Abstract
Avian influenza A (H5N1) viruses represent a growing threat for an influenza pandemic. The presence of widespread avian influenza virus infections further emphasizes the need for vaccine strategies for control of prepandemic H5N1 and other avian influenza subtypes. Influenza neuraminidase (NA) vaccines represent a potential strategy for improving vaccines against avian influenza H5N1 viruses. To evaluate a strategy for NA vaccination, we generated a recombinant influenza virus-like particle (VLP) vaccine comprised of the NA protein of A/Indonesia/05/2005 (H5N1) virus. Ferrets vaccinated with influenza N1 NA VLPs elicited high-titer serum NA-inhibition (NI) antibody titers and were protected from lethal challenge with A/Indonesia/05/2005 virus. Moreover, N1-immune ferrets shed less infectious virus than similarly challenged control animals. In contrast, ferrets administered control N2 NA VLPs were not protected against H5N1 virus challenge. These results provide support for continued development of NA-based vaccines against influenza H5N1 viruses.
Keywords: VLP vaccine, H5N1 influenza, Ferret, Neuraminidase (NA)
1. Introduction
Hemagglutinin (HA) and neuraminidase (NA) are key surface glycoproteins of influenza A viruses. Following influenza virus infection, antibodies to both glycoproteins are produced, but the HA functions as the major antigen that induces protective immunity against influenza (Wilson and Cox, 1990). HA-specific antibodies efficiently neutralize influenza viruses of the same HA subtype and thus are highly potent at inhibiting viral replication. NA is less abundant on the viral surface, but its enzymatic activity is important for cleaving sialic acid from the viral envelope, facilitating virus release from the surface of the infected cell (Matrosovich et al., 2004; Palese et al., 1974). Although less studied than anti-HA antibody responses, antibody to NA can block release of progeny viruses from the host cell and reduce the severity of influenza infection (Kilbourne et al., 1968; Murphy et al., 1972; Seto and Rott, 1966). NA-specific antibodies have been shown to reduce influenza virus replication and diminish lung pathology (Johansson et al., 1989; Schulman et al., 1968). Epidemiologic evidence in humans suggest that anti-NA antibodies induced by natural infection limit virus spread and reduce the severity of clinical illness (Beutner et al., 1979; Couch et al., 1974; Marcelin et al., 2011; Memoli et al., 2016; Monto and Kendal, 1973; Murphy et al., 1972; Smith and Davies, 1976).
Traditional inactivated influenza vaccines are composed of both HA and NA viral envelope glycoproteins. The amount of NA is not standardized in current influenza vaccines, and antibody responses to NA are generally weaker than those to the HA (Kilbourne et al., 1987; Wohlbold and Krammer, 2014). However, NA-based vaccines are a promising approach to improve influenza vaccines and offer a target of cross-protective immune responses against antigenic variants (Sylte and Suarez, 2009; Wohlbold and Krammer, 2014). NA antibodies are subtype-specific, binding to homologous and heterologous NAs within the same subtype (Lu et al., 2014; Sandbulte et al., 2007). A number of NA vaccines have been developed, including recombinant NA (rNA) proteins generated by expression systems that result in functional NA and protection against viral challenge (Bosch et al., 2010; Johansson et al., 1998; Martinet et al., 1997; Wohlbold et al., 2015). rNA vaccines are capable of inducing NI antibodies that have been reported to suppress viral replication and disease in experimental animals (Deroo et al., 1996; Kilbourne et al., 2004). Lei et al. recently showed that NA presented on the surface of Lactococcus lactis provided protection against highly pathogenic avian influenza (HPAI) H5N1 virus in mice (Lei et al., 2015).
Repeated human exposure to the HPAI H5N1 virus has been well documented since 1997 (http://www.who.int/en). This subtype presents a serious public health concern due to limited immunity in the population along with the ability of H5N1 virus to cause severe respiratory illness (Gambotto et al., 2008; Yu et al., 2008). From 2003 through May 2017, the World Health Organization (WHO) has confirmed 859 human cases of H5N1 infection with 453 deaths (http://www.who.int/en) and if this virus were to gain the capacity to spread efficiently among humans, a major pandemic would occur. Adhering to WHO’s recommendations, inactivated H5N1 virus vaccines for humans have been developed and licensed (http://www.who.int/influenza/en/). Like other inactivated influenza vaccines, H5N1 vaccines are based upon the induction of antibodies to the HA that are neutralizing and are often used to assess vaccine immunogenicity (Subbarao and Luke, 2007). However, less attention has been placed on developing NA vaccines, particularly for H5N1 virus. A NA vaccine may reduce the large disease burden of H5N1 virus infection and its associated high mortality. In this report, we describe the development and preclinical evaluation in a ferret model of an experimental NA virus-like particle (VLP) vaccine. Influenza VLPs are non-infectious, self-assembling protein structures that represent a useful recombinant vaccine approach with advantages in safety and manufacturing (Bright et al., 2008; Galarza et al., 2005; Perrone et al., 2009; Tretyakova et al., 2013). Here, VLP vaccines were constructed from the N1 NA of A/Indonesia/05/2005 (clade 2.1.3.2) H5N1 virus or control N2 NA of A/Brisbane/10/2007 H3N2 virus. The N1 vaccines included two VLP groups (referred to as H3/N1/M1 and N1/M1) were directly compared to control N2 VLPs (H3/N2/M1). N1/M1 VLPs were designed to study the effect of N1 without HA interference, while hybrid H3/N1/M1 VLPs were designed to serve as a control for the effect of heterosubtypic H3 and conserved M1. VLPs expressing homologous H5 HA (H5/N1/M1) representing a monovalent pandemic vaccine were also generated and used as the positive control vaccine for optimal protection. The investigational non-infectious N1 NA VLPs exhibited functional NA properties and induced high titers of NA-inhibiting (NI) antibodies. This work demonstrates the efficacy of a novel recombinant influenza NA-based VLP vaccine in ferrets to protect against HPAI H5N1 viral challenge.
