported inverse associations between coffee and both alcoholic and non-alcoholic cirrhosis development (24–26). With the largest number of liver disease cases to date, our results are consistent with these smaller studies. Serum levels of several indicators of altered hepatic function-including the enzymes ALP, ALT, AST, and GGT were lower among coffee drinkers compared to non-consumers/low consumers in the current analysis - observations that were consistent with prior data (24, 27); suggesting that coffee drinking may potentially have beneficial effects on hepatic function and health. Several lines of experimental evidence suggest that caffeine has anti-fibrotic effects on hepatocytes and hepatic stellate cells (28). In hepatocytes, caffeine has been demonstrated to lower levels of transforming growth factor-beta (TGF-β) which activates connective tissue growth factor, a potent fibrogenic promoting molecule (29). In hepatic stellate cells (HSC), which when activated are a major driver of liver fibrosis, caffeine has also been shown to exert anti-fibrotic effects by lowering proliferation, stimulating apoptosis, and inhibiting adhesion (30). Coffee has also been demonstrated to impede progression of fatty liver disease by reducing fat accumulation, oxidative stress and liver inflammation in murine models (31), and a possible beneficial role for coffee on liver disease progression in hepatitis-C patients has also been reported (32).
The observed inverse associations between coffee drinking and mortality from circulatory disease are consistent with the prior NIH-AARP analysis (10). We note that this relation was stronger among women than men with the difference between sexes driven by a strong inverse association for cerebrovascular mortality risk in women; a finding consistent with previous studies which reported lower incidence of stroke in women consuming coffee (33, 34). Interestingly, levels of HDL-C, which has been inversely related to risks of stroke and other circulatory disease outcomes (35), were higher among coffee drinkers compared to non-consumers in women but not in men. Further, among women only, lipoprotein(a), CRP, and HbA1c – factors that have been positively associated with cardiovascular disease outcomes (36–39) - were generally lower among coffee drinkers compared to non-consumers. Given that the inverse relation between coffee drinking and circulatory disease mortality was primarily restricted to females, it may be hypothesized that this association might be driven by female-specific beneficial effects of coffee on lipid, inflammatory, and metabolic profiles.
Interestingly, we observed a positive association between coffee drinking and overall cancer mortality among women in this population. This relationship was primarily driven by a statistically significant positive association between coffee and mortality from ovarian cancer, with suggestive positive associations for lung and colorectal cancer. To our knowledge, there is no strong hypothesis on why coffee drinking should specifically raise the risk of death from ovarian cancer. While this result may be spurious and requires follow-up in additional studies on ovarian cancer survival, we note that a positive association between coffee consumption and ovarian cancer incidence has previously been observed in the Iowa Women’s Health Study (40), though other prospective studies did not report similar relationships (41, 42).
We also note a statistically significant inverse relationship between coffee consumption and death from suicide for men, but not women. Consistent with this finding, coffee consumption was previously associated with lower suicide risk in a recent pooled analysis of the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (43). Discordant with these results, a Finnish prospective study reported greater suicide risk among heavy coffee consumers (44). Overall, our analysis only included 418 suicides, and importantly we lacked information on other factors related to suicide risk, such as antidepressant medication use and mental health status (e.g. depressive symptoms, anxiety, and stress) which may confound the coffee and suicide relationship. Further investigations on the effects of long-term coffee consumption on suicides are warranted.
Our prospective study was the largest to date to investigate the coffee-mortality relationship, and we controlled for important potential confounding factors. However we recognize that the associations may be biased due to residual confounding. In our analyses, smoking was the most important and influential confounder of the coffee-mortality relationship. However, the large number of participants and recorded deaths meant that our analyses could be restricted to never smokers. These analyses revealed that the inverse all-cause mortality association was stronger among never smokers, indicating that if residual confounding by smoking did occur, it would likely have attenuated the lower all-cause mortality risks observed among high coffee consumers. Generally, although residual confounding cannot be excluded as a potential explanation of our findings, our data revealed limited evidence that our results were the result of confounding bias due to smoking or other established mortality risk factors. Another possible explanation for our results is reverse causality, whereby participants experiencing early disease symptoms at baseline may have recorded lower coffee consumption or became non-consumers; thus artificially inflating the mortality risks amongst these individuals. However, we excluded participants from our analysis who self-reported previous ill-health. Further, similar associations were observed when the analyses were limited to those individuals who self-reported being in ‘excellent’ or ‘good’ health at baseline, and when participants who died during the first 5 and 8 years of follow-up were excluded. An additional limitation is that coffee consumption information was only measured once at baseline and it is possible that changes in consumption may have occurred during the follow-up period. However, other studies in Western populations which measured diet repeatedly over the study follow-up period have recorded relatively stable coffee consumption patterns over time indicating that a single measure likely captures medium to long-term drinking habits (11). Finally, as coffee drinking was self-reported using dietary questionnaires some degree of measurement error and misclassification is expected. However, due to the prospective nature of the study, such misclassification would be expected to be non-differential (not correlated with mortality risk), and if at all would lead to an under-estimate of the true association.
