Abstract
Fibroblast-like synoviocytes (FLS) reside in the synovial membrane of diarthrodial joints and are exposed to a dynamic fluid environment that presents both physical and chemical stimuli. The ability of FLS to sense and respond to these stimuli plays a key role in their normal function, and is implicated in the alterations to function that occur in osteoarthritis (OA). The present work characterizes the response of FLS to fluid flow-induced shear stress via real-time calcium imaging, and tests the hypothesis that this response is modulated by interleukin-1α (IL-1α), a cytokine elevated in OA. FLS demonstrated a robust calcium signaling response to fluid shear that was dose dependent upon stress level and required both external and internal calcium sources. Preconditioning with 10 ng/mL IL-1α for 24 hrs heightened this shear stress response by significantly increasing the percent of responding cells and peak magnitude, while significantly decreasing the time for a peak to occur. Intercellular communication via gap junctions was found to account for a portion of the FLS population response in normal conditions, and was significantly increased by IL-1α preconditioning. IL-1α was also found to significantly increase average length and incidence of the primary cilia, an organelle commonly implicated in shear mechanosensing. These findings suggest that the elevated levels of IL-1α found in the OA environment heighten FLS sensitivity to fluid shear by altering both intercellular communication and individual cell sensitivity, which could affect downstream functions and contribute to progression of the disease state.
Keywords: calcium signaling, shear stress, gap junction communication, primary cilia, osteoarthritis
INTRODUCTION
The synovium is a specialized connective tissue that envelops diarthrodial joints and maintains the fluid-filled cavity to provide a lubricating environment for the articulating surfaces within. It is comprised of fibroblast-like synoviocytes (FLS), which produce the lubricating molecules hyaluronan and lubricin, embedded within an extracellular matrix composed of hyaluronan, collagen and proteoglycans that acts as a semi-permeable membrane to mediate solute transport (Blewis, Lao et al. 2010, Hui, McCarty et al. 2012). FLS reside in the intimal layer of this membrane and are thus directly exposed to shear stresses induced in a thin layer of synovial fluid by the relative movement between the synovium and cartilage surfaces during joint articulation (Rattner, Sciore et al. 2010). Changes in normal FLS function have been implicated in osteoarthritis (OA), where pro-inflammatory cytokines such as interleukin-1α (IL-1α) released by the inflamed synovium shift chondrocyte activity to elevate degradative enzymes, breaking down cartilage and inhibiting tissue repair and regeneration (Abramson and Attur 2009, Bartok and Firestein 2010). FLS have been shown to respond to IL-1α themselves with increased production of degradative enzymes, and this response was modulated by gentle oscillatory shear (Sun, Nalim et al. 2009). These results point to a coupling between FLS mechanosensing in the normal joint environment, and enhanced chemical stimuli present in pathological conditions. The specific mechanisms of FLS mechanosensing however, and the influence of the OA environment thereupon, remain to be elucidated. It is anticipated that a more complete understanding of the synovium’s contribution to cartilage degeneration in OA can be obtained through elucidation of the FLS response to physiochemical stimuli associated with the disease environment.
The current study employs an in vitro model system to test the hypothesis that FLS mechanosensitivity is modulated by cytokines. Specifically, we characterize the real-time intracellular calcium ([Ca2+]i) response of a dense FLS monolayer to applied fluid shear and determine how this response is modulated by IL-1α. Calcium is an ubiquitous second messenger that is one of the earliest cell signaling events triggered by applied physical and chemical stimuli (Yellowley, Jacobs et al. 1999). Intracellular calcium transients in response to fluid-induced shear have been observed for cells residing in a variety of musculoskeletal tissues, including bone (Hung, Pollack et al. 1995, Jacobs, Yellowley et al. 1998), ligament (Hung, Allen et al. 1997), tendon (Wall and Banes 2005), meniscus (Eifler, Blough et al. 2006) and cartilage (Edlich, Yellowley et al. 2001). Toward efforts to better understand the underlying mechanisms that mediate the shear-induced calcium response, a subset of studies was undertaken to determine the role of extracellular and intracellular calcium sources.
Intracellular calcium has been implicated in synoviocyte mechanosignaling, with calcium wave propagation mediated in part by intercellular communication via gap junctions (D’Andrea, Calabrese et al. 1998). The role of cell-to-cell communication via gap junctions was thus investigated as a mechanism of both normal shear sensing and IL-1α modulation thereof. To further explore potential mechanisms of shear sensing modulation by this cytokine, the effect of IL-1α on the incidence and length of primary cilia was studied. Primary cilia are non-motile microtubule-based structures that emanate from the cell surface of many mammalian cells (Hoey, Downs et al. 2012) including synoviocytes (Ou, Ruan et al. 2009, Rattner, Sciore et al. 2010), and are considered critical effectors of cell mechanosensation (Lu, Du et al. 2008, Malone, Anderson et al. 2008, Besschetnova, Kolpakova-Hart et al. 2010). We hypothesize that the OA environment influences both intercellular communication and individual cell mechanosensing mechanisms, heightening sensitivity to shear stress and pointing to further investigation of gap junctions and primary cilia as potential therapeutic targets to modulate FLS within the OA disease state.
