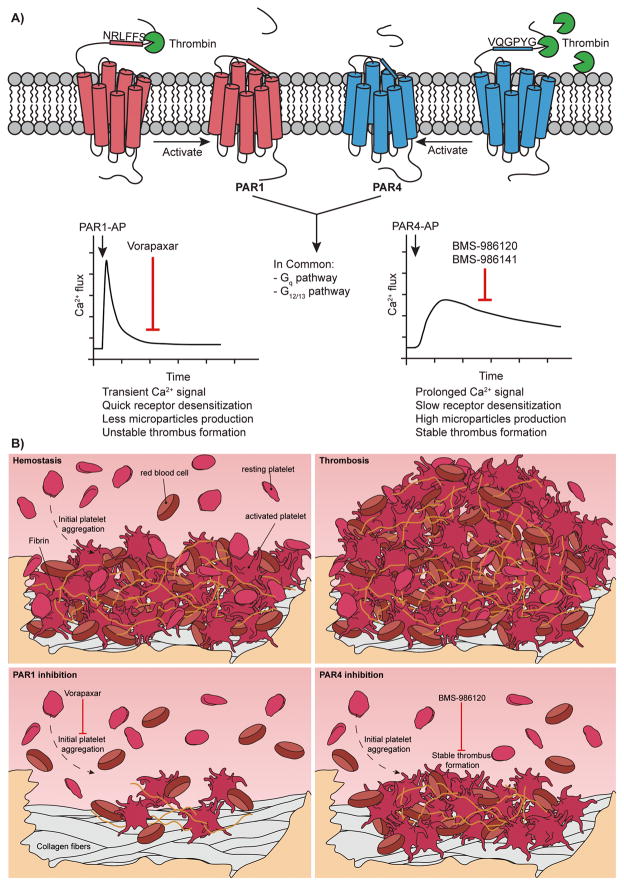
Thrombin, the most potent platelet agonist in vivo, activates human platelets through protease activated receptors 1 and 4 (PAR1 and PAR4) by proteolysis of the N-terminus, which exposes the tethered ligands.1 Although these receptors are activated by the same mechanism, the overall sequence identity is only ~33% and each has a distinct mode of interaction with thrombin, which impacts the subsequent rates of proteolysis.2–4 PAR1 is an efficient substrate and responds to sub-nanomolar thrombin. In contrast, PAR4 requires ~10-fold more thrombin for proteolysis. This difference led to the hypothesis that PAR4 was a redundant backup thrombin receptor, which limited the interest in developing PAR4 antagonists.
Upon further examination, the dual receptor system offers the intriguing possibility of pharmacologically fine tuning thrombin signaling in platelets by taking advantage of the individual contributions of PAR1 and PAR4.5 Both receptors initiate signaling through Gq and G12/13 pathways, but with distinct kinetics. PAR1 activation results in a rapid transient signal. In contrast, PAR4 mediates prolonged signaling that is required for stable thrombus formation (Figure A). Blocking the sustained signaling from PAR4 may limit thrombosis, while leaving the transient PAR1 signaling mechanism available to initiate hemostasis and limit bleeding (Figure B). With this goal in mind, PAR4 has been targeted with inhibitory antibodies, intracellular peptides, and small molecules.6–8 Several compounds that achieve PAR4 antagonism, such as YD-3 and ML354, are currently used as tools to study PAR4 in vitro.9–11 The major reasons these compounds have not reached beyond pre-clinical studies are due to their pharmacological properties, low selectivity between PAR1 and PAR4, or both.
Figure.
The unique characteristics of PAR1 and PAR4 may provide an opportunity to fine tune thrombin signaling to optimize platelet response. A) PAR1 and PAR4 are distinct thrombin receptors that have different rates of activation and signaling kinetics. These receptors have complementary roles in hemostasis and cooperate to mediate thrombin signaling in platelets. B) Hemostasis (top left) starts from initial platelet adhesion and activation to form an unstable thrombus. Following additional thrombin generation and fibrin deposition, a stable thrombus forms to stop the bleeding. Thrombosis (top right) is the uncontrolled growth of the thrombus that obstructs blood flow. Inhibition of PAR1 (bottom left) with vorapaxar targets the initial stages of platelet activation at low thrombin concentrations. Inhibition of PAR4 (bottom right) with BMS-986120 targets the later stages of thrombus growth, which preserves the initial platelet aggregation mediated by PAR1.