In summary, our results suggest that higher levels of coffee drinking are associated with lower risks of death from a variety of causes and specifically from digestive and circulatory diseases. The consistency of the results of this European study with those from other cohort studies around the world, as well as biomarker data that indicate coffee drinkers have a more favorable liver function and inflammatory biomarker profile than non-consumers/low consumers, offers support to the hypothesis that coffee may confer chemopreventive properties. Since coffee is so ubiquitously consumed, and intakes are modifiable, the potentially beneficial clinical implications of coffee consumption should be given careful consideration and deserve to be further explored for its potential major impact on population health.
Supplementary Material
Table S1. Analytical methods used to measure the liver function, circulatory disease and metabolic biomarkers.
Table S2. Descriptive information of the European Prospective Investigation into Cancer and Nutrition study participant countries.
Table S3. Multivariable associations of daily caffeinated coffee consumption and all-cause and cause-specific mortality.
Table S4. Multivariable associations of daily decaffeinated coffee consumption and all-cause and cause-specific mortality.
Table S5. Associations of daily coffee consumption and overall and individual cancer mortality.
Table S6. Associations of daily coffee consumption and cause-specific mortality by smoking status.
Table S7. Associations of daily coffee consumption and all-cause mortality by follow-up time categories.
Table S8. Multivariable associations of daily coffee consumption and all-cause and cause-specific mortality among men and women after deaths which occurred during the first 5 years of follow-up (n=5,247) were excluded.
Table S9. Multivariable associations of daily coffee consumption and all-cause and cause-specific mortality among men and women after deaths which occurred during the first 8 years of follow-up (n=10,790) were excluded.
Table S10. Associations of daily coffee consumption and all-cause and cause-specific mortality among participants who self-reported being in ‘excellent’ or ‘good’ health at baseline (n=119,609).
Figure S1. Adjusted cumulative incidence of all-cause mortality, by coffee consumption categories among men and women.
Figure S2. Multivariable associations of serum liver function, circulatory disease, and metabolic biomarkers and all-cause mortality (n=1,597 deaths) among men and women using sex specific quartiles.
Acknowledgments
We would like to thank the EPIC study participants and staff for their valuable contribution to this research.
Funding: The coordination of EPIC is financially supported by the European Commission (DG-SANCO); and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer; Institut Gustave Roussy; Mutuelle Générale de l’Education Nationale; and Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum; and Federal Ministry of Education and Research (Germany); Hellenic Health Foundation; Stavros Niarchos Foundation; and the Hellenic Ministry of Health and Social Solidarity (Greece); Italian Association for Research on Cancer (AIRC); National Research Council; and Associazione Iblea per la Ricerca Epidemiologica (AIRE-ONLUS) Ragusa, Associazione Volontari Italiani Sangu (AVIS) Ragusa, Sicilian Government (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS); Netherlands Cancer Registry (NKR); LK Research Funds; Dutch Prevention Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); and Statistics Netherlands (the Netherlands); European Research Council (ERC) (grant number ERC-2009-AdG 232997) and Nordforsk; and Nordic Center of Excellence Programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS); Regional Governments of Andalucía, Asturias, Basque Country, Murcia (No. 6236) and Navarra; and the Centro de Investigación Biomédica en Red en Epidemiología y Salud Pública and Instituto de Salud Carlos II (ISCIII RETIC) (RD06/0020) (Spain); Swedish Cancer Society; Swedish Scientific Council; and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK; Medical Research Council; Stroke Association; British Heart Foundation; Department of Health; Food Standards Agency; and the Wellcome Trust (UK). Funding for the biomarker measurements in the random sub-cohort was provided by grants to EPIC-InterAct from the European Community Framework Programme 6 and to EPIC-Heart from the Medical Research Council and British Heart Foundation (Joint Award G0800270). We thank Nicola Kerrison (MRC Epidemiology Unit, Cambridge) for managing the data for the InterAct Project. Funding for the InterAct project was provided by the EU FP6 programme (grant number LSHM_CT_2006_037197). The work undertaken by David C Muller was done during the tenure of an IARC, Australia postdoctoral fellowship, supported by the Cancer Council Australia. Domenico Palli was supported by a grant from the Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Mailing Addresses
Marc J. Gunter, Neil Murphy, Laure Dossus, Idlir Licaj, Paul Brennan - International Agency for Research on Cancer (IARC), 150 cours Albert Thomas, 69372 Lyon CEDEX 08, France.