METHODS
Cell Isolation and Culture
Fibroblast-like synoviocytes were isolated from the synovium of 3–6 juvenile bovine knee joints (2–4 weeks old) via type II collagenase (Worthington) digestion. Cells were cultured in αMEM containing 10% fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 5 ng/mL FGF (Life Technologies) for two passages to obtain a pure population of FLS (Sampat, O’Connell et al. 2011, Silverstein, Stefani et al. 2017).
Fluid Shear Setup
FLS were plated in silicone wells (Grace Bio-Labs) on glass slides coated with 5 μg/cm2 collagen type 1 at 30×103 cells/well and allowed to attach overnight, then preconditioned with 10 ng/mL IL-1α for 24 hrs prior to imaging, with parallel untreated controls. Changes in intracellular calcium ([Ca2+]i) were tracked with Fura Red-AM (Life Technologies) at 5 μM incubated for 40 min at 37 °C. Fluid flow-induced shear stress was applied in a parallel plate flow chamber at 0.5 dyne/cm2 (or otherwise indicated), calculated at the wall of the chamber (Hung, Allen et al. 1997). Chambers were allowed to rest on the microscope stage for 10 min, followed by a time lapse consisting of a 2 min pre-flow baseline, 2 min flow, and 2 min post-flow observation. Experiments were performed at room temperature in Hank’s Buffered Salt Solution (HBSS) supplemented with 0.5% fetal bovine serum (or otherwise indicated). 100 μM ATP was added as a positive chemical control in conditions where no response to shear was observed. Calcium Source Experiments: To isolate external and internal calcium sources respectively, 10 mM EGTA in calcium-free HBSS and 1 μM thapsigargin in normal HBSS were used for chamber setup and flow. To allow for complete emptying of internal stores by thapsigargin, rest time prior to imaging was increased to 20 minutes for all groups. Vehicle controls were examined for each treatment: Ca2+-free HBSS alone for EGTA, and 0.1% v/v ethanol in normal HBSS for thapsigargin. Gap Junction Experiments: To isolate contribution of intercellular communication to shear response, the gap junction blocker octanol was included at 1mM for chamber setup, 20 min equilibration, and flow. All fluid shear data were collected for 100 cells per slide, pooled across 3 slides per group (or otherwise indicated).
Calcium Imaging
Fura Red fluorescence intensity was tracked for individual cells, where increasing [Ca2+]i resulted in decreased fluorescence, and was normalized to the average intensity prior to flow and inverted to represent relative intracellular calcium concentration. A custom Matlab (Mathworks) code calculated percent responding cells, peak magnitude, and peak latency (time to max value after flow onset). Area under curve (AUC), a measure of the total intracellular calcium flux, was calculated to compare not only peak magnitude but overall size of transient. A responding cell was defined as having a peak magnitude greater than 20% above the baseline average to exclude any false responses due to natural variation in baseline levels of calcium.
Quantification of Gap Junction Communication
A dye transfer assay was employed to confirm blocking of gap junctions by octanol and determine the influence of IL-1α on intercellular communication. FLS monolayers were cultured for 24 hrs prior to low-density plating of separate ‘parachute’ FLS loaded with 2 μM calcein AM and 4 μM DiI (Invitrogen). Neighboring cells exhibiting transferred calcein were counted after 4 hrs for 30 parachute cells (identified by non-transferred DiI), pooled across triplicate slides per group. IL-1α-preconditioned and control FLS were treated with 1mM octanol for 20 min prior to addition of parachute cells and during imaging, with non-octanol-treated controls imaged for each group.
Primary Cilia Length Measurements
FLS were preconditioned with 10 ng/mL IL-1α for 24 hrs prior to fixation in 4% paraformaldehyde and immunohistochemical staining with 2 μg/mL Alexa-488-tagged alpha-acetylated tubulin (Santa Cruz Biotechnology) for visualization of primary cilia, and counterstained with TRITC-conjugated phalloidin (Millipore) and DAPI (Life Technologies) for cytoskeleton and nuclear visualization respectively. Primary cilia counting and length measurement was performed with Zen Blue (Zeiss).
Statistical Analysis
Peak magnitude, AUC, peak latency, calcein transfer quantification, and cilia length were evaluated via ANOVA with Tukey HSD post-hoc testing (p < 0.05 or lower), presented as mean ± standard deviation with outliers removed under the criteria of ± 2 standard deviations. Categorical data (percent responding cells, primary cilia incidence) were analyzed via Fisher’s Exact Test with Holms-Sidak correction for multiple comparisons (p < 0.05 or lower).
RESULTS
FLS demonstrated a robust calcium signaling response to shear stress, characterized by a rapid increase in intracellular calcium and return to equilibrium levels (Fig. 1). This signaling occurred in a coordinated fashion among a subset of FLS with a slight delay after flow onset and was dependent upon a minimal level of serum in the flow media, which increased percentile response in a dose dependent manner. In the absence of serum, <5% of cells responded to 0.5 dyne/cm2 shear stress (Fig. 2), similar to the low-level activity observed in no-flow controls (not shown). Inclusion of FBS significantly increased percentile response to 16% with 0.1% FBS (Fig. 2, p < 0.001) and 46% with 0.5% FBS (Fig. 2, p < 0.001). The ability of FLS to mount a calcium signal in the absence of serum was confirmed with exposure to 100 μM ATP, which resulted in a 96% response (not shown).
