Abstract
Avian influenza viruses, notably H5 subtype viruses, pose a continuous threat to public health due to their pandemic potential. In recent years, influenza virus H5 subtype split vaccines with novel oil-in-water emulsion based adjuvants (e.g. AS03, MF59) have been shown to be safe, immunogenic, and able to induce broad immune responses in clinical trials, providing strong scientific support for vaccine stockpiling. However, whether such vaccines can provide protection from infection with emerging, antigenically distinct clades of H5 viruses has not been adequately addressed. Here, we selected two AS03-adjuvanted H5N1 vaccines from the US national prepandemic influenza vaccine stockpile and assessed whether the 2004–05 vaccines could provide protection against a 2014 highly pathogenic avian influenza (HPAI) H5N2 virus (A/northern pintail/Washington/40964/2014), a clade 2.3.4.4 virus responsible for mass culling of poultry in North America. Ferrets received two doses of adjuvanted vaccine containing 7.5 μg of hemagglutinin (HA) from A/Vietnam/1203/2004 (clade 1) or A/Anhui/1/2005 (clade 2.3.4) virus either in a homologous or heterologous prime-boost vaccination regime. We found that both vaccination regimens elicited robust antibody responses against the 2004–05 vaccine viruses and could reduce virus-induced morbidity and viral replication in the lower respiratory tract upon heterologous challenge despite the low level of cross-reactive antibody titers to the challenge H5N2 virus. This study supports the value of existing stockpiled 2004–05 influenza H5N1 vaccines, combined with AS03-adjuvant for early use in the event of an emerging pandemic with H5N2-like clade 2.3.4.4 viruses.
Keywords: H5N2 influenza virus, Ferret, Vaccine
1. Introduction
Human infections with avian influenza A viruses are rare, but can occur in persons who have direct unprotected contact with infected birds or contaminated surfaces (Uyeki, 2009). Highly pathogenic avian influenza (HPAI) H5N1 virus are often associated with severe disease, multi-organ failure, and high mortality rates (Beigel et al., 2005; Tran et al., 2004). As of January 16, 2017, more than 850 cases of human infections with HPAI H5N1 viruses have been identified in several countries in Africa, Asia and Europe since their reemergence in 2003 (WHO, 2016a). Although no sustained human-to-human transmission of avian H5N1 viruses has been documented to date, the lack of population-level immunity in humans and the continuing evolution of H5 viruses provides the opportunity for the virus to adapt to humans and cause a pandemic (Webster and Govorkova, 2006).
Vaccines have proven to be effective measures to mitigate illness from human seasonal influenza infection, and the development of efficacious avian influenza vaccines has become an important component in pandemic preparedness (Wood, 2002). To date, several countries including the US have approved the production of a number of H5N1 influenza vaccines from candidate clade 1 and 2 viruses for human vaccination and stockpiling (SAGE Working Group on Influenza Vaccines and Immunizations, 2013). The available data from preclinical and clinical studies have shown that the influenza virus H5 subtype hemagglutinin (HA) is less immunogenic compared to similarly prepared human seasonal H1 or H3 HA, and that a higher HA dose or the use of adjuvant is necessary to overcome its low immunogenicity (Bresson et al., 2006; Nicholson et al., 2001; Stephenson et al., 2003). The highly diverse genetic nature and the rapid evolution of H5 viruses has resulted in the emergence of viruses with antigenic characteristics that are distinct from stockpiled vaccines. As the rapid generation of a well-matched vaccine would represent a challenging task at the onset of a pandemic, one important parameter in evaluating the efficacy of stockpiled vaccines is their ability to provide cross-clade protection to newly emerged strains of H5 influenza viruses.
In late 2014, HPAI H5N8 Eurasian lineage viruses (clade 2.3.4.4) were introduced into North America for the first time. This event led to the emergence of H5N2 and H5N1 influenza viruses derived from the reassortment between HPAI H5N8 viruses and North American low-pathogenicity avian influenza viruses (Lee et al., 2016). The so-called H5Nx viruses (H5N8, H5N1 and H5N2) subsequently spread along the North American flyways for waterfowl, causing widespread poultry outbreaks and resulting in the culling of more than 40 million birds in the US alone (Krauss et al., 2016). Phylogenetic analysis showed that the H5Nx viruses from clade 2.3.4.4 are genetically distant from candidate H5 vaccine strain including A/Vietnam/1203/2004 (clade 1) and A/Anhui/1/2005 (clade 2.3.4) (Kwon et al., 2011). Although no human infections were reported in the US during H5Nx outbreaks in North America, 16 laboratory-confirmed cases of human infection including 6 deaths due to H5N6 virus from the same subclade have been reported to WHO from China since 2014 (Shen et al., 2016; WHO, 2016b; Yang et al., 2015). The emergence of H5Nx influenza viruses has provided a unique opportunity to evaluate the efficacy of available stockpiled H5N1 vaccines against an antigenically distinct clade of H5 viruses. Here, we selected two stockpiled AS03-adjuvanted H5N1 influenza vaccines and assessed their protective effectiveness against newly emerged H5N2 virus challenge in the ferret model.
