Originally identified as coagulation factor IV, calcium (Ca2+) is now well established as a key cofactor in the formation of the tenase and prothrombinase complexes on the extracellular surfaces of activated platelets to ultimately mediate fibrin generation and hemostasis.1 Similarly, Ca2+ has long been known to serve an important intracellular role in orchestrating the cell biological responses of platelets in hemostatic plug formation.2,3,4,5 Over the past several decades, as biochemical efforts have identified and refined roles for Ca2+ as an essential second messenger in virtually all cells,3 complementary studies of platelets have similarly detailed how spatiotemporal changes in intracellular Ca2+ levels regulate platelet granule secretion, cytoskeletal dynamics, aggregation and other cell biological outputs underying platelet physiology. On a general mechanistic level, changes in intracellular Ca2+ concentrations that trigger the cellular responses driving platelet function are solicited downstream of a variety of receptors that differentially activate phospholipase C (PLC) family members, resulting in inositol-1,4,5-triphosphate (IP3) production and IP3 receptor (IP3R)-mediated release of Ca2+ from intracellular stores (Figure 1).4,6,7,8,9
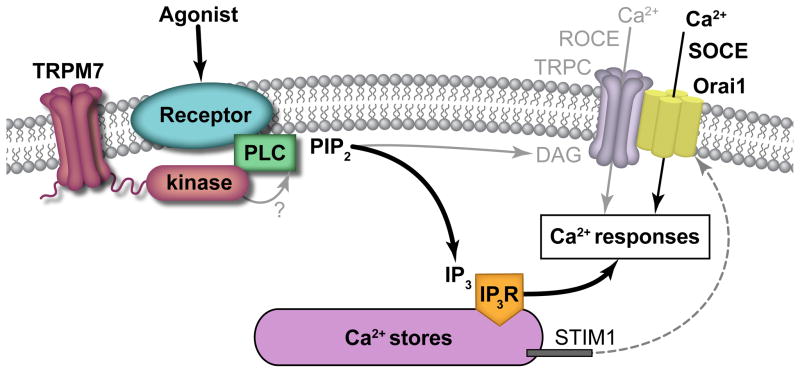
Figure 1. TRPM7 kinase domain interacts with PLC family members to regulate intracellular Ca2+ responses and platelet function (model).
Platelet activation downstream of GPVI, CLEC-2, PARs and other receptors results in intracellular signaling events that upregulate phospholipase C (PLCγ2, PLCβ3) phosphorylation and activity to drive the metabolism of phosphatidylinositol 4,5-bisphosphate (PIP2) on the cytosolic face of the platelet plasma membrane to produce IP3 and diacylglycerol (DAG). Rather than serving as a constitutively active Mg2+/Ca2+ channel or regulating DAG-mediated receptor-operated calcium entry (ROCE) through TRP channels (TRPC) and other associated processes, this study supports a model whereby the TRPM7 kinase domain interacts with PLC family members to modulate PLC phosphorylation and activation, IP3 production, intracellular Ca2+ mobilization and STIM1-Orai1-mediated store operated calcium entry (SOCE), ultimately modulating intracellular Ca2+ concentrations and associated Ca2+ responses underlying platelet function.10
Given the critical importance of intracellular Ca2+ concentration to platelet function, diverse endeavors have aimed to understand and target the myriad of molecular processes regulating and driven by Ca2+ dynamics. In addition to mobilization from internal stores following IP3 generation, extracellular Ca2+ also enters platelets via store- and receptor-operated calcium entry (SOCE and ROCE, respectively) routes involving more recently described players such as Orai1, STIM1 and transient receptor potential (TRP) family channels.3 In this issue of ATVB, Gotru et al. now uncover a novel mechanism by which the TRP subfamily member transient receptor potential melastatin-like 7 (TRPM7) modulates PLC phosphorylation and intracellular calcium mobilization to effect SOCE and platelet function.10 By taking advantage of a transgenic mouse model with a loss-of-function mutation in the cytosolic TRPM7 C-terminal serine/threonine kinase domain (Trpm7R/R),11 this study specifies a role for the TRPM7 kinase domain–rather than its constitutive Mg2+ and Ca2+ channel activity–in modulating the phosphorylation and activation of PLC family members and consequently intracellular calcium responses and platelet functional processes underlying both hemostasis and thrombosis (Figure 1).
Earlier work also from the Braun group suggested a general role for TRPM7 in regulating platelet function through pathways controlling megakaryocyte and platelet cytoskeletal dynamics.12 In the current study, Gotru et al. now more specifically show altered signaling responses in Trpm7R/R platelets, including a delay in phosphorylation of Syk, LAT, PLCγ2 and PKCε in response to the platelet GPVI receptor agonist collagen-related peptide (CRP).10 Similar effects are also found to be associated with rhodocytin→CLEC-2→PLCγ2 as well as thrombin→PAR→PLCβ3 signaling axes. To investigate the physiological consequences associated with these alterations in signaling kinetics in platelets, Gotru et al. also analyze a number of platelet phenotypes and functional responses in Trpm7R/R mice, revealing defects in granule secretion, integrin activation and platelet aggregation in vitro. In association with these altered platelet responses, Trpm7R/R mice display prolonged tail bleeding times, as well as significantly less vessel occlusion in response to arteriole injury as compared to wild-type (WT) counterparts, supporting roles for the TRPM7 kinase domain in modulating platelet function in vivo. Perhaps most remarkably, antithrombotic effects of mutating the TRPM7 kinase domain appear to be mediated through marrow derived cells, especially platelets, as WT chimeric mice transplanted with bone marrow from Trpm7R/R mice, or thrombocytopenic WT mice transfused with platelets from Trpm7R/R animals were similarly protected from infarct progression and showed overall improved neurological and motor function outcomes relative to matched controls following transient middle cerebral artery occlusion (tMCAO) to model ischemic stroke.