Figure 1.

Representative calcium transient of FLS in response to fluid-induced shear stress. [Ca2+]i is steady at equilibrium, rises sharply with flow onset to maximum value (peak magnitude) after slight delay (peak latency), then returns to equilibrium levels. Area under curve (AUC, dashed region) indicates total calcium flux. Fluorescence intensity of calcium indicator dye is normalized to average value prior to flow and set at 1 to represent relative changes in [Ca2+]i.
Figure 2.
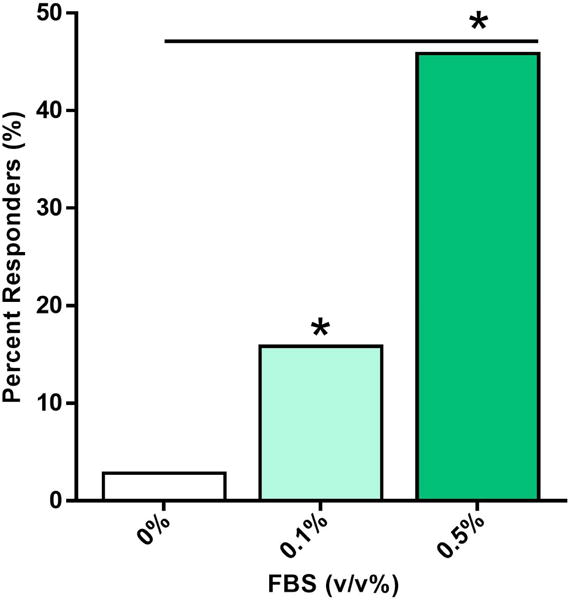
Dose dependence of percent responding FLS to shear stress on minimal concentration of fetal bovine serum (FBS) in flow solution. Significant increases in responding cells observed for 0.1% and 0.5% FBS over low response without FBS. *p<0.001 vs. 0% FBS or indicated groups. N=200 cells per group, pooled across duplicate slides.
FLS response to shear stress was dose dependent on magnitude with respect to percentile response, peak magnitude, AUC, and peak latency. FLS were subjected to 0.05, 0.5, and 5 dyne/cm2 shear stress. Percentile response increased significantly from 23% to 57% and 89% respectively (Fig. 3a, p < 0.001). Mean peak magnitude increased significantly for 5 dyne/cm2 at 1.53 ± 0.09 normalized [Ca2+]i compared to 1.41 ± 0.12 normalized [Ca2+]i and 1.43 ± 0.10 normalized [Ca2+]i for 0.05 and 0.5 dyne/cm2 respectively (Fig. 3b, p < 0.001). Mean AUC increased significantly with each stress level from 22.3 ± 6.3, to 31.2 ± 11.2, and 37.2 ± 10.2 normalized [Ca2+]i*s respectively (Fig. 3c, p<0.001). Mean peak latency significantly decreased from 112 ± 45 s with 0.05 dyne/cm2 to 76 ± 24.6 s with 0.5 dyne/cm2, and 51.2 ± 19 s with 5 dyne/cm2 (Fig. 3d, p < 0.001).
Figure 3.
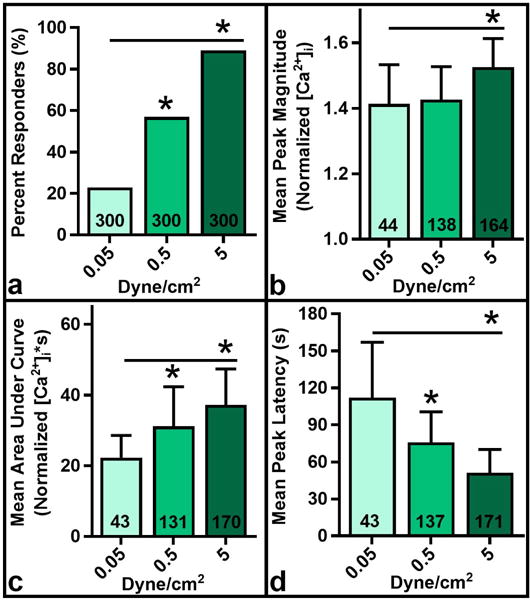
Dose dependence of response metrics on shear stress level. Percent responders (a), mean peak magnitude (b), and mean AUC (c) increased significantly with order of magnitude increases in shear stress, while mean peak latency significantly decreased (d). *p<0.001 vs. 0.05 dyne/cm2 or indicated groups. N reported on each bar, pooled across triplicate slides per group.
Modulation of FLS shear response by IL-1α was confirmed, with preconditioning resulting in a significantly higher percentile response to 0.5 dyne/cm2 shear stress of 97% compared to controls at 42% (Fig. 4a, p < 0.001). IL-1α preconditioning also significantly increased mean peak magnitude at 1.55 ± 0.05 normalized [Ca2+]i compared to 1.39 ± 0.14 normalized [Ca2+]i in controls (Fig. 4b, p < 0.001), and mean AUC at 43.3 ± 18 normalized [Ca2+]i*s versus 28.8 ± 9.4 normalized [Ca2+]i*s in controls (Fig. 4c, p < 0.001). Mean peak latency was significantly decreased from 73.2 ± 51.5 s in controls to 35 ± 10.7 s with IL-1α (Fig. 4d, p < 0.05).