Early this year, Bristol-Myers-Squibb reported a potent and reversible PAR4 specific antagonist, BMS-986120, in Science Translational Medicine.12 This compound was identified in a high-throughput screen of 1.1 million compounds from the Bristol-Myers-Squibb (BMS) library. Compounds that blocked calcium signaling induced by an optimized PAR4 activation peptide were further characterized for their ability to inhibit γ-thrombin induced platelet aggregation. This screen resulted in the discovery of an imidazothiadiazole compound that was further optimized for potency, specificity, and oral bioavailability to yield BMS-986120. This specific PAR4 antagonist demonstrated saturable and reversible binding to human PAR4. BMS-986120 blocked human platelet activation in platelet rich plasma stimulated by γ-thrombin or a PAR4 activation peptide with an IC50 <10 nM.12 The most compelling findings in this study resulted from a direct comparison of BMS-986120 to clopidogrel in a nonhuman primate thrombosis model. BMS-986120 administered orally at 1 mg/kg decreased thrombus weight by 80% with limited bleeding risk. In contrast, the dose of clopidogrel that achieved >80% reduction in thrombus weight (1 mg/kg) led to >8-fold increase in bleeding.12 This comprehensive study showed BMS-986120 to be an effective PAR4 antagonist with a lower bleeding risk and a wider therapeutic window compared to clopidogrel.
In this issue of ATVB, Wilson et al. performed a phase 1 trial (NCT02439190) to determine the effect of BMS-986120 on ex vivo human thrombus formation following oral administration to healthy volunteers. BMS-986120 showed no serious adverse side effects. The pharmacokinetic studies demonstrated that plasma concentration of BMS-986120 peaked at 2 h, with a half-life of 4 h, and elimination of this compound (<10% of peak concentration) took 24 h. The ex vivo platelet function studies confirmed that BMS-986120 is a potent reversible inhibitor of PAR4-induced platelet activation and aggregation at 2 and 24 h after oral administration. BMS-986120 did not inhibit platelet response to other agonists (SFLLRN, ADP, or arachidonic acid), which is consistent with the previous report that demonstrated BMS-986120 specifically blocks PAR4 signaling when added to platelets ex vivo.12 The authors demonstrated that BMS-986120 reduces thrombus growth to a similar degree as the combined treatment of aspirin and clopidogrel, signifying a potential major therapeutic advance. However, these treatments were not directly compared with statistical analysis (see Figure 3, panels B and C). Nonetheless, these data offer the exciting possibility that a single agent can be as effective as the current dual antiplatelet therapy. This hypothesis is strengthened by the report BMS-986120 reduced thrombus growth with less bleeding than clopidogrel, demonstrated by the nonhuman primate thrombosis data.12 A closer look at the individual components of the thrombus may shed light on the mechanism of BMS-986120. Interestingly, the platelet component of the thrombus appeared to be reduced to a lesser extent in the BMS-986120 treated individuals compared to those treated with aspirin and clopidogrel. However, BMS-986120 also led to a slight reduction in the fibrin component. This may be due to reduced phosphatidylserine (PS) exposure down stream of PAR4 inhibition that decreases the local thrombin generation and subsequent fibrin formation.8
In summary, this phase 1 PROBE designed clinical trial is the first to administer a PAR4 antagonist to human subjects. The study demonstrated the PAR4 selective potent antagonist, BMS-986120, can reduce ex vivo human thrombus formation in a model designed to represent deep arterial injury in a stenosed coronary artery. This study also supports the hypothesis that blocking PAR4-mediated sustained signaling for stable thrombus formation, while preserving PAR1 signaling for initial thrombus formation, may be a safe and effective antithrombotic strategy (Figure B). The first in-class antiplatelet therapy targeting PAR1, vorapaxar, was approved by the FDA in 2014 for secondary prevention of thrombotic events in stable patients.13–16 Importantly, vorapaxar was approved for use in addition to the standard antiplatelet therapy, but not as a stand-alone therapy or as a substitute for aspirin or clopidogrel. Vorapaxar has not gained widespread use clinically. One issue is difficult clinical management due to its pharmacokinetics and pharmacodynamics. The reversibility and elimination characteristics of BMS-986120 may prove to be advantageous in this regard. At the American Heart Association Scientific Sessions in November, the second PAR4 antagonist, BMS-986141, was revealed.17 This compound also showed great promise in nonhuman primate models of thrombosis. The true promise of these PAR4 antagonists will be revealed in further clinical trials. Moving in this direction, a Phase 2 trial with BMS-986141 was completed in March of 2017 (NCT02671461). In recent years, we have seen a dramatic shift in clinical management due to the introduction of direct oral anticoagulants.18 Now, there is also the potential for a new wave of antiplatelet agents targeting PAR4. Currently, the major hurdle is the large clinical trials necessary to move these forward into clinical practice.