Amanda J. Cross, David C Muller, Paolo Vineis, Elio Riboli - Department of Epidemiology and Biostatistics, Imperial College London, St Mary’s Campus, Norfolk Place, Paddington, London W2 1PG, United Kingdom.
Laureen Dartois, Guy Fagherazzi - Gustave Roussy, Espace Maurice Tubiana, Equipe E3N / E4N, 114 rue Edouard Vaillant, 94800 Villejuif, France.
Rudolf Kaaks, Tilman Kühn - Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Im Neuenheimer Feld 581, 69120 Heidelberg, Germany.
Heiner Boeing, Krasimira Aleksandrova - German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Arthur-Scheunert-Allee 114-116, 14558 Nuthetal, Germany.
Anne Tjønneland, Anja Olsen - Danish Cancer Society, Strandboulevarden 49, DK-2100 København Ø, Denmark.
Kim Overvad – Aarhus University, Bartholins Allé 2, building 1260, 2.26, 8000 Aarhus C, Denmark.
Sofus Christian Larsen - The Parker Institute, Copenhagen University Hospital, Bispebjerg og Frederiksberg, Nordre Fasanvej 57, Road 8-entrance 19, DK-2000 Frederiksberg, Denmark.
Maria Luisa Redondo Cornejo - Public Health and Health Planning Directorate, Consejeria de Salud y Servicios Sanitarios del Principado de Asturias General Elorza 32, 33001 Oviedo, Spain.
Antonio Agudo - Institut Català d’Oncologia (ICO), Cancer Epidemiology Research Programme, Av. Gran Via de l’Hospitalet 199–203, L’Hospitalet de Llobregat, Barcelona, 08908, Spain.
María José Sánchez Pérez - Escuela Andaluza de Salud Pública, Cuesta del Observatorio, 4 - Campus Universitario de Cartuja s/n, Apdo. de Correos 2070 - 18080 Granada, Spain.
Jone M Altzibar - Public Health Department of Gipuzkoa, Basque Government, Avenida de Navarra, 4-20013 Donostia, San Sebastián, Spain.
Carmen Navarro - Epidemiology and Public Health Department, Murcia Health Council, Ronda de Levante 11, Murcia 3008, Spain.
Eva Ardanaz - Epidemiology, Prevention and Promotion Health Service, Institute of Public Health Navarra, Leyre 15, 31003 Pamplona, Navarra, Spain.
Kay-Tee Khaw, Adam Butterworth - Department of Public Health & Primary Care, Strangeways Research Laboratory, Worts Causeway, Cambridge, CB1 8RN, United Kingdom.
Kathryn E Bradbury - Nuffield Department of Population Health, University of Oxford, Richard Doll Building, Old Road Campus, Oxford OX3 7LF, United Kingdom.
Antonia Trichopoulou – Hellenic Health Foundation, Kaisareias 13 & Alexandroupoleos, GR-115 27, Athens, Greece.
Pagona Lagiou - Department of Epidemiology, Harvard TH Chan School of Public Health,677 Huntington Avenue, Kresge, Room 901, Boston, Massachusetts 02115, USA.
Dimitrios Trichopoulos – deceased.
Domenico Palli - Molecular and Nutritional Epidemiology Unit, ISPO (Cancer Study and Prevention Centre), Via delle Oblate 2, 50141, Florence, Italy.
Sara Grioni - Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Via Giacomo Venezian, 1, 20133 Milano, Italy.
Salvatore Panico - Dipartimento di Medicina Clinica e Sperimentale, Federico II University, Corso Umberto I, 40, 80138 Napoli, Italy.