Figure 4.

Effect of 24 hr preconditioning with 10 ng/mL IL-1α (IL1) on FLS response to shear stress. IL1 significantly increased percent responders (a), mean peak magnitude (b), and AUC (c), while significantly decreasing mean peak latency (d). *p<0.001 vs. CTL. N reported on each bar, pooled across triplicate slides per group.
To elucidate the dynamics of intracellular calcium contributing to the observed response in normal FLS and the effect of IL-1α on these dynamics, experiments were performed in the absence of external calcium sources (calcium-free HBSS with 10 mM EGTA) and with internal calcium stores depleted (1 μM thapsigargin). Removal of external calcium resulted in a complete abolishment of response to 0.5 dyne/cm2 shear stress in both IL-1α-preconditioned and control FLS (Table 1). Stimulation with 100μM ATP resulted in characteristic calcium peaks in 9% of FLS compared to 96% with ATP in control conditions (not shown). A vehicle control of calcium-free HBSS only resulted in 14% response to fluid shear, demonstrating a minimal level of response occurs with residual calcium in the system (not shown). Treatment with thapsigargin significantly reduced percentile response to 13% and 3% in control and preconditioned FLS respectively (Table 1, p<0.001). The thapsigargin vehicle had no significant effect on percentile response to fluid shear compared to controls (not shown). Calcium transients of control FLS in the presence of thapsigargin were characterized by a gradual rise to lower peak magnitudes in comparison to the sharp peaks observed in normal conditions (Fig. 5). Though fewer IL-1α-preconditioned FLS responded in the presence of thapsigargin and achieved lower maximum values, the shape of calcium transients retained a characteristic sharp peak (Fig. 5).
Table 1.
Effect of removing external and internal calcium sources on FLS response to shear, for control and IL1-preconditioned cells. No response observed with external calcium removed (EGTA). Low percentile response observed with internal calcium depleted (thapsigargin).
| Flow Condition | Percent Responders (%) | |
|---|---|---|
| CTL | IL1 | |
| Control (HBSS) | 43% | 96%# |
| Ca2+-free HBSS + 10mM EGTA | 0%* | 0%* |
| 1 μM thapsigargin | 13%*ˆ | 3%*# |
p<0.001 vs. control within column.
p<0.001 vs. control within row.
Response characterized by slow rise, not characteristic peak. N = 300 cells per group across triplicate slides.
Figure 5.
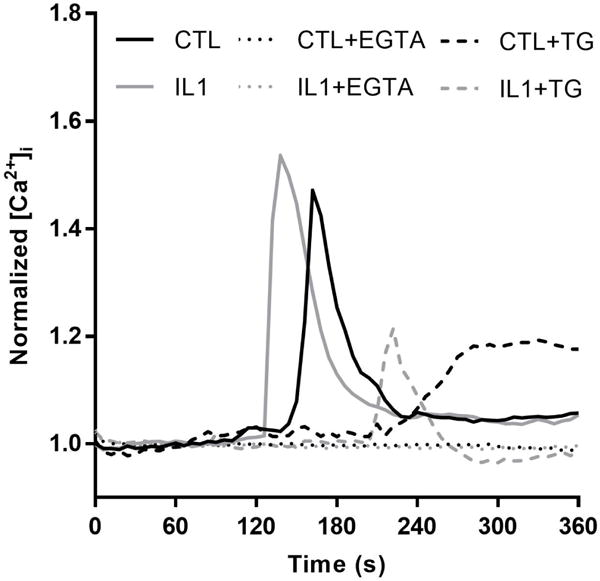
Representative transients for control (black) and IL1-preconditioned (gray) FLS to shear stress in control (solid), EGTA (dotted), and thapsigargin (dashed) conditions. Removal of external calcium (EGTA) resulted in no response to fluid shear in both groups, while removal of internal calcium stores (thapsigargin) resulted in low level responses that differed in shape between control and IL1-preconditioned FLS.
Preconditioning with IL-1α led to a significant increase in gap junction communication compared to control FLS, as indicated by calcein transfer between cells (Fig. 6a,c), with an average of 81 ± 26 cells per parachute compared to 40 ± 12 cells in control populations (Fig. 7, p < 0.001). Blocking of gap junctions by 1mM octanol was confirmed in both groups (Fig. 6b,d), with a significant reduction in dye transfer to 10 ± 3 cells and 13 ± 6 for control and preconditioned cells respectively (Fig. 7, p<0.001).
Figure 6.

Representative images showing calcein dye transfer, an indicator of gap junction communication, after 4 hr to surrounding cells from ‘parachute’ cells (arrow). IL1-preconditioning (c) significantly increased calcein transfer compared to control FLS (a). Blocking of dye transfer by 1mM octanol (OCT) was confirmed for both groups (b, d).