Acknowledgments
We thank Emma G. Bouck and Maria de la Fuente for helpful comments and suggestions.
Sources of funding: M.T. Nieman and X. Han are supported by the National Institutes of Health Grant R01HL098217.
References
- 1.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–87. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacques SL, LeMasurier M, Sheridan PJ, Seeley SK, Kuliopulos A. Substrate-assisted catalysis of the PAR1 thrombin receptor. Enhancement of macromolecular association and cleavage. J Biol Chem. 2000;275:40671–8. doi: 10.1074/jbc.M004544200. [DOI] [PubMed] [Google Scholar]
- 3.Jacques SL, Kuliopulos A. Protease-activated receptor-4 uses dual prolines and an anionic retention motif for thrombin recognition and cleavage. Biochem J. 2003;376:733–40. doi: 10.1042/BJ20030954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieman MT, Schmaier AH. Interaction of thrombin with PAR1 and PAR4 at the thrombin cleavage site. Biochemistry. 2007;46:8603–10. doi: 10.1021/bi700597p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidhu TS, French SL, Hamilton JR. Differential signaling by protease-activated receptors: implications for therapeutic targeting. Int J Mol Sci. 2014;15:6169–83. doi: 10.3390/ijms15046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8:1161–5. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 7.Mumaw MM, de la Fuente M, Noble DN, Nieman MT. Targeting the anionic region of human protease-activated receptor 4 inhibits platelet aggregation and thrombosis without interfering with hemostasis. J Thromb Haemost. 2014;12:1331–41. doi: 10.1111/jth.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French SL, Arthur JF, Lee H, Nesbitt WS, Andrews RK, Gardiner EE, Hamilton JR. Inhibition of protease-activated receptor 4 impairs platelet procoagulant activity during thrombus formation in human blood. J Thromb Haemost. 2016;14:1642–54. doi: 10.1111/jth.13293. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Huang SW, Hwang TL, Kuo SC, Lee FY, Teng CM. YD-3, a novel inhibitor of protease-induced platelet activation. Br J Pharmacol. 2000;130:1289–96. doi: 10.1038/sj.bjp.0703437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CC, Hwang TL, Liao CH, Kuo SC, Lee FY, Lee CY, Teng CM. Selective inhibition of protease-activated receptor 4-dependent platelet activation by YD-3. Thromb Haemost. 2002;87:1026–33. [PubMed] [Google Scholar]
- 11.Young SE, Duvernay MT, Schulte ML, Nance KD, Melancon BJ, Engers J, Wood MR, Hamm HE, Lindsley CW. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. A Novel and Selective PAR4 Antagonist: ML354. [PubMed] [Google Scholar]
- 12.Wong PC, Seiffert D, Bird JE, et al. Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Science translational medicine. 2017:9. doi: 10.1126/scitranslmed.aaf5294. [DOI] [PubMed] [Google Scholar]
- 13.Tricoci P, Huang Z, Held C, et al. Thrombin-Receptor Antagonist Vorapaxar in Acute Coronary Syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–13. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 15.Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, Tendera M, Van de Werf F, Wilcox R, Morrow DA Investigators TRAdP-TSC. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet. 2012;380:1317–24. doi: 10.1016/S0140-6736(12)61269-0. [DOI] [PubMed] [Google Scholar]
- 16.Baker NC, Lipinski MJ, Lhermusier T, Waksman R. Overview of the 2014 food and drug administration cardiovascular and renal drugs advisory committee meeting about vorapaxar. Circulation. 2014;130:1287–94. doi: 10.1161/CIRCULATIONAHA.114.011471. [DOI] [PubMed] [Google Scholar]
- 17.Wong PC, Watson C, Bostwick JS, Banville J, Wexler RR, Priestley ES, Marinier A, Bouvier M, Gordon D, Schumacher WA, Yang J. Abstract 13794: An Orally-Active Small-Molecule Antagonist of the Platelet Protease-Activated Receptor-4, BMS-986141, Prevents Aeterial Thrombosis With Low Bleeding Liability in Cynomolgus Monkeys. Circulation. 2017;136:A13794. [Google Scholar]
- 18.Weitz JI, Harenberg J. New developments in anticoagulants: Past, present and future. Thromb Haemost. 2017;117:1283–1288. doi: 10.1160/TH16-10-0807. [DOI] [PubMed] [Google Scholar]