Rosario Tumino - Tumor Registry, Department of preventive medicine, provincial health Ragusa, Via Dante 109, 97100 Ragusa, Italy.
Bas Bueno-de-Mesquita - RIVM, Antonie van Leeuwenhoeklaan 9, 3721 MA, Bilthoven, PO Box 1, 3720 BA Bilthoven, The Netherlands.
Peter Siersema, Max Leenders - Department of Gastroenterology and Hepatology, University Medical Centre, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
Joline WJ Beulens, Cuno U Uiterwaal - Department of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Universiteitsweg 100, 3584 CG Utrecht, The Netherlands.
Peter Wallström - Departmental Office for Clinical Sciences, Malmö, Clinical Research Centre, Post box 50332, SE-202 13 Malmö, Sweden.
Lena Maria Nilsson - Department of Public Health and Clinical Medicine, University Hospital, 901 85 Umeå, Sweden.
Rikard Landberg - Department of Food Science, BioCenter, Swedish University of Agricultural Sciences, Almas Allé 8, 750 07 Uppsala, Sweden.
Elisabete Weiderpass, Guri Skeie, Tonje Braaten - Fakturaadresse: UiT Norges arktiske universitet, Fakturamottak, Postboks 6050 Langnes, 9037 Tromsø, Norway.
Rashmi Sinha - 9609 Medical Center Drive, MSC 9776, Bethesda, Maryland 20892, USA.
Nick Wareham - MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Box 285 Institute of Metabolic Science, Cambridge Biomedical Campus, Cambridge, CB2 0QQ, United Kingdom.
Ethical approval
Informed consent was provided by all participants and ethical approval for entire EPIC cohort was obtained from the internal review board of the International Agency for Research on Cancer in Lyon, France under the protocol numbers SC/24/4 and SC/24/6, as well as from local ethics committees in the participating countries.
Contributors
MJG, NM and ER conceived and designed the study. All authors contributed to recruitment, data collection/acquisition and/or biological sample collection, and are responsible for the ongoing follow-up and management of the EPIC cohort. MJG, NM, and ER analyzed the data and wrote the manuscript. All authors critically evaluated the data, reviewed the manuscript and approved the final version. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors are not affiliated with the listed funding institutions. MJG and NM act as the guarantors of this paper.
References
- 1.Gomez-Ruiz JA, Leake DS, Ames JM. In Vitro Antioxidant Activity of Coffee Compounds and Their Metabolites. Journal of Agricultural and Food Chemistry: American Chemical Society. 2007:6962–9. doi: 10.1021/jf0710985. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Garcia E, Van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84(4):888–93. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 3.Wedick N, Brennan A, Sun Q, Hu F, Mantzoros C, van Dam R. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutrition Journal. 2011;10(1):93. doi: 10.1186/1475-2891-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loopstra-Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ. Associations between the intake of caffeinated and decaffeinated coffee and measures of insulin sensitivity and beta cell function. Diabetologia. 2011;54(2):320–8. doi: 10.1007/s00125-010-1957-8. [DOI] [PubMed] [Google Scholar]
- 5.Van Dam RM. Coffee and type 2 diabetes: from beans to beta-cells. Nutr Metab Cardiovasc Dis. 2006;16(1):69–77. doi: 10.1016/j.numecd.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 6.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care. 2006;29(2):398–403. doi: 10.2337/diacare.29.02.06.dc05-1512. [DOI] [PubMed] [Google Scholar]
- 7.Lindsted KD, Kuzma JW, Anderson JL. Coffee consumption and cause-specific mortality association with age at death and compression of mortality. Journal of Clinical Epidemiology. 1992;45(7):733–42. doi: 10.1016/0895-4356(92)90051-n. [DOI] [PubMed] [Google Scholar]
- 8.Andersen LF, Jacobs DR, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. The American Journal of Clinical Nutrition. 2006;83(5):1039–46. doi: 10.1093/ajcn/83.5.1039. [DOI] [PubMed] [Google Scholar]