2. Materials and methods
2.1. Vaccinations and Challenge
The Biomedical Advanced Research and Development Authority (BARDA)/US Department of Health and Human Services (HHS) provided the vaccines and adjuvant from the National Prepandemic Influenza Vaccine Stockpile. Influenza H5N1 subtype monovalent subvirion vaccines contained the HA and NA from A/Vietnam/1203/2004 (VN/04, HPAI, H5N1, clade 1) (manufactured by Sanof Pasteur Ltd, Toronto, Canada) or A/Anhui/1/2005 (Anhui/05, HPAI, H5N1, clade 2.3.4) (manufactured by Seqirus Vaccines Limited, Speke, Liverpool, UK) virus. The stockpiled pre-pandemic H5N1 influenza virus vaccines were periodically tested under a formal stability program and the HA content of the influenza vaccines was measured by the single-radial-immunodiffusion (SRID) assay. Vaccines were mixed with AS03 adjuvant (GlaxoSmithKline Biologicals S.A., Rixensart, Belgium) in a 1:1 ratio immediately prior to vaccination according to manufacturer’s instructions. Eighteen male Fitch ferrets, 10 months of age (Triple F Farms, Sayre, PA) and serologically negative by hemagglutination-inhibition (HI) assay for currently circulating influenza viruses, were used in these studies. H5N1 vaccinations were conducted under BSL-2 conditions and H5N1 virus challenges were performed in a biosafety level 3 containment laboratory with enhancements (BSL-3E) under the guidance of the Centers for Disease Control and Prevention’s Institutional Animal Care and Use Committee and the Select Agent Program (Chosewood LC, 2009). Ferrets were housed within a Duo-Flo Bioclean unit (Lab Products, Seaford, DE) with a maximum capacity of 18 ferrets. Two groups of 6 ferrets were vaccinated by the intramuscular route (i.m.) into the quadriceps muscle of the hind legs with two doses of AS03-adjuvanted vaccine (spaced 28 days apart) containing 7.5 μg of HA in 0.5 ml of volume in either a homologous or heterologous prime-boost vaccination regime. For the homologous vaccination regime, two doses of Anhui/05 (Anhui/Anhui) vaccines were administered, and for the heterologous vaccination regime, VN/04 and Anhui/05 (VN/Anhui) were administered as prime and boost vaccination, respectively. In ferrets, the AS03-adjuvanted H5N1 split vaccines did not cause any detectable irritation or swelling at the injection site. Because it has been previously shown that animals that receive AS03 adjuvant only (without vaccine) do not elicit HI antibody titers and are not protected from influenza virus challenge (Baras et al., 2008; Leroux-Roels, 2009; Morel et al., 2011), PBS (0.5 ml) was used as the control in place of vaccine and adjuvant.
Baseline temperature and weight measurements were obtained before virus challenge. Temperatures were measured with a subcutaneous (s.c.) implantable temperature transponder (BioMedic Data Systems, Seaford, DE). On day 63 post-prime, all ferrets were challenged intranasally (i.n.) with 1 ml of 106.0 50% egg infectious dose (EID50) of A/Northern pintail/Washington/40964/2014 (Pin/2014) (H5N2, clade 2.3.4.4) virus diluted in PBS as described previously (Pulit-Penaloza et al., 2015). Ferrets were monitored for changes in body temperature, weight loss, and the presence of clinical signs. To evaluate the effect of vaccination on viral replication in both the upper and lower respiratory tract, the presence of infectious virus was determined in nasal washes collected on days 2 and 3 post-challenge (p.c.), and in nasal turbinate, trachea, and lung tissues collected on day 3 p.c. following ferret necropsy. Titers of infectious virus in the abovementioned samples were expressed as EID50/ml or g and calculated by the method of Reed and Muench (Reed LJ, 1938) following serial titration in eggs as described previously (Maines et al., 2005).
2.2. Humoral and cell-mediated immune responses
Antibody titers from sera collected on 28 days post-prime as well as 7 and 28 days post-boost vaccination were determined by microneutralization (MN) assay (WHO, 2011) and a modified HI assay with horse red blood cells, shown to possess higher sensitivity in detecting antibody responses against H5 influenza viruses compared to MN assay (Levine et al., in press). Sera were initially treated with receptor-destroying enzyme (RDE) from Vibrio cholerae (Denka Seiken, Tokyo, Japan) overnight at 37 °C. The enzyme was then inactivated at 56 °C for 30 min, and PBS was added to the sera for a final dilution of 1:10. Ferret sera were tested for MN and HI antibody titers against the following H5 viruses: Pin/2014 (H5N2), Anhui/05, and A/Vietnam/1194/2004 (VN/1194; H5N1), the latter of which is an antigenically related surrogate virus for the detection of anti-VN/04 antibodies.