While Gotru et al. have astutely elucidated platelet-associated roles for the TRPM7 kinase domain in complete and proper platelet function, a number of intriguing questions remain regarding how TRPM7 impacts platelet physiology in hemostasis and thrombosis. For instance, it is not clear how mutation of this serine/threonine kinase domain translates into a delay in the tyrosine phosphorylation of receptor-proximal components of platelet signaling systems, especially as physiological substrates of TRPM7 are very limited and have not been yet demonstrated in platelets. Furthermore, these findings remain to be placed into the context of the Braun group’s former studies that made use of megakaryocyte/platelet-lineage specific deletions of TRPM7 that resulted in altered cytoskeletal phenotypes.12 Along these lines, as platelets from Trpm7R/R mice exhibit delayed PKC phosphorylation kinetics, a number of other signaling processes around the platelet cytoskeleton may be disrupted, especially given the spatial and temporal link between intracellular calcium mobilization and PKC activation in regulating Rho GTPases and platelet function.13,14 Finally, from a translational perspective, the rather unique TRPM7 kinase domain may represent a specialized therapeutic or biomarker target; however, validation of the relevance of TRPM7 within human platelet biology remains to be shown. Regardless, whether TRPM7 serves as a kinase, a molecular scaffold, an effector, or some combination thereof, Gotru et al. provide a rich contribution to the ever-evolving and increasingly important understanding of mechanisms regulating intracellular Ca2+ dynamics in health and disease in platelet physiology and beyond.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (R01HL101972 and R01GM116184 to O.J.T.M) and the American Heart Association (17SDG33350075 to J.E.A. and 13EIA12630000 to O.J.T.M.). Illustration provided by Inky Mouse Studios ©2018 – all rights reserved.
References
- 1.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131–142. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Wright JH, Minot GR. THE VISCOUS METAMORPHOSIS OF THE BLOOD PLATELETS. J Exp Med. 1917;26:395–409. doi: 10.1084/jem.26.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Delaney MK, O’Brien KA, Du X. Signaling During Platelet Adhesion and Activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslan JE. Platelets in Thrombotic and Non-Thrombotic Disorders. Cham: Springer International Publishing; 2017. Platelet Shape Change; pp. 321–336. [DOI] [Google Scholar]
- 6.Feinstein MB, Fraser C. Human platelet secretion and aggregation induced by calcium ionophores: Inhibition by PGE, and dibutyryl cyclic AMP. JGenPhysiol. 1975;66:561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallach DF, Surgenor DM, Steele BB. Calcium-lipid complexes in human platelets. Blood. 1958;13:589–598. [PubMed] [Google Scholar]
- 8.Hathaway DR, Adelstein RS. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979;76:1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mccarty OJ, Zhao Y, Andrew N, Machesky LM, Staunton D, Frampton J, Watson SP. Evaluation of the role of platelet integrins in fibronectin-dependent spreading and adhesion. J Thromb Haemost. 2004;2:1823–1833. doi: 10.1111/j.1538-7836.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 10.Gotru SK, Chen W, Kraft P, Becker IC, Wolf K, Stritt S, Zierler S, Hermanns HM, Rao D, Perraud A-L, Schmitz C, Zahedi RP, Noy PJ, Tomlinson MG, Dandekar T, Matsushita M, Chubanov V, Gudermann T, Stoll G, Nieswandt B, Braun A. TRPM7 (Transient Receptor Potential Melastatin-Like 7 Channel) Kinase Controls Calcium Responses in Arterial Thrombosis and Stroke in Mice. Arterioscler Thromb Vasc Biol. 2017 doi: 10.1161/ATVBAHA.117.310391. In Press:ATVBAHA.117.310391. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei F-Y, Tomizawa K, Matsui H, Chait BT, Cahalan MD, Nairn AC. Channel Function Is Dissociated from the Intrinsic Kinase Activity and Autophosphorylation of TRPM7/ChaK1. J Biol Chem. 2005;280:20793–20803. doi: 10.1074/jbc.M413671200. [DOI] [PubMed] [Google Scholar]
- 12.Stritt S, Nurden P, Favier R, Favier M, Ferioli S, Gotru SK, van Eeuwijk JMM, Schulze H, Nurden AT, Lambert MP, Turro E, Burger-Stritt S, Matsushita M, Mittermeier L, Ballerini P, Zierler S, Laffan MA, Chubanov V, Gudermann T, Nieswandt B, Braun A. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg(2+) homeostasis and cytoskeletal architecture. Nat Commun. 2016;7:11097. doi: 10.1038/ncomms11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslan JE, McCarty OJ. Rho GTPases in platelet function. J Thromb Haemost. 2013;11:35–46. doi: 10.1111/jth.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngo AT, Thierheimer ML, Babur Ö, Rocheleau AD, Huang T, Pang J, Rigg RA, Mitrugno A, Theodorescu D, Burchard J, Nan X, Demir E, McCarty OJ, Aslan JE. Assessment of roles for the Rho-specific guanine nucleotide dissociation inhibitor (RhoGDI) Ly-GDI in platelet function: a spatial systems approach. Am J Physiol - Cell Physiol. 2017;312:C527–C536. doi: 10.1152/ajpcell.00274.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]