2. Materials and methods
2.1. Viruses and cloning of HA, NA, and M1 genes
Influenza H5 HA, N1 NA and matrix M1 sequences of A/Indonesia/05/2005 (H5N1) virus were obtained from the NCBI Genbank with accession numbers ABP51969, ABW06107 and ABI36004, respectively. Influenza H3 HA and N2 NA sequences of A/Brisbane/10/2007 (H3N2) were obtained from the Genbank with accession numbers ACI26318 and ACI26321. All virus genes were codon-optimized for high-level expression in Spodoptera frugiperda (Sf9) insect cells (ATCC, Manassas, VA) and synthesized biochemically by Geneart (Regensburg, Germany). To generate constructs to express VLPs, full-length HA, NA and M1 genes were cloned into baculovirus (rBV) pFastBac1 transfer vectors between BamHI–HindIII sites downstream from a polyhedrin promoter and subcloned into double/triple tandem vectors including HA/NA/M1 genes as described elsewhere (Smith et al., 2013). rBV expressing H3, H5, N1, N2 and M1 genes were generated by using a Bac-to-Bac baculovirus expression system (Life Technologies, Carlsbad, CA). The titers of rBV stocks were determined using plaque assays on Sf9 cells, expressed as plaque forming units (PFU)/ml. For expression of VLPs, Sf9 cells were maintained as suspension cultures in HyQ-SFX insect serum free medium (HyClone, Logan, UT) at 27 ± 2 °C. Recombinant baculovirus stocks were prepared as described (Liu et al., 2015). Briefly, Sf9 cells were infected at a low multiplicity of infection (MOI) of ≤ 0.01 pfu per cell and harvested at 68–72 h post infection.
2.2. Production and purification of VLPs
The following four VLP vaccines were generated: H5/N1/M1, H3/N1/M1, H3/N2/M1, and N1/M1. Sf9 cells at 2 × 106 cells/ml were co-infected at an MOI of 0.5 pfu per cell with rBVs expressing H3, H5, N1, N2 and M1 genes. For the H5 HA gene, the site encoding RRKK amino acid residues was deleted from the natural polybasic furin cleavage site. After 68–72 h, VLPs were harvested from the medium of baculovirus-infected Sf9 cells, incubated with continuous agitation at 27 ± 2 °C and harvested by centrifugation at 4000 × g for 15 min. Cell culture supernatants containing VLPs were filtered with 0.45 μm membrane and purified with 25% sucrose gradient centrifugation. The purified VLPs were 0.2 μm sterile-filtered, characterized and stored at 4 °C until vaccinations.
2.3. Characterization of VLP vaccine
VLPs were analyzed by SDS-PAGE using 4–12% gradient polyacrylamide gel from Life Technologies (Carlsbad, CA), followed by staining with GelCode Blue commassie reagent (Pierce, Rockford, IL). Total protein concentrations of VLPs were determined by BCA bicinchoninic acid protein assay (Pierce Biochemicals, Rockford, IL). Particle size was determined by dynamic light scattering (DLS) with a Zetasizer Nano ZS (Malvern Instruments, PA). Western blot was performed using the primary antibodies of in house produced sheep anti-H5, rabbit anti-N1 and mouse anti-influenza A M1 (AbD Serotec, Kidlington, UK), and alkaline phosphatase labelled secondary antibodies rabbit anti-sheep, goat anti-rabbit and goat anti-mouse (KPL, Gaithersburg, MD).
The HA content in purified VLPs was measured using the single radial immunodiffusion (SRID) assay based on the protocol and reference standards from US Food and Drug Administration Center for Biologics Evaluation and Research (CBER). The SRID assay was performed as outlined in the original protocol (Schild et al., 1975). Sheep antisera raised in house against HA from A/Indonesia/05/2005 (H5N1) or A/Brisbane/10/07 (H3N2) and standard antigen of purified H5/N1/M1 or H3/N2/M1 VLPs were used in the H5N1 or H3N2 SRID assay.
Stained VLPs were observed using transmission electron microscopy (TEM). Purified VLPs were applied to glow-discharged copper grids (400-mesh) that previously had been coated with carbon parlodion (Poly-Sciences, Warrington, PA). The grids were rinsed with buffer containing 10 mM Tris-HCl buffer (pH 7.4), negatively stained with 1% phosphotungstic acid, and dried by aspiration. VLPs were examined directly by TEM using a Hitachi H-7600 (Hitachi High Technologies America, Schaumburg, IL) operating at 80 kV and incorporating a charge-coupled device (CCD) detector at 1k × 1k resolution (Advanced Microscopy Techniques Corp., Danvers, MA).
The NA-Fluor™ Influenza Neuraminidase Assay Kit (Life Technologies) was used for monitoring NA enzyme activity of VLPs according to manufacturer’s instructions. To generate a standard curve, 4-methylumbelliferone sodium salt (4-MU(SS), Sigma-Aldrich, St. Louis, MO) was used according to the NA-Fluor kit instructions. This kit is based on a 96-well microplate format and includes the fluorescent methyl umbelliferone N-acetyl neuraminic acid (MUNANA) neuraminidase substrate.
2.4. Vaccinations and challenge
All experiments using H5N1 viruses, including work with animals, were performed in biosecurity level-3 enhanced (BSL-3E and ABSL-3E) facilities. Ferret experiments were performed under the guidance of the Centers for Disease Control and Prevention’s Institutional Animal Care and Use Committee and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Adult male Fitch ferrets, 5–6 months of age (Triple F Farms, Sayre, PA), serologically negative by hemagglutination inhibition (HI) assay for currently circulating influenza viruses, were used in this study. Two vaccine experiments were conducted where groups of 4–5 ferrets received two intramuscular (i.m.) inoculations (4–5 weeks apart) of the VLPs in a 0.5 ml volume. Ferrets were vaccinated with identical doses (2300 mU/ml) of VLPs based on NA activity. PBS treated ferrets served as unimmunized controls.
Prior to primary vaccination, vaccine boost, and viral challenge, all ferrets were bled for collection of serum to assess responses to vaccination. Ferrets were challenged intranasally (5 weeks following boost) with 106 PFU of A/Indonesia/05/2005 (H5N1) virus in a total volume of 1 ml (500 μl per nostril). Following challenge, ferrets were monitored daily for changes in body weight, temperature and clinical signs of illness. Any ferret that reached a clinical end point prior to the end of the 14 day experiment was humanely euthanized. Nasal wash samples were collected post-challenge (p.c.) on the days indicated, snap frozen on dry ice, and stored at −80 °C. Nasal wash samples were titrated in eggs to determine viral titers shed from infected ferrets, as previously described (Maines et al., 2005). The limit of virus detection was 101.5 EID50/ml. Weight loss and clinical signs of sneezing and nasal discharge, other respiratory distress, and level of activity were assessed daily. A scoring system was used to assess activity level where 0 = alert and playful; 1 = alert but playful only when stimulated; 2 = alert but not playful when stimulated; 3=neither alert nor playful when stimulated. Based on the daily scores for each animal in a group, a relative inactivity index was calculated (Zitzow et al., 2002). The statistical significance of differences in weight loss, temperature changes, and virus titers between vaccinated and PBS-control animals were determined by ANOVA.