To measure total HA binding antibodies induced by vaccination, the flu antibody biosensor assay (f-AbBA) was performed with biolayer interferometry (BLI) technology on an Octet Red instrument (Pall ForteBio, CA) as described previously (Carney et al., 2010). In brief, recombinant HA (rHA) of influenza VN/04 (H5N1) and Pin/2014 (H5N2) were generated and purified following procedures as described previously (Stevens et al., 2006; Yang et al., 2016). The rHA proteins were successfully expressed, trimeric proteins were assessed and purified using analytical size exclusion chromatography (SEC). The rHA for Anhui/05 was obtained from International Reagent Resource (IRR, Manassas, VA). rHA was bound to anti-penta-His biosensors by incubating the tip of biosensors in solutions of rHA (25 μg/ml) in ForteBio’s kinetics buffer with the usage of a sidekick biosensor immobilization station (Pall ForteBio, CA). RDE-treated ferret sera collected at 28 days post boost were serially diluted in two-fold increments beginning at 1:80 with kinetics buffer and their association/disassociation to rHA was analyzed by incubating the tip of rHA-loaded biosensors into diluted sera. Data were analyzed using the system software and the result was presented as shifted wavelength (nm) at the end of the association step.
To measure cell-mediated immunity (CMI), ferret peripheral blood leukocytes were stimulated overnight with positive control (PMA/ionomycin), negative control (allantoic fluid, or no stimulation), or with recombinant HA from Anhui/05, VN/04, or Pin/2014 virus, in the presence of Golgi-blocker for the last 6 h. The cells were then permeabilized, stained with anti-ferret CD4 (Sino Biological Inc, Beijing, China), anti-human CD8 (OKT8; eBioscience, Hatfield, UK), and anti-bovine interferon gamma (“IFNγ”) (MorphoSys, AbD Serotec, Oxford, UK), and analyzed by flow cytometry (Reber et al. submitted). A response in which more than 0.1% of cells expressed IFNγ was considered positive.
3. Results
3.1. AS03-adjuvanted H5N1 vaccines are efficient at inducing HI and MN antibody responses
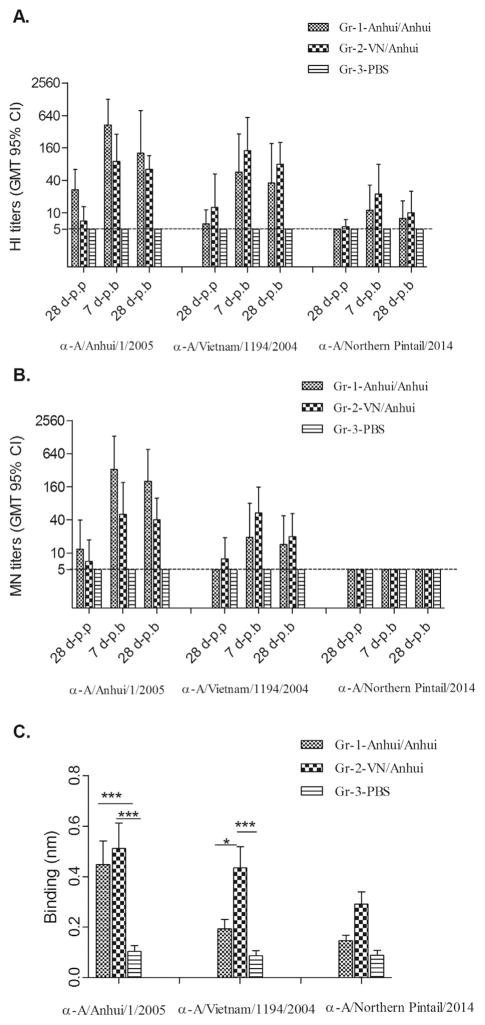
The immunogenicity of the H5N1 influenza vaccines was assessed by HI and MN assays. We found that two doses of AS03-adjuvanted H5N1 vaccine delivered in either a homologous (Anhui/Anhui) or heterologous (VN/Anhui) prime-boost regimen were necessary to induce adequate antibody responses to the vaccine viruses (Fig. 1). A homologous boost with Anhui/05 adjuvanted vaccine (Group 1) substantially enhanced the immune response against the Anhui/05 vaccine virus, as the HI (Fig. 1A) and MN (Fig. 1B) geometric mean titers (GMT) increased from 27 and 12 before boost to 427 (95% confidence interval [CI]=144–1270) and 329 (81–1338), respectively, on day 7 post-boost (7 d-p.b). Antibody titers dropped slightly by 28 days post-boost (28 d-p.b) with HI and MN GMT of 127 (21–985) and 201 (53–766), respectively. Furthermore, two doses of Anhui/05 adjuvanted vaccine also elicited a moderate cross-clade antibody response to the A/VN/1194/2004 (clade 1) virus, which shares high HA sequence similarity with the VN/04 vaccine virus. Here, Anhui/Anhui vaccination resulted in peak HI and MN titers to VN/1194 virus of 57 (11–293) and 19 (5–80), respectively, on day 7 post boost, declining less than two-fold by day 28 post boost.