Figure 7.
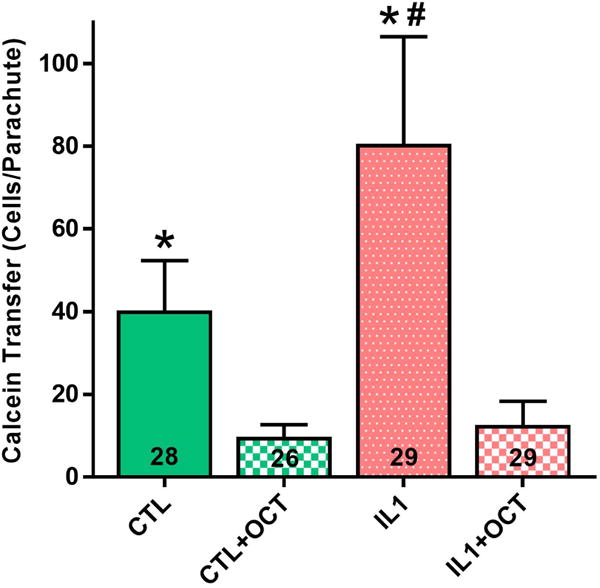
Quantification of dye transfer to surrounding cells in parachute assay. IL1 preconditioning significantly increased transfer rate compared to control FLS. Blocking gap junctions with octanol (OCT) significantly decreased transfer for both control and preconditioned groups. *p<0.001 vs. octanol treatment within preconditioning group. #p<0.001 vs. CTL. N reported on each bar, pooled across triplicate slides per group.
Treatment of control FLS populations with octanol to block gap junction communication significantly reduced percentile response to 0.5 dyne/cm2 shear stress from 42% in controls to 21% (Fig. 8a, p < 0.001), with no significant effect on mean peak magnitude, AUC, or latency. For IL-1α-preconditioned FLS, octanol significantly decreased percentile response to 76%, mean peak magnitude to 1.48 ± 0.06 normalized [Ca2+]i and AUC to 30.1 ± 8.5 normalized [Ca2+]i*s, while increasing mean peak latency to 52.7 ± 15.7 s (Fig. 8a–d). For octanol-treated groups, IL-1α preconditioning still significantly increased percentile response, mean peak magnitude and AUC while decreasing latency versus control FLS (Fig. 8a–d).
Figure 8.
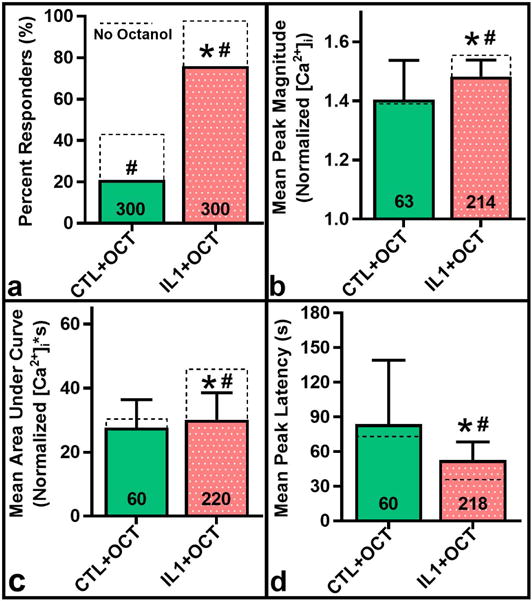
Effect of gap junction blocking by octanol (OCT) on shear response for control and IL1-preconditioned FLS. Percent responders decreased significantly for both control and IL1-preconditioned groups with octanol (a), compared to without octanol (dotted boxes). Mean peak magnitude (b) and AUC (c) significantly decreased, while mean peak latency (d) significantly increased with octanol treatment for IL1-preconditioned FLS. Octanol-treated IL1 group also exhibited significance versus octanol-treated control FLS for all metrics. *p<0.001 vs. CTL+OCT. #p<0.001 vs. respective non-octanol group. N reported on each bar, pooled across triplicate slides per group.
IL-1α preconditioning significantly increased primary cilia length in FLS, with an average 4.22 ± 1.37 μm compared to 3.17 ± 1.04 μm in controls (Fig. 9a, p < 0.001). Primary cilia incidence was also significantly increased, with 27% of preconditioned FLS exhibiting cilia compared to 19% in controls (Fig. 9b, p<0.001). Representative images show primary cilia in IL-1α-preconditioned and control FLS with characteristic cilia lengths for each group (Fig. 9c,d).
Figure 9.

Effect of IL1 preconditioning on FLS primary cilia. Preconditioning significantly increased mean cilia length (a) and percent of cells expressing cilia (b) compared to control FLS. *p<0.001 vs. control. N reported on each bar, pooled across triplicate slides per group. Representative images of FLS primary cilia (arrows), with actin and nuclear counterstaining for visualization (c, d).