2.5. Serological assays
All sera were initially diluted 1:10 in receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Tokyo, Japan). The HI assay was performed using 0.5% turkey or 1% horse red blood cells (RBCs) with 4 hemagglutination units (HAU) of homologous viruses using standard methods (Rowe et al., 1999). Titers of HI antibody are expressed as the reciprocal of the highest dilution of serum titer in which complete inhibition occurred. The HI titers are presented as the geometric mean titers (GMT) from vaccinated or control ferrets.
Antibodies with neuraminidase inhibition activity were detected using an Enzyme-Linked Lectin assay (ELLA) as previously described (Couzens et al., 2014). Briefly, two-fold serially diluted ferret sera were incubated with live H5N1 virus bearing target NA in 96 well plates coated with fetuin for 16–18 h. Next, horse radish peroxidase-labelled peanut agglutinin (lectin) was added to the reaction followed by TMB substrate to reveal enzymatic cleavage of fetuin by viral NA. The percent inhibition of NA enzymatic activity was calculated by comparison with values from virus control wells (virus but no serum). NA inhibition (NI) titer is expressed as the reciprocal of the highest dilution that exhibited at least 50% inhibition of NA activity.
3. Results
3.1. Expression and characterization of NA VLP Vaccines
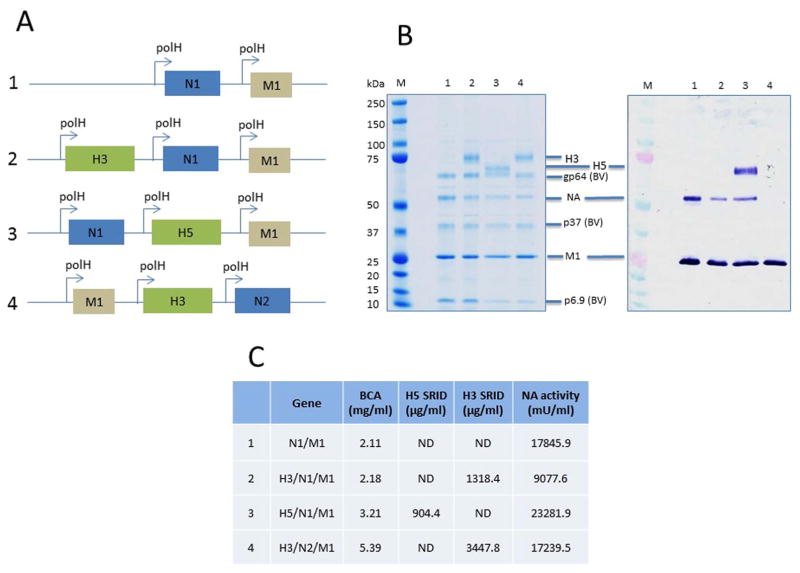
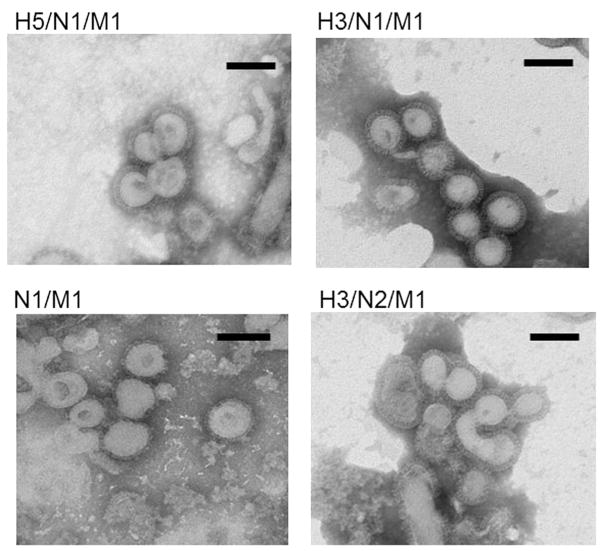
For expression of VLPs, the baculovirus expression system in Sf9 cells was used. NA proteins from A/Indonesia/05/2005 (H5N1) and A/Brisbane/10/2007 (H3N2) viruses were used to generate NA VLP vaccines containing M1, which has been previously shown to be a major structural component of influenza VLP complexes (Pushko et al., 2011, 2005). Experimental NA/M1 VLPs without HA were included along with a homologous H5 HA VLP (H5/N1/M1) that was used as the positive control vaccine for optimal protection (Fig. 1A). The presence of specific H5 or H3 HA, N1 or N2 NA and M1 proteins in VLPs were confirmed by SDS-PAGE and Western blot with antibodies specific for the proteins. The protein composition of VLP preparations were analyzed by using equal protein quantities for Western blot analysis. The expressed H5 and H3 HA of the VLPs represented uncleaved HA0 polypeptides of approximately 65 and 75 kilodaltons (kDa), respectively (Fig. 1B). VLP preparations were prepared by sucrose centrifugation methods and expected to contain the residual infectious baculovirus that potentially can also include HA and NA antigens on the surface of recombinant baculoviruses. Baculovirus proteins were detected in the VLP preparations shown as baculovirus (BV) proteins gp64, p37 and p6.9 (Fig. 1B). Functionally, the NA VLPs displayed NA enzymatic activity after incubation with the MUNANA neuraminidase substrate. Comparable activity levels were observed between N1 NA VLPs and the control N2 NA VLP vaccine (Fig. 1C). Negative-staining transmission electron microscopy (TEM) identified the NA VLPs as largely spherical, typical influenza-like enveloped particles with characteristic spikes of NA protruding from the VLP envelope, which ranged from less than 100 nm to approximately 150 nm in diameter. TEM image of the four vaccines used in this study; H5/N1/M1, N1 VLPs (H3/N1/M1 & N1/M1), and control H3/N2/M1 VLPs are shown in Fig. 2.
Fig. 1. Cloning and characterization of NA recombinant influenza virus-like particle (VLP).