Fig. 1. Ferret antibody responses following AS03-adjuvanted H5N1 vaccination.
Ferret serum samples collected at 28 days post prime vaccination (28 d-p.p), 7 days post boost (7 d-p.b), and 28 days post boost (28 d-p.b) were pretreated with receptor-destroying enzyme (RDE) and tested by HI and MN assays against A/Anhui/1/2005, A/Vietnam/1194/2004, and A/Northern pintail/2014 viruses for determination of antibody responses. HI antibody titers (A) and MN antibody titers (B) are shown as GMT with 95% CI. The detection limit (5) is shown with a dashed line. Serum samples with antibody titers below the detection limit were assigned values of 5 for purposes of GMT calculations. (C) For the measurement of total binding antibodies to recombinant HA’s, ferret sera collected on day 28 post boost were RDE-treated and serially diluted in two-fold increments before binding to recombinant HA coated biosensor tips. The shifted wavelength (nm) resulted from the binding of 80-fold diluted ferret serum samples to the recombinant HA’s are shown as the mean plus standard deviation. Statistical analysis was performed using two-way ANOVA with GraphPad prism, ***, p < 0.001 and *p < 0.05.
For the heterologous (VN/Anhui) prime-boost group (Group 2), an Anhui/05 vaccine boost following VN/04 prime not only induced potent antibody responses to Anhui/05 virus, but also enhanced antibody responses against the priming vaccine virus, VN/04 (Fig. 1). The ferrets from this group exhibited a peak HI GMT of 143 (35–585) and 90 (28–288) against priming VN/04 and booster Anhui/05, respectively on day 7 post boost (Fig. 1A). HI titers remained at 63 (35–115) and 80 (32–201) against Anhui/05 and VN/04, respectively on day 28 post boost. In general, the MN titers from the heterologous VN/Anhui vaccination group were slightly lower than HI titers with peak GMT of 53 and 50 against the priming and boosting virus strains, which declined to 20 and 40, respectively, on day 28 post boost (Fig. 1B).
In addition to measuring HI and neutralizing specific antibodies induced by vaccination, we also assessed the total HA binding antibodies with the f-AbBA assay, in which antibody binding to HA-coated biosensor tips results in an increase of biosensor tip thickness that can be measured as shifted wavelength (nm). As shown in Fig. 1C, on 28 days post boost both the homologous (Anhui/Anhui) and heterologous (VN/Anhui) vaccination induced a significantly higher (p < 0.01) level of Anhui/05 HA binding antibodies compared to PBS control groups. Interestingly, compared to homologous vaccination, heterologous (VN/Anhui) vaccination induced higher total HA binding antibodies to all three antigens tested, (Anhui/05, VN/04, and Pin/2014), although only the differences in responses to Anhui/05 and VN/04 antigens reached statistical significance (p < 0.05).
3.2. Protective efficacy of stockpiled H5N1 influenza virus vaccines against clade 2.3.4.4 (H5N2) virus challenge
Next, we evaluated the cross-reactivity of both vaccination regimens against the Pin/2014 (H5N2) virus from clade 2.3.4.4. We found that both homologous and heterologous vaccination regimes elicited relatively low levels of antibody responses to Pin/2014 virus; only 2/6 and 3/6 ferrets from homologous and heterologous vaccine groups, respectively, exhibited an HI titer ≥40 at peak response (Fig. 1A). A ≥40 titer in humans has been associated with an approximately 50% reduction in infection with seasonal influenza viruses (Baz et al., 2013; Potter and Oxford, 1979) and is considered to be a seroprotective titer, but it is unknown if this titer cutoff represents a comparable correlate of protection for H5 viruses. Both vaccination approaches also induced low levels of cross-clade binding antibodies to recombinant Pin/2014 HA; heterologous prime-boost induced slightly higher HA-binding antibodies than those from homologous prime-boost animals (Fig. 1C). Although we detected low levels of HI and HA-binding antibodies to Pin/2014 virus, we did not detect cross-reactive MN antibodies against the H5N2 virus (Fig. 1B). Taken together, we conclude that homologous vaccination with two doses of Anhui/05 (clade 2.3.4) vaccine or heterologous vaccination primed with VN/04 (clade 1) followed by boost with Anhui/05 (clade 2.3.4) can elicit sufficient antibody responses (HI titer ≥40) against the H5N1 vaccine strains. However, both vaccination regimens induced only limited cross-reactive antibody responses against the Pin/2014 H5N2 virus with ≤50% seroprotective rates based on HI titers, confirming that the recently emerged H5Nx viruses are antigenically divergent from candidate vaccine strains from clade 1 and clade 2.3.4 (Levine et al., in press).