- 9.Woodward M, Tunstall-Pedoe H. Coffee and tea consumption in the Scottish Heart Health Study follow up: conflicting relations with coronary risk factors, coronary disease, and all cause mortality. Journal of Epidemiology and Community Health. 1999;53(8):481–7. doi: 10.1136/jech.53.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of Coffee Drinking with Total and Cause-Specific Mortality. New England Journal of Medicine: Massachusetts Medical Society. 2012:1891–904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Satija A, Bhupathiraju SN, Hu Y, Sun Q, Han J, et al. Association of Coffee Consumption with Total and Cause-Specific Mortality in Three Large Prospective Cohorts. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftfield E, Freedman ND, Graubard BI, Guertin KA, Black A, Huang W-Y, et al. Association of Coffee Consumption With Overall and Cause-Specific Mortality in a Large US Prospective Cohort Study. American Journal of Epidemiology. 2015;182(12):1010–22. doi: 10.1093/aje/kwv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamakoshi A, Lin Y, Kawado M, Yagyu K, Kikuchi S, Iso H. Effect of coffee consumption on all-cause and total cancer mortality: findings from the JACC study. Eur J Epidemiol. 2011;26(4):285–93. doi: 10.1007/s10654-011-9548-7. [DOI] [PubMed] [Google Scholar]
- 14.Tverdal A, Stensvold I, Solvoll K, Foss OP, Lund-Larsen P, Bjartveit K. Coffee Consumption And Death From Coronary Heart Disease In Middle Aged Norwegian Men And Women. BMJ: British Medical Journal. 1990;300(6724):566–9. doi: 10.1136/bmj.300.6724.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosengren A, Wilhelmsen L. Coffee, coronary heart disease and mortality in middle-aged Swedish men: findings from the Primary Prevention Study. Journal of internal medicine. 1991;230(1):67–71. doi: 10.1111/j.1365-2796.1991.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The Relationship of Coffee Consumption with Mortality. Annals of Internal Medicine. 2008;148(12):904–14. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama K, Kuriyama S, Akhter M, Kakizaki M, Nakaya N, Ohmori-Matsuda K, et al. Coffee Consumption and Mortality Due to All Causes, Cardiovascular Disease, and Cancer in Japanese Women. The Journal of Nutrition. 2010;140(5):1007–13. doi: 10.3945/jn.109.109314. [DOI] [PubMed] [Google Scholar]
- 18.Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee Consumption and Mortality From All Causes, Cardiovascular Disease, and Cancer: A Dose-Response Meta-Analysis. American Journal of Epidemiology. 2014;180(8):763–75. doi: 10.1093/aje/kwu194. [DOI] [PubMed] [Google Scholar]
- 19.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. International Journal of Epidemiology. 1997;26(suppl 1):S6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 20.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutrition. 2002;5(6b):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S, Longnecker MP. Methods for Trend Estimation from Summarized Dose-Response Data, with Applications to Meta-Analysis. American Journal of Epidemiology. 1992;135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 22.Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Statistics in Medicine. 2002;21(15):2175–97. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 23.ONS. [on 1st April 2017];2017 Pages. Accessed at Office for National Statistics-UK at w http://webarchive.nationalarchives.gov.uk/20160105160709/http:/www.ons.gov.uk/ons/guide-method/user-guidance/health-and-life-events/revised-european-standard-population-2013--2013-esp-/index.html.
- 24.Klatsky ALMC. Coffee, cirrhosis, and transaminase enzymes. Archives of Internal Medicine. 2006;166(11):1190–5. doi: 10.1001/archinte.166.11.1190. [DOI] [PubMed] [Google Scholar]
- 25.Tverdal A, Skurtveit S. Coffee Intake and Mortality from Liver Cirrhosis. Annals of Epidemiology. 2003;13(6):419–23. doi: 10.1016/s1047-2797(02)00462-3. [DOI] [PubMed] [Google Scholar]
- 26.Corrao G, Zambon A, Bagnardi V, D’Amicis A, Klatsky A. Coffee, Caffeine, and the Risk of Liver Cirrhosis. Annals of Epidemiology. 2001;11(7):458–65. doi: 10.1016/s1047-2797(01)00223-x. [DOI] [PubMed] [Google Scholar]
- 27.Casiglia E, Spolaore P, Ginocchio G, Ambrosio GB. Unexpected Effects of Coffee Consumption on Liver Enzymes. European Journal of Epidemiology. 1993;9(3):293–7. doi: 10.1007/BF00146266. [DOI] [PubMed] [Google Scholar]
- 28.Saab S, Mallam D, Cox GA, 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34(4):495–504. doi: 10.1111/liv.12304. [DOI] [PubMed] [Google Scholar]
- 29.Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol. 2008;49(5):758–67. doi: 10.1016/j.jhep.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Shim SG, Jun DW, Kim EK, Saeed WK, Lee KN, Lee HL, et al. Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. Journal of Gastroenterology and Hepatology. 2013;28(12):1877–84. doi: 10.1111/jgh.12317. [DOI] [PubMed] [Google Scholar]