VLPs were constructed in a baculovirus genetic background with the sequences of the genes for HA and NA sequences of A/Indonesia/05/2005 (H5N1) or A/Brisbane/10/2007 (H3N2) virus and produced in recombinant baculovirus infected Spodoptera frugiperda (Sf9) cells. (A) Influenza HA, NA and M1 genes were cloned into pFastBac1 baculovirus transfer vector in a tandem manner with each gene under a polyhedron promoter. The H5, N1, and M1 genes were from A/Indonesia/05/2005 (H5N1); and H3 and N2 genes were from A/Brisbane/10/2007 (H3N2). (B) Purified VLPs were analyzed using SDS-PAGE (left panel) and a Western blot against a mixture of sheep anti-H5, rabbit anti-N1 and mouse anti-influenza A M1 serum (right panel). The identity of protein bands are labelled for influenza protein including H3 and H5 HA, N1 NA and M1, and baculovirus proteins gp64, p37 and p6.9. VLP samples were loaded at the same total protein levels. The sample in lane 1 represents only 2 proteins (N1 and M1) compared to samples in lanes 2, 3, 4, which express 3 proteins (HA, M1, N1). (C) Total protein concentration (BCA), SRID values for H5 and H3 HA; N1 and N2 NA activity by MUNANA assay. ND, not done.
Fig. 2. Selected transmission electron microscope (TEM) micrographs of NA VLPs.
VLPs containing NA were negatively stained with 1% phosphotungstic acid, and examined directly by TEM using a Hitachi H-7600. Bar, 100 nm.
3.2. Protective efficacy of N1 NA VLP vaccine to H5N1 virus challenge
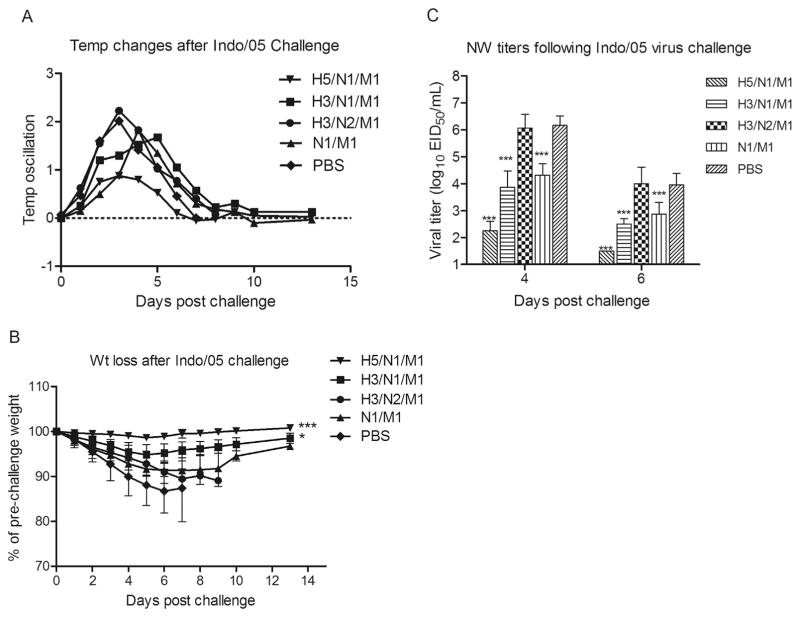
We determined the level of protection in ferrets against H5N1 virus challenge induced by N1 NA VLP vaccines. Vaccine protection was measured by reduction in (i) fever, (ii) weight loss, (iii) viral shedding, and (iv) mortality following intranasal A/Indonesia/05/2005 virus challenge. Overall, ferrets that were vaccinated with N1 VLPs (H3/N1/M1 and N1/M1) exhibited less fever compared to N2 VLP vaccinated (H3/N2/M2) and mock (PBS) vaccinated control animals (Table 1; experiment 1). Control vaccinated ferrets displayed high fever (mean maximum fever of 2.0–2.2 °C over baseline) peaking on day 3 p.c. (Fig. 3A). N1-immune ferrets also experienced high fever, but there was a slight delay (days 4–5 p.c.) in peak temperatures that were less than 1.9 °C over baseline. Vaccination with both N1 VLP vaccine formulations resulted in significantly (p < 0.01) less weight loss compared to H3/N2/M1 vaccinated and mock control groups (Fig. 3B and Table 1). Following H5N1 virus challenge, all six PBS mock-vaccinated ferrets showed a progressive loss of body weight and had to be euthanized by 6–7 days p.c. due to neurologic signs (uncontrolled movement or hind limb paralysis). Control N2 NA VLP-vaccinated ferrets also experienced substantial weight loss ranging from 9.9% to 13.7% with 100% (4 of 4) mortality (Fig. 3B and Table 1). In contrast, N1 VLP vaccines protected against severe H5N1 disease; H3/N1/M1 and N1/M1 vaccinated animals did not exhibit neurological signs and displayed survival rates of 100% and 75%, respectively (Table 1). The surviving ferrets showed moderate morbidity that reached a mean maximum weight loss of 5.2% and 8.6% for H3/N1/M1 and N1/M1 vaccinated animals, respectively (Fig. 3B and Table 1). As anticipated, ferrets that received the VLP vaccine expressing homologous (Indonesia/05) H5 HA (H5/N1/M1 VLP) were well protected against substantial weight loss ( < 1.5%) and high fever (mean maximum fever of < 1.0 °C over baseline).
Table 1.
Clinical signs observed in NA-immune ferrets challenged with H5N1 virus.
| VLP vaccine groupa | Clinical signs of H5N1 challenged ferrets
|
|||
|---|---|---|---|---|
| Max temp (°C)b | % Weight Lossc | Lethargyd | Lethalitye | |
| Experiment 1 | ||||
| H5/N1/M1 | 0.9 | 1.4 | 1.04 | 0/4 |
| H3/N1/M1 | 1.7 | 5.2 | 1.07 | 0/4 |
| H3/N2/M1 | 2.2 | 10.9 | 1.43 | 4/4 |
| N1/M1 | 1.8 | 8.6 | 1.45 | 1/4 |
| PBS | 2.0 | 13.3 | 1.47 | 6/6 |
| Experiment 2 | ||||
| H5/N1/M1 | 1.1 | 2.1 | 1.00 | 0/4 |
| H3/N1/M1 | 1.5 | 11.9 | 1.22 | 1/5 |
| H3/N2/M1 | 1.6 | 19.3 | 1.93 | 5/5 |
| N1/M1 | 1.5 | 20.0 | 1.42 | 2/4 |
Ferrets were intramuscularly vaccinated twice (4–5 weeks apart) with NA VLP vaccines.
Mean maximum temperature increase over baseline.