To evaluate the cross-protective efficacy of H5N1 vaccination, all three groups of ferrets were challenged with the heterologous Pin/2014 (H5N2) virus and subjected to post-mortem necropsy on day 3 p.c. for collection of tissues throughout the respiratory tract. As characterized previously, HPAI Pin/2014 virus was not lethal in ferrets, but the virus exhibited enhanced virulence compared to another 2014 North American clade 2.3.4.4 virus, A/gyrfalcon/Washington/41088/2014 (H5N8) virus (Pulit-Penaloza et al., 2015). Unvaccinated ferrets from the PBS control group (Gr-3) reached a mean maximum weight loss of 10% on day 3 p.i. Conversely, Anhui/Anhui and VN/Anhui vaccinated groups lost only 4.3% and 2.3% of their pre-challenge body weight, respectively, which was significantly lower compared to the control ferrets (p < 0.05, by one-way ANOVA with Dunett post-test). Ferrets from all three groups shed comparable amounts of infectious virus (approximately 104.0 EID50/ml) on days 2 and 3 p.c. in nasal washes (NW) and exhibited similar viral titers in nasal turbinates (NT) on day 3 p.c. (Fig. 2). However, differences in viral titers were observed in trachea and lung tissues. High viral loads (average titer of 104.8 EID50/g) were detected in both the trachea and lungs of unvaccinated control ferrets. In contrast, only 1 of 6 (103.4 EID50/g) and 2 of 6 (103.0, 104.0 EID50/g) ferrets from the Anhui/Anhui and VN/Anhui vaccinated groups, respectively, possessed low viral titers in the trachea, and only 2 of 6 ferrets (102.2, 103.6 EID50/g) from Anhui/Anhui group and 1 of 6 (102.3 EID50/g) from VN/Anhui group possessed detectable virus in the lung (Fig. 2). Thus, compared to the unvaccinated control group, viral titers in the trachea and lungs of both vaccinated groups were reduced by over 100-fold, which achieved significance compared to unvaccinated ferrets (p < 0.001). Collectively, we found that either homologous prime-boost Anhui/05 vaccination or heterologous prime-boost with VN/04 and Anhui/05 vaccination could protect ferrets from H5N2 virus challenge by reducing viral morbidity as measured by weight loss, and viral replication in the lower respiratory tract of ferrets.
Fig. 2. Viral replication in ferret respiratory tract samples following heterologous virus challenge.
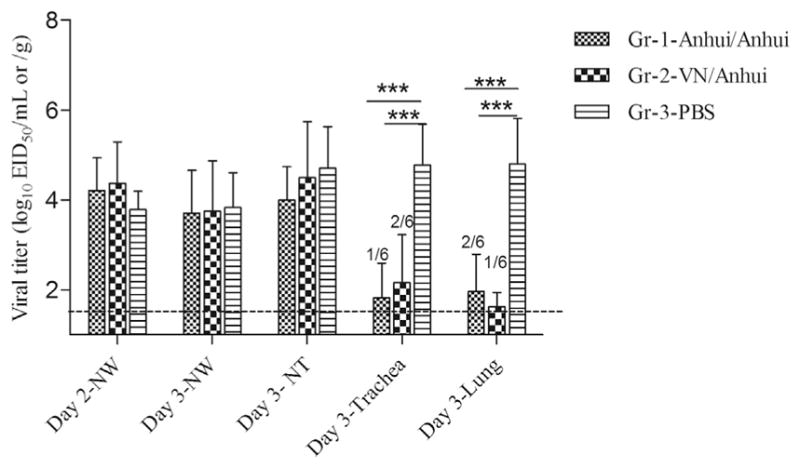
Groups of six ferrets were administered two doses of homologous (Anhui/Anhui) or heterologous (VN/Anhui) AS03-adjuvanted H5 split vaccines or PBS, and challenged intranasally with the Pin/2014, H5N2 clade 2.3.4.4 virus. Nasal washes were collected on days 2 and 3 post challenge. On day 3 post challenge, ferrets were euthanized to collect nasal turbinates, trachea and lung tissues. Viral titers are shown as mean plus standard deviation. The limit of detection of 1.5 log10EID50/ml [nasal wash, nasal turbinate] or g [trachea, lung]) was indicated with a dashed line. Tissue samples with viral titers below the detection limit were assigned values of 1.5 log10EID50/ml or g for purposes of calculating mean titers. Titers are indicative of 6 ferrets unless specified otherwise. Statistical analysis was performed using two-way ANOVA with GraphPad Prism, ***, p < 0.001.