DISCUSSION
Synovial fibroblasts are key contributors to the articular cartilage environment, producing molecules important for lubrication and extracellular matrix turnover (Hung, Pollack et al. 1995, Denko, Boja et al. 1996, Harsha and Joyce 2011, Hoey, Downs et al. 2012). FLS both respond and contribute to the secretion of inflammatory cytokines into the synovial fluid (Denko, Boja et al. 1996) and their response to the pro-inflammatory cytokine IL-1α has been shown to be modulated by fluid shear (Sun, Nalim et al. 2009). In the current study, we sought to further the understanding of this interplay between chemical and mechanical stimuli in the OA joint environment by characterizing the calcium signaling response of FLS to fluid shear, testing the hypothesis that IL-1α modulates key aspects of this response, and exploring mechanisms for both normal shear sensing and its modulation by IL-1α.
The robust, dose dependent response to fluid shear stress exhibited by FLS (Fig. 1,3) was reminiscent of other cell types in the musculoskeletal system (Hung, Pollack et al. 1995, Hung, Allen et al. 1997). Under the conditions of the current study, serum in the flow media was required to elicit a calcium transient response to applied shear stress, while calcium transients in response to ATP were observed in media with or without serum (Fig. 2). These findings are reminiscent of studies on endothelial mechanobiology that have indicated a requirement for agonists such as adenosine triphosphate in the flow media to elicit fluid shear-induced calcium transients (Mo, Eskin et al. 1991, Nollert and McIntire 1992). The dose dependence of percentile response on serum concentration observed here compares with our earlier work on bone cells that demonstrated serum can modulate the calcium response, increasing mechanosensitivity to applied shear (Allen, Hung et al. 2000). Other work on synovial joint biomechanics such as wear testing protocols specifying bovine serum as the lubricant (Harsha and Joyce 2011) point to the inclusion of serum as a relevant physiological parameter for in vitro model systems, as synovial fluid is an ultrafiltrate of plasma and includes growth factors found in serum (Denko, Boja et al. 1996). Our team has studied the maintenance of native cartilage explants in synovial fluid (Albro, Durney et al. 2014), and shown that shearing of native synovial fluid can activate growth factors such as latent TGF-beta that may modulate biological behavior of the surrounding joint tissues (Albro, Cigan et al. 2012). Future work will include shear studies using native synovial fluid as the perfusion media to investigate FLS mechanosensitivity in a more physiologic model system.
Previous experiments determined that in the current conditions, simple exposure to IL-1α without fluid shear does not elicit a calcium response in FLS. Rather, we observed here that IL-1α preconditioning influenced FLS response to fluid shear, increasing percentile response, peak magnitude, and AUC, while decreasing both the mean and variance of peak latency (Fig. 4). This points to enhanced mechanosensitivity at the population and individual level, with more FLS responding to applied shear with larger calcium transients, in a more immediate and coordinated fashion. Together, these results led us to accept our hypothesis that the FLS response to applied fluid shear is modulated by cytokines.
Drug experiments were performed to determine the key sources of calcium during a fluid shear-induced peak, and the effect of IL-1α on these basic dynamics. For both control and preconditioned FLS, elimination of external calcium with EGTA resulted in a complete abolishment of shear response, while depletion of internal calcium stores with thapsigargin led to a significant decrease in percentile response (Table 1, Fi. 5). In control FLS the response with thapsigargin was characterized by a slow rise in intracellular calcium, while IL-1α-preconditioned FLS still exhibited characteristic transients with sharp peaks. Together these results suggest the calcium signaling event depends on both external and internal stores, and is characterized by an initial intake of external calcium (the slow rise observed when internal stores are depleted), which is required to trigger the release from internal calcium stores necessary to produce the sharp peak observed. This scenario is supported by other examples of calcium-induced calcium release as a signaling mechanism in cells of the musculoskeletal system (Endo 2009). The differences observed in responses with thapsigargin suggest that IL-1α may influence specific pathways that enable FLS to mount a sharper calcium transient in the absence of internal stores, which will be the subject of future work.
The decreased calcium response of FLS to fluid-shear in the presence of octanol (Fig. 8), a chemical inhibitor of gap junctions (Spray, White et al. 1985, Kolomytkin, Marino et al. 2002), suggests that cell-to-cell communication contributes in part to the overall synovial monolayer response to applied shear. Intercellular communication allows the coordination of cell metabolism between tissues as well as sensitivity to extracellular stimuli (Guo, Takai et al. 2006). Cell-to-cell coupling through gap junctions support the formation of complex cellular networks that favor the intercellular exchange of nutrients and second messengers (D’Andrea, Calabrese et al. 1998). Gap junctions are present in the synovium (Kolomytkin, Marino et al. 2000), and have been shown to affect increased production of matrix metalloproteinases by FLS subjected to pro-inflammatory cytokines (Kolomytkin, Marino et al. 2002, Marino, Waddell et al. 2004) as well as constitutively in clinical OA samples (Marino, Waddell et al. 2004).