Percentage mean maximum weight loss after challenge.
Relative inactivity index of ferrets during the first 10 days post-challenge.
Number of animals euthanized before the end of the 14 day experimental period
Fig. 3. Protective efficacy of NA VLP vaccines to A/Indonesia/05/2005 (H5N1) virus challenge in ferrets.
Ferrets (4/vaccine group) received two intramuscular inoculations of VLP vaccines in a 0.5 ml volume. Six ferrets received PBS in place of vaccine. Vaccine groups are as follows: H5/N1/M1 (▼), H3/N1/M1 (■), H3/N2/M1 (●), N1/M1 (▲), and mock (PBS) (◆). Ferrets were challenged intranasally (5 weeks following boost) with 106 PFU of A/Indonesia/05/2005 (H5N1) virus and observed daily for body temperatures (A), and weight loss for 14 days (B). Virus shedding was measured on 4 and 6 days post-challenge and is expressed as the log10 EID50/ml + SD (C). The weight loss among groups was analyzed by one-way ANOVA based on calculated Area Under the Curve (AUC) by day 6 post-challenge. Nasal wash titers among vaccine groups were analyzed by two-way ANOVA with GraphPad Prism software. ***p < 0.001, *p < 0.01, compared to PBS group.
The extent of virus shedding from the respiratory tract was determined by titrating nasal wash samples collected from immune and control ferrets following H5N1 virus challenge. In comparison to unimmunized PBS control animals, N1-immune (H3/N1/M1 and N1/M1) ferrets had a statistically significant reduction in viral shedding on days 4 and 6 p.c. (p < 0.001) (Fig. 3C). Conversely, the control N2 NA VLP-vaccinated ferrets had similar titers to the mock-vaccinated control animals on both days measured. The positive control H5/N1/M1 VLP provided a high degree of protection against virus shedding on day 4 p.c. (p < 0.001) which fell below detectable limits by day 6 p.c. (Fig. 3C). These data indicate that although N1 VLPs do not provide sterilizing immunity against H5N1 virus challenge, these vaccines offered a significant level of protection against viral shedding and severe morbidity.
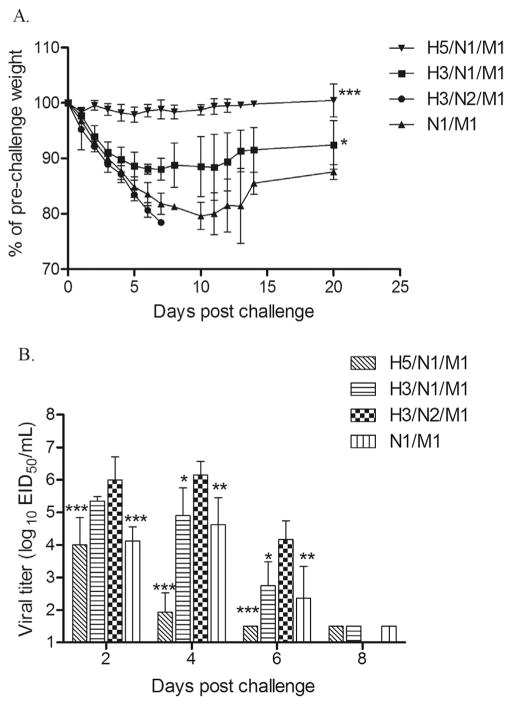
Similar protection results against H5N1 virus were observed in a repeat vaccine experiment. Because the two control groups (mock and N2 NA VLP-vaccinated) yielded similar levels of protection in the first study, only one control group, the N2 VLP vaccine, was included in the second experiment. This allowed for a greater number of animals (4–5) for each experimental group. As expected, the homologous H5/N1/M1 VLP vaccine offered excellent protection against A/Indonesia/05/2005 (H5N1) virus challenge (Fig. 4A). All H5/N1/M1 VLP vaccinated ferrets survived challenge and lost minimal body weight (Table 1; experiment 2). Moreover, H5/N1/M1 vaccinated animals shed less infectious virus as compared to the control animals on days 2, 4 and 6 p.c. (Fig. 4B). In contrast, all ferrets that received the control N2 NA VLP vaccine showed severe weight loss and succumbed to H5N1 virus infection by day 7 p.c. (Table 1). Similar to the first experiment, H3/N1/M1 VLP vaccinated ferrets were protected from severe disease and exhibited significantly less weight loss (p < 0.01) by day 5 post challenge. Although these vaccinated ferrets exhibited mean weight loss up to 11.9% and virus shedding titers up to 5.3 log10 EID50/ml, the H3/N1/M1 VLP vaccine protected 4 of 5 ferrets from neurological signs and death. The N1/M1 vaccinated ferrets were partially protected against H5N1 challenge, as indicated by reduced virus shedding on days 2, 4 and 6 p.c. (Fig. 4B) and 50% survival (Table 1). These results further support the use of recombinant NA VLPs as vaccines against H5N1 viruses.
Fig. 4. Weight loss and viral shedding in NA-immune and control ferrets following H5N1 virus challenge.
Ferrets (4–5/vaccine group) received two intramuscular inoculations of VLP vaccines in a 0.5 ml volume and were challenged with 106 pfu of A/Indonesia/05/2005 (H5N1) virus five weeks after final vaccine boost. (A) Mean weight loss of ferrets following H5N1 virus challenge are shown at various days post challenge (p.c.). (B) Nasal washes were collected on indicated days p.c. and titrated for virus and are expressed as the log10 EID50/ml + SD. Weight loss by day 5 post challenge was analyzed by one-way ANOVA based on calculated AUC. Differences in viral titers of nasal washes between groups were analyzed by two-way ANOVA with GraphPad Prism software. ***p < 0.001, **p < 0.01, *p < 0.05 compared to control group.
3.3. NA VLP vaccines are efficient at inducing NA reactive antibodies
The immunogenicity of the NA VLP influenza vaccines in ferrets was assessed by hemagglutination and neuraminidase inhibition assays. Ferret sera were collected four (experiment 1) or five weeks (experiment 2) after the first immunization and prior to H5N1 virus challenge. As expected, ferrets that received NA VLP influenza vaccines without H5 HA did not exhibit any HI antibodies to the challenge H5N1 virus at any time point. The administration of one dose of H5/N1/M1 VLP vaccine resulted in HI titers that ranged from 80 to 160 for both experiments (Table 2). Following the second immunization of H5/N1/M1 VLP vaccine, the serum antibody response was further boosted and all the ferrets achieved HI titers of ≥160 to the homologous H5N1 virus. H3/N1/M1 and H3/N2/M1 vaccinated ferrets generated prechallenge HI antibody titers to H3N2 (A/Brisbane/10/2007) virus that ranged from 320 to 640 (not shown).