4. Discussion
Producing and stockpiling H5N1 vaccines has been an important part of pandemic preparedness efforts. Human H5N1 vaccines are available in the US federal vaccine stockpile (https://www.medicalcountermeasures.gov/barda.aspx) and may provide protection against a newly emerging H5 pandemic virus before a strain-specific vaccine becomes available. However, the level of cross-protection of these stockpiled vaccines against antigenically distinct H5 subtype viruses is poorly understood. Here, we determined whether AS03-adjuvanted stockpiled 2004–05 H5N1 influenza virus vaccines provide cross-protection against the more recently emerged H5N2 clade 2.3.4.4 virus challenge in ferrets. AS03 is an oil-in-water emulsion consisting of squalene, alpha-tocopherol, and polysorbate-80 and has been shown to be well tolerated in laboratory animals and humans (Segal et al., 2015). AS03 has been shown to significantly enhance serum HI titers to candidate pre-pandemic influenza vaccines and allows for antigen sparing (Leroux-Roels et al., 2007, 2008). The robustness of antibody responses elicited by AS03-adjuvanted H5N1 vaccines demonstrated that our ferret model is in good agreement with previous data from human clinical trials, in which two doses of adjuvanted A/Vietnam/1194/2004, clade 1 vaccine containing 3.8 μg of HA could induce > 82% seroprotective rates in humans aged 18–60 (Leroux-Roels et al., 2007, 2008). Moreover, Langley et al. showed that AS03 formulated with split VN/1194 inactivated virus induced sufficient immune responses in adults with as few as two doses of 3.75 μg of HA (Langley et al., 2010). Importantly, we found in this study that despite low cross-reactive antibody responses to the challenge Pin/2014 virus, both vaccination regimens could reduce virus-induced morbidity and viral replication in the respiratory tract of ferrets following challenge with the antigenically distant H5N2 virus from clade 2.3.4.4.
The heterologous prime-boost vaccine group was included in the study because this strategy can induce broader cross-reactive antibodies (Galli et al., 2009; Khurana et al., 2014; Lu, 2009). Levine et al. recently showed that heterologous, but not homologous prime-boost vaccination with VN/1194 and an adjuvanted Anhui/05 stockpiled H5N1 vaccine induced modest cross-reactive HI antibody to Pin/2014 H5N2 virus (Levine et al., in press). The study also showed that the 2004–05 H5N1 vaccine and 2014 H5N2 viruses are antigenically distinct from each other; ferret sera to Anhui/05 (H5N1) reacted with Pin/2014 (H5N2) virus at titers that were > 16-fold lower than titers against homologous virus. In the current study, serum HI GMT titers to Pin/2014 virus following heterologous prime-boost were slightly higher than those from homologous prime-boost animals; however, the improved cross-reactivity to the clade 2.3.4.4 H5N2 virus was not statistically significant. Based on previous data from human clinical trials, the length of the interval between priming and boosting affects the magnitude of the immune response to vaccination, and a heterologous booster given 6 months after priming can induce higher immune responses compared to two doses of vaccines given 28 days apart (Belshe et al., 2011). In the current study, we studied the most rapid sequence of prime and boost (28 days apart) to emulate what may occur during a pandemic. Further investigation is warranted to ascertain whether longer intervals of heterologous boost after initial prime would improve cross-immune response to antigenically distant H5 viruses.
Protection was likely due to cross-reactive HA antibodies, which were detected by both f-AbBA and HI assays. The 2004–05 influenza H5N1 vaccines containing the N1 NA shares only 43–45% amino acid identity with the NA protein of Pin/2014 (H5N2) virus. Therefore, the protection induced by the AS03-adjuvanted H5N1 vaccines makes it unlikely to be due to cross-reactive responses to viral antigens on the N1 NA. In accord with our findings, a recent study with alumadjuvanted inactivated whole H5N1 vaccines prepared using the recombinant H5 viruses from clade 1, 2.2 and 2.5 also demonstrated strong protection from the H5N8 clade 2.3.4.4 virus challenge in both mouse and ferret models (Park et al., 2016). Although cross-reactive antibody levels to Pin/2014 virus were low, the generation of HI titers ≥10 against the H5N2 virus likely played a role in decreasing virus replication in trachea and lung tissues. Previous vaccine studies have shown that at antibody titers as low as 10 or 20 are sufficient to confer protection in ferrets (Mahmood et al., 2008; Pearce et al., 2012), suggesting that the HI titer threshold of 40 (titer ≥1:40), considered to be protective for H3N2 virus in humans (Baz et al., 2013), may not hold true for all subtypes and species. Other immunological mechanisms may also have contributed to the cross-clade protection against H5N2 virus. Studies have suggested that both cell-mediated immunity and non-neutralizing antibodies, such as antibodies with antibody-dependent cell-mediated cytotoxicity (ADCC) activity, contribute to cross-clade protection in the absence of a detectable neutralizing antibody response (Lipatov et al., 2006; Santiago et al., 2011). ADCC assays measure HA-binding, non-neutralizing antibodies and thus it is conceivable that non-neutralizing antibodies from vaccinated ferrets in our current study confer protection against H5N2 virus through the ADCC pathway. However, ADCC activity has been difficult to establish in some species due to the requirement of species-specific functional effector cells that mediate ADCC such as natural killer (NK) cells, monocytes, or macrophages. To understand the immunologic basis of protection against heterologous challenge, we attempted to test whether ADCC activity accounted for the cross-protection observed in ferrets. Although our laboratory has recently developed an improved ADCC NK cell activation assay utilizing human NK cell lines as effector cells (Zhong et al., 2016), the assay failed to detect H5-specific ADCC titers in ferret sera, most likely due to the incompatibility of the ferret immunoglobulin Fc region with human NK cell FcγRIIIa receptor (unpublished observation).