Previous work has shown increases in gap junction communication with IL-1β in lapine synovial fibroblasts (Niger, Howell et al. 2009). The current work confirmed this result with IL-1α for bovine FLS (Fig. 6,7), as this isoform of interleukin has been shown to be more effective in the juvenile bovine model (Lima, Tan et al. 2008). This increase in cell-cell communication may contribute to the enhanced shear response with IL-1α preconditioning, as this phenomenon was shown to contribute to the overall FLS population response. However, the persistence of a significant effect of IL-1α on all response metrics when gap junctions were blocked (Fig. 8) indicates that modulation of individual cell mechanosensing may play a dominant role. To that end the effect of IL-1α preconditioning on the primary cilia was investigated, as this organelle is considered an effector of individual cell mechanosensing, and alterations to its length have been implicated in fluid shear sensing (Lu, Du et al. 2008, Malone, Anderson et al. 2008, Besschetnova, Kolpakova-Hart et al. 2010). A significant increase in both cilia length and incidence with IL-1α preconditioning were confirmed here for FLS (Fig. 9), as previously seen in chondrocytes and 3T3 fibroblasts (Wann and Knight 2012).
In addition to further elucidating the respective roles of gap junction communication and primary cilia, future work will explore other putative mechanisms of mechanosensing that may apply to FLS. Specifically, the glycocalyx has been shown to play a role in mechanosensing of smooth muscle cells and fibroblasts of the vascular system (Ainslie, Garanich et al. 2005, Tarbell and Pahakis 2006, Shi and Tarbell 2011), and may be a relevant mechanism in the synovium during OA as changes occur to the interstitial matrix. Other work investigating the mechanisms addressed in this study already provide examples of potential strategies for controlling mechanosensitivity, such as manipulation of primary cilia length (Ou, Ruan et al. 2009, Besschetnova, Kolpakova-Hart et al. 2010) and gap junctional communication (Alves, Nihei et al. 2000, Kurtenbach and Zoidl 2014). As such, we anticipate that a better understanding of these mechanisms will not only shed light on how the pathological sensitization of FLS to shear stress observed here contributes to the pathophysiology of OA, but point to therapeutic interventions that restore normal mechanosensitivity and hinder progression of the disease.
Acknowledgments
This work was funded in part by the Orthopedic Scientific Research Foundation (02-2015), NIH 1R01AR068133, and T32 AR059038. Special thanks to Adam B. Nover, Ph.D. for development of the custom Matlab code for peak analytics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
References
- Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie KM, Garanich JS, Dull RO, Tarbell JM. Vascular smooth muscle cell glycocalyx influences shear stress-mediated contractile response. J Appl Physiol (1985) 2005;98(1):242–249. doi: 10.1152/japplphysiol.01006.2003. [DOI] [PubMed] [Google Scholar]
- Albro MB, Cigan AD, Nims RJ, Yeroushalmi KJ, Oungoulian SR, Hung CT, Ateshian GA. Shearing of synovial fluid activates latent TGF-beta. Osteoarthritis Cartilage. 2012;20(11):1374–1382. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Durney KM, Shim JJ, Singh A, Cigan AD, Nims RJ, Jones BK, Hung CT, Ateshian GA. Synovial Fluid and Physiologic Levels of Cortisol, Insulin, and Glucose in Media Maintain the Homeostasis of Immature Bovine Cartilage Explants over Long Term Culture. Orthopedic Research Society Annual Meeting 2014 [Google Scholar]
- Allen FD, Hung CT, Pollack SR, Brighton CT. Serum modulates the intracellular calcium response of primary cultured. J Biomech. 2000;33(1–2):1585–1591. doi: 10.1016/s0021-9290(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Alves LA, Nihei OK, Fonseca PC, Campos-de-Carvalho AC, Savino W. Gap junction modulation by extracellular signaling molecules-the thymus model. Brazilian Journal of Medical and Biological Research. 2000;33:457–465. doi: 10.1590/s0100-879x2000000400012. [DOI] [PubMed] [Google Scholar]
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20(2):182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewis ME, Lao BJ, Jadin KD, McCarty WJ, Bugbee WD, Firestein GS, Sah RL. Semi-permeable membrane retention of synovial fluid lubricants hyaluronan and proteoglycan 4 for a biomimetic bioreactor. Biotechnol Bioeng. 2010;106(1):149–160. doi: 10.1002/bit.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea P, Calabrese A, Grandolfo M. Intercellular calcium signalling between chondrocytes and synovial cells in co-culture. Biochem J. 1998;329:681–687. doi: 10.1042/bj3290681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko CW, Boja B, Moskowitz RW. Growth factors, insulin-like growth factor-1 and growth hormone, in synovial fluid and serum of patients with rheumatic disorders. Osteoarthritis Cartilage. 1996;4(4):245–249. doi: 10.1016/s1063-4584(05)80102-5. [DOI] [PubMed] [Google Scholar]
- Edlich M, Yellowley CE, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates cytosolic calcium concentration in bovine articular chondrocytes. J Biomechanics. 2001;34:59–65. doi: 10.1016/s0021-9290(00)00158-5. [DOI] [PubMed] [Google Scholar]