Table 2.
Serum hemagglutination inhibition (HI) and enzyme-linked lectin assay (ELLA) antibody titers following NA VLP vaccination in ferretsa.
| Vaccine group | HI titerb
|
ELLA titerb
|
||
|---|---|---|---|---|
| Pre-boost | Pre-challenge | Pre-vaccine | Pre-challenge | |
| Experiment 1 | ||||
| H5/N1/M1 | 113 (80–160) | 226 (160–320) | 5 (5) | 10240 (10240) |
| H3/N1/M1 | < 10 | < 10 | 5 (5) | 905 (640–1280) |
| H3/N2/M1 | < 10 | < 10 | 5 (5) | 47.6 (20–80) |
| N1/M1 | < 10 | < 10 | 5 (5) | 3620 (2560–10240) |
| PBS | < 10 | < 10 | 5 (5) | 5 (5) |
| Experiment 2 | ||||
| H5/N1/M1 | 135 (80–160) | 320 (320) | 14.1 (5–20) | 4305 (2560–5120) |
| H3/N1/M1 | < 10 | < 10 | 11.9 (5–20) | 403.2 (320–640) |
| H3/N2/M1 | < 10 | < 10 | 17.4 (10–20) | 30.3 (20–40) |
| N1/M1 | < 10 | < 10 | 10 (5–20) | 2153 (1280–2560) |
Ferrets were intramuscularly vaccinated twice (4–5 weeks apart).
Samples were tested for HI and ELLA antibody activity against A/Indonesia/05/2005 virus. Geometric mean titers (range in parentheses) are shown as the dilution of serum that inhibits agglutination of horse or turkey RBC’s or that exhibits ≥ 50% inhibition of NA enzymatic activity.
We next determined if NA-inhibiting (NI) antibody levels correlated with reduced viral shedding and disease severity among H5N1 challenged ferrets. We used the enzyme-linked lectin assay (ELLA) to titer functional NI antibodies in ferrets immunized with VLPs. Prior to viral challenge, ferret sera were collected and examined by ELLA. As shown in Table 2, the ferret pre-vaccine serum did not show any significant NI titers to N1 of H5N1 (A/Indonesia/05/2005) virus. Preimmune sera from experiment 2 showed higher background titers compared to the same sera in experiment 1, but these values were considerably lower than pre-challenge titers. All three N1 VLP immunized groups expressed high NI antibody titers against N1 compared to NI titers elicited by N2 VLP vaccination (Table 2). In both experiments, pre-challenge NI titers were highest in ferrets that had been immunized with a H5/N1/M1 VLP vaccine (GMT titers of 10240 and 4305) followed by the N1/M1 VLP (without HA) vaccinated group that elicited high-titer NI antibodies; GMT titers of 3620 and 2153 were measured for experiments 1 and 2, respectively. NI GMTs following H3/N1/M1 VLP vaccination also inhibited N1 (A/Indonesia/05/2005) NA activity efficiently that ranged from 320 to 1280 for the nine ferrets in both experiments. The ferrets administered control N2 NA VLPs, which were not protected against H5N1 virus challenge, had the lowest NI antibody titers in both experiments. Collectively, our results demonstrate that recombinant VLP vaccines comprised of NA protein of H5N1 virus generated substantially higher N1-inhibiting antibodies among surviving ferrets, compared to NI titers of N2 NA-vaccinated ferrets that needed to be euthanized due to neurologic signs.
4. Discussion
The influenza virus HA is the target of antibodies for neutralization of infectivity. As a consequence, currently licensed inactivated influenza vaccines are standardized only by HA content. Although influenza vaccines predominantly induce protective anti-HA antibody titers, antibody responses to NA protein are formed following immunization or infection and can contribute to protection (Beutner et al., 1979; Couch et al., 1974; Marcelin et al., 2011; Monto and Kendal, 1973; Murphy et al., 1972; Smith and Davies, 1976). However, NA antibodies do not neutralize virus infectivity and anti-NA antibody levels induced by vaccination are variable, owing to the lack of standardization of NA protein content. More work is needed to clearly define the role of NA in protection against influenza and studies evaluating novel NA vaccine candidates will facilitate a greater understanding of the immune correlates of NA vaccine-induced protection. Here, we used the ferret model to demonstrate that N1 NA VLP vaccination could provide effective protection against an H5N1 (A/Indonesia/05/2005) clade 2.1.3.2 virus isolated from a fatal case in 2005. A/Indonesia/05/2005 virus is among a group of highly virulent viruses able to spread to the brain of infected ferrets (Maines et al., 2006, 2005), and therefore provides a stringent challenge model for preclinical evaluation of influenza vaccines. The comparability of ELLA antibody titers for the analysis of NA VLP vaccine immunogenicity and the potential contribution of NI antibodies to protection demonstrated that high NI serum antibodies correlated with protection of ferrets against lethal H5N1 virus challenge.
Influenza vaccination against H5N1 is considered the best approach to prevent disease and its complications, including death (Subbarao and Luke, 2007). VLP technology allows for the safe production of influenza vaccines containing one or more surface glycoproteins stabilized by the matrix (M1) assembly protein (Kang et al., 2009; Pushko et al., 2010; Quan et al., 2016). Our approach in this study was to generate VLP vaccines expressing NA, without homologous HA, and compare the protection efficacy conferred by a conventional HA-based VLP vaccine expressing homologous A/Indonesia/05/2005 HA protein. As demonstrated previously, VLPs containing H5 HA protect ferrets against lethal H5N1 challenge as confirmed by reduced virus replication in the respiratory tract and protection against death (Pushko et al., 2011). In the current study, vaccination with N1 NA VLPs (H3/N1/M1 and N1/M1) did not reduce viral replication to the level observed among H5 HA (H5/N1/M1) vaccinated ferrets; however N1 NA VLP vaccination resulted in a 10- to 195-fold reduction in viral load compared to control ferrets. Importantly, such a reduction was sufficient to protect against neurological signs and death in this model. Compared to the N1/M1 vaccine group, the N1 NA VLPs containing H3 HA (H3/N1/M1) provided slightly better protection following H5N1 virus challenge. The marginal improvement in body weight change and survival of H3/N1/M1 vaccinated ferrets may be due to certain limited pan-influenza A and/or stem based antibody or cellular responses elicited by H3. Conversely, the VLP vaccine containing control N2 NA (from seasonal H3N2 virus), which shares amino acid identity of only 42% to the N1 NA protein of A/Indonesia/05/2005 virus, and also the M1 contained in each VLP vaccine, failed to protect against neurological signs and death. The N2 NA was selected as the control VLP because NA genes exhibiting little sequence homology (40–46%) would not be expected to induce protective immunity (Colman and Ward, 1985; Wohlbold and Krammer, 2014; Wohlbold et al., 2015). The low cross-reactive NI antibody responses among N2 NA VLP vaccinated ferrets correlated with the lack of protection in this control group. Therefore, protection induced by the H3/N1/M1 vaccine but not H3/N2/M1 VLPs makes it unlikely to be due to cross-reactive responses to viral antigens on the H3 HA, as the seasonal influenza A/Brisbane/10/2007 (H3) HA are present in both vaccines. Moreover, the N1/M1 VLP vaccine, without HA also provided protection against virus shedding and death.