To further explore the role of cell-mediated protection in the setting of heterologous challenge, T cell responses following vaccination were additionally examined. However, only very low levels of IFN-γ-producing CD4+ and CD8+ T cells from leukocytes prepared from peripheral blood samples on days 7 and 28 post-boost were detected upon stimulation with the recombinant HA proteins from Anhui/05, VN/04 or Pin/2014 viruses; no significant difference among three groups were observed (data not shown). Overall, the ferret CMI responses were too low to draw conclusions regarding cross-reactivity. Future studies pertaining to ferret cell-mediated immunity may require the development of ferret specific reagents to better understand the immunology associated with vaccine protection, in particular cross-protectiveness of H5 vaccines.
As currently circulating H5Nx viruses continue to diverge from vaccine strains, there is a heightened need to assess the efficacy of stockpiled vaccines against newly emergent H5 viruses with pandemic potential. Our study provides evidence for the broad cross-protectiveness of two existing stockpiled H5N1 vaccines, which have been evaluated in clinical trials and proven to be highly immunogenic (Belshe et al., 2014; Bresson et al., 2006). Altogether, we conclude that current split H5N1 vaccines for stockpiling or inactivated whole virus vaccines remain useful tools in pandemic preparedness.
Acknowledgments
We wish to thank our colleagues at BARDA for helpful discussions and Ruben Donis for critical review of the manuscript. The authors thank Weimin Zhong for his expertise and for his performance of the ADCC assays. The authors also thank Adrian Reber and Nedzad Music for their expertise and analysis of ferret peripheral blood leukocytes. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. H.M.C was supported by the Oak Ridge Institute for Science and Education.
References
- Baras B, Stittelaar KJ, Simon JH, Thoolen RJ, Mossman SP, Pistoor FH, van Amerongen G, Wettendorff MA, Hanon E, Osterhaus AD. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One. 2008;3:e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res. 2013;178:78–98. doi: 10.1016/j.virusres.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY Writing Committee of the World Health Organization Consultation on Human Influenza AH. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Frey SE, Graham I, Mulligan MJ, Edupuganti S, Jackson LA, Wald A, Poland G, Jacobson R, Keyserling HL, Spearman P, Hill H, Wolff M National Institute of A, Infectious Diseases-Funded V, Treatment Evaluation U. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis. 2011;203:666–673. doi: 10.1093/infdis/jiq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe RB, Frey SE, Graham IL, Anderson EL, Jackson LA, Spearman P, Edupuganti S, Mulligan MJ, Rouphael N, Winokur P, Dolor RJ, Woods CW, Walter EB, Chen WH, Turley C, Edwards KM, Creech CB, Hill H, Bellamy AR National Institute of A, Infectious Diseases-Funded V, Treatment Evaluation U. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA. 2014;312:1420–1428. doi: 10.1001/jama.2014.12609. [DOI] [PubMed] [Google Scholar]
- Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Hoschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- Carney PJ, Lipatov AS, Monto AS, Donis RO, Stevens J. Flexible label-free quantitative assay for antibodies to influenza virus hemagglutinins. Clin Vaccin Immunol. 2010;17:1407–1416. doi: 10.1128/CVI.00509-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosewood LC, WD . Biosafety in microbiological and medical laboratories. U.S. Department of Health and Human Services; Washington, DC: 2009. [Google Scholar]
- Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, Del Giudice G, Banzhoff A, Brauer V, Montomoli E, Zambon M, Katz J, Nicholson K, Stephenson I. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci USA. 