- 31.Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, et al. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52(5):1652–61. doi: 10.1002/hep.23902. [DOI] [PubMed] [Google Scholar]
- 32.Freedman ND, Everhart JE, Lindsay KL, Ghany MG, Curto TM, Shiffman ML, et al. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50(5):1360–9. doi: 10.1002/hep.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke; a journal of cerebral circulation. 2011;42(4):908–12. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, Logroscino G, Hu FB, van Dam RM. Coffee Consumption and Risk of Stroke in Women. Circulation. 2009;119(8):1116–23. doi: 10.1161/CIRCULATIONAHA.108.826164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collaboration* TERF. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and Stroke: A Meta-Analysis of Observational Studies. Stroke. 2007;38(6):1959–66. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 37.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. Plasma Concentration of C-Reactive Protein and Risk of Ischemic Stroke and Transient Ischemic Attack: The Framingham Study. Stroke. 2001;32(11):2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. New England Journal of Medicine. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 39.Khaw KT, Wareham N. Glycated hemoglobin as a marker of cardiovascular risk. Curr Opin Lipidol. 2006;17(6):637–43. doi: 10.1097/MOL.0b013e3280106b95. [DOI] [PubMed] [Google Scholar]
- 40.Lueth NA, Anderson KE, Harnack LJ, Fulkerson JA, Robien K. Coffee and caffeine intake and the risk of ovarian cancer: the Iowa Women’s Health Study. Cancer Causes & Control. 2008;19(10):1365–72. doi: 10.1007/s10552-008-9208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braem MG, Onland-Moret NC, Schouten LJ, Tjønneland A, Hansen L, Dahm CC, et al. Coffee and tea consumption and the risk of ovarian cancer: a prospective cohort study and updated meta-analysis. The American Journal of Clinical Nutrition. 2012;95(5):1172–81. doi: 10.3945/ajcn.111.026393. [DOI] [PubMed] [Google Scholar]
- 42.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008;112(5):1169–77. doi: 10.1002/cncr.23275. [DOI] [PubMed] [Google Scholar]
- 43.Lucas M, O’Reilly EJ, Pan A, Mirzaei F, Willett WC, Okereke OI, et al. Coffee, caffeine, and risk of completed suicide: Results from three prospective cohorts of American adults. The World Journal of Biological Psychiatry. 2014;15(5):377–86. doi: 10.3109/15622975.2013.795243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanskanen A, Tuomilehto J, Viinamaki H, Vartiainen E, Lehtonen J, Puska P. Heavy coffee drinking and the risk of suicide. Eur J Epidemiol. 2000;16(9):789–91. doi: 10.1023/a:1007614714579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Analytical methods used to measure the liver function, circulatory disease and metabolic biomarkers.
Table S2. Descriptive information of the European Prospective Investigation into Cancer and Nutrition study participant countries.
Table S3. Multivariable associations of daily caffeinated coffee consumption and all-cause and cause-specific mortality.
Table S4. Multivariable associations of daily decaffeinated coffee consumption and all-cause and cause-specific mortality.
Table S5. Associations of daily coffee consumption and overall and individual cancer mortality.
Table S6. Associations of daily coffee consumption and cause-specific mortality by smoking status.
Table S7. Associations of daily coffee consumption and all-cause mortality by follow-up time categories.
Table S8. Multivariable associations of daily coffee consumption and all-cause and cause-specific mortality among men and women after deaths which occurred during the first 5 years of follow-up (n=5,247) were excluded.
Table S9. Multivariable associations of daily coffee consumption and all-cause and cause-specific mortality among men and women after deaths which occurred during the first 8 years of follow-up (n=10,790) were excluded.
Table S10. Associations of daily coffee consumption and all-cause and cause-specific mortality among participants who self-reported being in ‘excellent’ or ‘good’ health at baseline (n=119,609).
Figure S1. Adjusted cumulative incidence of all-cause mortality, by coffee consumption categories among men and women.
Figure S2. Multivariable associations of serum liver function, circulatory disease, and metabolic biomarkers and all-cause mortality (n=1,597 deaths) among men and women using sex specific quartiles.