NA activity is essential for efficient influenza virus replication and antibodies to neuraminidase inhibit viral spread (Matrosovich et al., 2004). The presence of NI titers in humans may offer partial protection during influenza epidemics and pandemics by reducing the clinical and epidemiological impact of influenza (Beutner et al., 1979; Brett and Johansson, 2005; Johansson, 1999; Kilbourne et al., 2002; Monto and Kendal, 1973; Murphy et al., 1972). In the current study, the presence of NI titers in vaccinated ferrets suggest that antibodies are binding to the globular NA head domains due to their ability to inhibit NA activity. Thus, the protection afforded by the N1 NA VLP vaccines is thought to be due to NI antibodies which likely function by reducing the efficiency of release of progeny virus from the surface of the infected cell (Johansson et al., 1998; Rott et al., 1974; Sylte et al., 2007; Webster et al., 1988). A protective NI titer is not clearly defined in animal vaccine studies and humans, however studies suggest that high levels of NI titers against influenza viruses are needed to provide protection in mice (Chen et al., 2000) and humans (Beutner et al., 1979; Kilbourne et al., 1995). In our hands, high NI titers were measured in protected ferrets against the homologous NA by the ELLA assay.
The relative conservation and slower antigenic evolution of NA, at least compared to HA1, suggest the use of NA as an influenza vaccine antigen (Frobert et al., 2010; Martinez et al., 1983; Sandbulte et al., 2007; Westgeest et al., 2012). Influenza VLPs continue to provide attractive candidates for influenza vaccines (Bright et al., 2008; Galarza et al., 2005; Perrone et al., 2009; Tretyakova et al., 2013). The protection demonstrated by using the stringent challenge model described here highlights the potential benefits of N1 NA candidate vaccines in a VLP platform against emerging avian influenza viruses. Because H5N1, and more recently H7N9 viruses, continue to cause outbreaks in poultry populations while posing a threat to humans, a better understanding of the potential of NA as a protective immunogen against these viruses may be an important step toward improving influenza vaccines.
Acknowledgments
H.M.C was supported by the Oak Ridge Institute for Science and Education. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
References
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. J Infect Dis. 1979;140:844–850. doi: 10.1093/infdis/140.6.844. [DOI] [PubMed] [Google Scholar]
- Bosch BJ, Bodewes R, de Vries RP, Kreijtz JH, Bartelink W, van Amerongen G, Rimmelzwaan GF, de Haan CA, Osterhaus AD, Rottier PJ. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J Virol. 2010;84:10366–10374. doi: 10.1128/JVI.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett IC, Johansson BE. Immunization against influenza A virus: comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology. 2005;339:273–280. doi: 10.1016/j.virol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, Ross TM. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kadowaki S, Hagiwara Y, Yoshikawa T, Matsuo K, Kurata T, Tamura S. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine. 2000;18:3214–3222. doi: 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- Colman PM, Ward CW. Structure and diversity of influenza virus neuraminidase. Curr Top Microbiol Immunol. 1985;114:177–255. doi: 10.1007/978-3-642-70227-3_5. [DOI] [PubMed] [Google Scholar]
- Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. 1974;129:411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- Couzens L, Gao J, Westgeest K, Sandbulte M, Lugovtsev V, Fouchier R, Eichelberger M. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods. 2014;210:7–14. doi: 10.1016/j.jviromet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Deroo T, Jou WM, Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14:561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- Frobert E, Bouscambert-Duchamp M, Escuret V, Mundweiler S, Barthelemy M, Morfin F, Valette M, Gerdil C, Lina B, Ferraris O. Anti N1 crossprotecting antibodies against H5N1 detected in H1N1 infected people. Curr Microbiol. 2010;61:25–28. doi: 10.1007/s00284-009-9571-z. [DOI] [PubMed] [Google Scholar]
- Galarza JM, Latham T, Cupo A. Virus-like particle vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:365–372. doi: 10.1089/vim.2005.18.365. [DOI] [PubMed] [Google Scholar]
- Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- Johansson BE. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine. 1999;17:2073–2080. doi: 10.1016/s0264-410x(98)00413-7. [DOI] [PubMed] [Google Scholar]
- Johansson BE, Bucher DJ, Kilbourne ED. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989;63:1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Matthews JT, Kilbourne ED. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine. 1998;16:1009–1015. doi: 10.1016/s0264-410x(97)00279-x. [DOI] [PubMed] [Google Scholar]
- Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza viruslike particles as pandemic vaccines. Curr Top Microbiol Immunol. 2009;333:269–289. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- Kilbourne ED, Cerini CP, Khan MW, Mitchell JW, Jr, Ogra PL. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I studies in human vaccines. J Immunol. 1987;138:3010–3013. [PubMed] [Google Scholar]
- Kilbourne ED, Couch RB, Kasel JA, Keitel WA, Cate TR, Quarles JH, Grajower B, Pokorny BA, Johansson BE. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine. 1995;13:1799–1803. doi: 10.1016/0264-410x(95)00127-m. [DOI] [PubMed] [Google Scholar]
- Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968;2:281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne ED, Pokorny BA, Johansson B, Brett I, Milev Y, Matthews JT. Protection of mice with recombinant influenza virus neuraminidase. J Infect Dis. 2004;189:459–461. doi: 10.1086/381123. [DOI] [PubMed] [Google Scholar]