- Eifler RL, Blough ER, Dehlin JM, Haut Donahue TL. Oscillatory fluid flow regulates glycosaminoglycan production via an intracellular calcium pathway in meniscal cells. J Orthop Res. 2006;24(3):375–384. doi: 10.1002/jor.20028. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89(4):1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- Guo XE, Takai E, Jiang X, Xu Q, Whitesides GM, Yardley JT, Hung CT, Chow EM, Hantschel T, Costa KD. Intracellular calcium waves in bone cell networks under single cell nanoindentation. Mol Cell Biomech. 2006;3(3):95–107. [PubMed] [Google Scholar]
- Harsha AP, Joyce TJ. Challenges associated with using bovine serum in wear testing orthopaedic biopolymers. Proc Inst Mech Eng H. 2011;225(10):948–958. doi: 10.1177/0954411911416047. [DOI] [PubMed] [Google Scholar]
- Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012;45(1):17–26. doi: 10.1016/j.jbiomech.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;4(1):15–37. doi: 10.1002/wsbm.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CT, Allen FD, Pollack SR, Attia ET, Hannafin JA, Torzilli PA. Intracellular Calcium Response of ACL and MCL Ligament Fibroblasts to Fluid-Induced Shear Stress. Cell Signal. 1997;9(8):587–594. doi: 10.1016/s0898-6568(97)00050-8. [DOI] [PubMed] [Google Scholar]
- Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop. 1995;313:256–269. [PubMed] [Google Scholar]
- Jacobs C, Yellowley C, Davis B, Zhou Z, Donahue H. Differential effect of steady versus oscillating flow on bone cells. J Orthop Res. 1998;23(2):543. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomytkin OV, Marino AA, Sadasivan KK, Meek WD, Wolf RE, Hall V, McCarthy KJ, Albright JA. Gap junctions in human synovial cells and tissue. J Cell Physiol. 2000;184(1):110–117. doi: 10.1002/(SICI)1097-4652(200007)184:1<110::AID-JCP12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kolomytkin OV, Marino AA, Waddell DD, Mathis JM, Wolf RE, Sadasivan KK, Albright JA. IL-1beta-induced production of metalloproteinases by synovial cells depends on gap junction conductance. Am J Physiol Cell Physiol. 2002;282(6):C1254–1260. doi: 10.1152/ajpcell.01166.2000. [DOI] [PubMed] [Google Scholar]
- Kurtenbach S, Zoidl G. Gap junction modulation and its implications for heart function. Front Physiol. 2014;5:82. doi: 10.3389/fphys.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EG, Tan AR, Tai T, Bian L, Ateshian GA, Cook JL, Hung CT. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41(15):3253–3259. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31(3):171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. PNAS. 2008;15(2):13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino AA, Waddell DD, Kolomytkin OV, Meek WD, Wolf R, Sadasivan KK, Albright JA. Increased intercellular communication through gap junctions may contribute to progression of osteoarthritis. Clin Orthop Relat Res. 2004;(422):224–232. doi: 10.1097/01.blo.0000129346.29945.3b. [DOI] [PubMed] [Google Scholar]
- Mo M, Eskin SG, Schilling WP. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: effect of shear stress and ATP. Am J Physiol. 1991;260:H1698–H1707. doi: 10.1152/ajpheart.1991.260.5.H1698. [DOI] [PubMed] [Google Scholar]
- Niger C, Howell FD, Stains JP. Interleukin-1beta increases gap junctional communication among synovial fibroblasts via the extracellular-signal-regulated kinase pathway. Biol Cell. 2009;102(1):37–49. doi: 10.1042/BC20090056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollert MU, McIntire LV. Convective mass transfer effects on the intracellular calcium response of endothelial cells. J Biomech Eng. 1992;114:321–326. doi: 10.1115/1.2891390. [DOI] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315(16):2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner JB, Sciore P, Ou Y, van der Hoorn FA, Lo IK. Primary cilia in fibroblast-like type B synoviocytes lie within a cilium pit: a site of endocytosis. Histol Histopathol. 2010;25(7):865–875. doi: 10.14670/HH-25.865. [DOI] [PubMed] [Google Scholar]
- Sampat SR, O’Connell GD, Fong JV, Alegre-Aguaron E, Ateshian GA, Hung CT. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17(17–18):2259–2265. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng. 2011;39(6):1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AM, Stefani RM, Sobczak E, Tong EL, Attur MG, Shah RP, Bulinski JC, Ateshian GA, Hung CT. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthritis Cartilage. 2017 doi: 10.1016/j.joca.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, White RL, Mazet F, Bennett MV. Regulation of gap junctional conductance. Am J Physiol. 1985;248(6 Pt 2):H753–764. doi: 10.1152/ajpheart.1985.248.6.H753. [DOI] [PubMed] [Google Scholar]
- Sun HB, Nalim R, Yokota H. Expression and Activities of Matrix Metalloproteinases under Oscillatory Shear in IL-1-Stimulated Synovial Cells. Connective Tissue Research. 2009;44(1):42–49. [PubMed] [Google Scholar]
- Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259(4):339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact. 2005;5(1):70–84. [PubMed] [Google Scholar]
- Wann AK, Knight MM. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci. 2012;69(17):2967–2977. doi: 10.1007/s00018-012-0980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellowley CE, Jacobs CR, Donahue HJ. Mechanisms Contributing to Fluid-Flow-Induced Ca2+ Mobilization in Articular Chondrocytes. Journal of Cellular Physiology. 1999;180:402–408. doi: 10.1002/(SICI)1097-4652(199909)180:3<402::AID-JCP11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]