- Kilbourne ED, Smith C, Brett I, Pokorny BA, Johansson B, Cox N. The total influenza vaccine failure of 1947 revisited: major intrasubtypic antigenic change can explain failure of vaccine in a post-World War II epidemic. Proc Natl Acad Sci USA. 2002;99:10748–10752. doi: 10.1073/pnas.162366899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Peng X, Zhao D, Ouyang J, Jiao H, Shu H, Ge X. Lactococcus lactis displayed neuraminidase confers cross protective immunity against influenza A viruses in mice. Virology. 2015;476:189–195. doi: 10.1016/j.virol.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Liu YV, Massare MJ, Pearce MB, Sun X, Belser JA, Maines TR, Creager HM, Glenn GM, Pushko P, Smith GE, Tumpey TM. Recombinant virus-like particles elicit protective immunity against avian influenza A(H7N9) virus infection in ferrets. Vaccine. 2015;33:2152–2158. doi: 10.1016/j.vaccine.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Liu F, Zeng H, Sheu T, Achenbach JE, Veguilla V, Gubareva LV, Garten R, Smith C, Yang H, Stevens J, Xu X, Katz JM, Tumpey TM. Evaluation of the antigenic relatedness and cross-protective immunity of the neuraminidase between human influenza A (H1N1) virus and highly pathogenic avian influenza A (H5N1) virus. Virology. 2014;454–455:169–175. doi: 10.1016/j.virol.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai le Q, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci USA. 2006;103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G, DuBois R, Rubrum A, Russell CJ, McElhaney JE, Webby RJ. A contributing role for anti-neuraminidase antibodies on immunity to pandemic H1N1 2009 influenza A virus. PLoS One. 2011;6:e26335. doi: 10.1371/journal.pone.0026335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W, Saelens X, Deroo T, Neirynck S, Contreras R, Min Jou W, Fiers W. Protection of mice against a lethal influenza challenge by immunization with yeast-derived recombinant influenza neuraminidase. Eur J Biochem. 1997;247:332–338. doi: 10.1111/j.1432-1033.1997.00332.x. [DOI] [PubMed] [Google Scholar]
- Martinez C, del Rio L, Portela A, Domingo E, Ortin J. Evolution of the influenza virus neuraminidase gene during drift of the N2 subtype. Virology. 1983;130:539–545. doi: 10.1016/0042-6822(83)90108-3. [DOI] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT, Jr, Taubenberger JK. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio. 2016;7:e00417-00416. doi: 10.1128/mBio.00417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet. 1973;1:623–625. doi: 10.1016/s0140-6736(73)92196-x. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Kasel JA, Chanock RM. Association of serum antineuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- Palese P, Tobita K, Ueda M, Compans RW. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, Pushko P, Tumpey TM. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83:5726–5734. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushko P, Kort T, Nathan M, Pearce MB, Smith G, Tumpey TM. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine. 2010;28:4771–4776. doi: 10.1016/j.vaccine.2010.04.093. [DOI] [PubMed] [Google Scholar]
- Pushko P, Pearce MB, Ahmad A, Tretyakova I, Smith G, Belser JA, Tumpey TM. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011;29:5911–5918. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–5759. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Quan FS, Lee YT, Kim KH, Kim MC, Kang SM. Progress in developing virus-like particle influenza vaccines. Expert Rev Vaccin. 2016;15:1281–1293. doi: 10.1080/14760584.2016.1175942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R, Becht H, Orlich M. The significance of influenza virus neuraminidase in immunity. J Gen Virol. 1974;22:35–41. doi: 10.1099/0022-1317-22-1-35. [DOI] [PubMed] [Google Scholar]
- Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild GC, Wood JM, Newman RW. A single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen. Proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull World Health Organ. 1975;52:223–231. [PMC free article] [PubMed] [Google Scholar]
- Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968;2:778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto JT, Rott R. Functional significance of sialidose during influenza virus multiplication. Virology. 1966;30:731–737. doi: 10.1016/0042-6822(66)90178-4. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Davies JR. Natural infection with influenza A (H3N2). The development, persistance and effect of antibodies to the surface antigens. J Hyg. 1976;77:271–282. doi: 10.1017/s0022172400024712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, Kpamegan E, Courbron D, Fries LF, 3rd, Glenn GM. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013;31:4305–4313. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Luke C. H5N1 viruses and vaccines. PLoS Pathog. 2007;3:e40. doi: 10.1371/journal.ppat.0030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylte MJ, Hubby B, Suarez DL. Influenza neuraminidase antibodies provide partial protection for chickens against high pathogenic avian influenza infection. Vaccine. 2007;25:3763–3772. doi: 10.1016/j.vaccine.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Sylte MJ, Suarez DL. Influenza neuraminidase as a vaccine antigen. Curr Top Microbiol Immunol. 2009;333:227–241. doi: 10.1007/978-3-540-92165-3_12. [DOI] [PubMed] [Google Scholar]
- Tretyakova I, Pearce MB, Florese R, Tumpey TM, Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013;442:67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Webster RG, Reay PA, Laver WG. Protection against lethal influenza with neuraminidase. Virology. 1988;164:230–237. doi: 10.1016/0042-6822(88)90640-x. [DOI] [PubMed] [Google Scholar]
- Westgeest KB, de Graaf M, Fourment M, Bestebroer TM, van Beek R, Spronken MI, de Jong JC, Rimmelzwaan GF, Russell CA, Osterhaus AD, Smith GJ, Smith DJ, Fouchier RA. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J Gen Virol. 2012;93:1996–2007. doi: 10.1099/vir.0.043059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol. 1990;8:737–771. doi: 10.1146/annurev.iy.08.040190.003513. [DOI] [PubMed] [Google Scholar]
- Wohlbold TJ, Krammer F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses. 2014;6:2465–2494. doi: 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio. 2015;6:e02556. doi: 10.1128/mBio.02556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Gao Z, Feng Z, Shu Y, Xiang N, Zhou L, Huai Y, Feng L, Peng Z, Li Z, Xu C, Li J, Hu C, Li Q, Xu X, Liu X, Liu Z, Xu L, Chen Y, Luo H, Wei L, Zhang X, Xin J, Guo J, Wang Q, Yuan Z, Zhou L, Zhang K, Zhang W, Yang J, Zhong X, Xia S, Li L, Cheng J, Ma E, He P, Lee SS, Wang Y, Uyeki TM, Yang W. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One. 2008;3:e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]