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Coyle EM, Dimitrova M, Castellino F, Nicholson K, Del Giudice G, Golding H. Heterologous prime-boost vaccination with MF59-adjuvanted H5 vaccines promotes antibody affinity maturation towards the hemagglutinin HA1 domain and broad H5N1 cross-clade neutralization. PLoS One. 2014;9:e95496. doi: 10.1371/journal.pone.0095496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Stallknecht DE, Slemons RD, Bowman AS, Poulson RL, Nolting JM, Knowles JP, Webster RG. The enigma of the apparent disappearance of Eurasian highly pathogenic H5 clade 2.3.4.4 influenza A viruses in North American waterfowl. Proc Natl Acad Sci USA. 2016;113:9033–9038. doi: 10.1073/pnas.1608853113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HI, Song MS, Pascua PN, Baek YH, Lee JH, Hong SP, Rho JB, Kim JK, Poo H, Kim CJ, Choi YK. Genetic characterization and pathogenicity assessment of highly pathogenic H5N1 avian influenza viruses isolated from migratory wild birds in 2011, South Korea. Virus Res. 2011;160:305–315. doi: 10.1016/j.virusres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, Johnson C, Li P, Kenney R, Innis B, Fries L. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–1653. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- Lee DH, Bahl J, Torchetti MK, Killian ML, Ip HS, DeLiberto TJ, Swayne DE. Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014–2015. Emerg Infect Dis. 2016;22:1283–1285. doi: 10.3201/eid2207.160048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux-Roels G. Prepandemic H5N1 influenza vaccine adjuvanted with AS03: a review of the pre-clinical and clinical data. Expert Opin Biol Ther. 2009;9:1057–1071. doi: 10.1517/14712590903066695. [DOI] [PubMed] [Google Scholar]
- Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- Levine MZ, Holiday C, Liu F, Jefferson S, Gillis E, Bellamy A, Tumpey T, Katz J. Cross-reactive antibody responses to novel H5Nx Influenza viruses Following homologous and heterologous prime boost vaccination with a prepandemic Stockpiled A(H5N1) vaccine in humans. J Infect Dis. 2017 doi: 10.1093/infdis/jix001. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis. 2006;194:1040–1043. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood K, Bright RA, Mytle N, Carter DM, Crevar CJ, Achenbach JE, Heaton PM, Tumpey TM, Ross TM. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, Kielland A, Chomez P, Garcon N, Van Mechelen M. Adjuvant system AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Park SJ, Si YJ, Kim J, Song MS, Kim SM, Kim EH, Kwon HI, Kim YI, Lee OJ, Shin OS, Kim CJ, Shin EC, Choi YK. Cross-protective efficacies of highly-pathogenic avian influenza H5N1 vaccines against a recent H5N8 virus. Virology. 2016;498:36–43. doi: 10.1016/j.virol.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Pearce MB, Belser JA, Gustin KM, Pappas C, Houser KV, Sun X, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. Seasonal trivalent inactivated influenza vaccine protects against 1918 Spanish influenza virus infection in ferrets. J Virol. 2012;86:7118–7125. doi: 10.1128/JVI.00674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- Pulit-Penaloza JA, Sun X, Creager HM, Zeng H, Belser JA, Maines TR, Tumpey TM. Pathogenesis and Transmission of Novel Highly Pathogenic Avian Influenza H5N2 and H5N8 Viruses in Ferrets and Mice. J Virol. 2015;89:10286–10293. doi: 10.1128/JVI.01438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. J Hyg. 1938;27:493–497. [Google Scholar]
- SAGE Working Group on Influenza Vaccines and Immunizations. Influenza A (H5N1) Vaccine Stockpile and Inter-Pandemic Vaccine Use 2013 [Google Scholar]
- Santiago FW, Fitzgerald T, Treanor JJ, Topham DJ. Vaccination with drifted variants of avian H5 hemagglutinin protein elicits a broadened antibody response that is protective against challenge with homologous or drifted live H5 influenza virus. Vaccine. 2011;29:8888–8897. doi: 10.1016/j.vaccine.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal L, Wouters S, Morelle D, Gautier G, Le Gal J, Martin T, Kuper F, Destexhe E, Didierlaurent AM, Garcon N. Non-clinical safety and biodistribution of AS03-adjuvanted inactivated pandemic influenza vaccines. J Appl Toxicol. 2015;35:1564–1576. doi: 10.1002/jat.3130. [DOI] [PubMed] [Google Scholar]
- Shen YY, Ke CW, Li Q, Yuan RY, Xiang D, Jia WX, Yu YD, Liu L, Huang C, Qi WB, Sikkema R, Wu J, Koopmans M, Liao M. Novel Reassortant Avian Influenza A(H5N6) Viruses in Humans, Guangdong, China, 2015. Emerg Infect Dis. 2016;22:1507–1509. doi: 10.3201/eid2208.160146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, Zambon M. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/ Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–1693. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VV, Pham TS, Vo CD, Le TQ, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen KT, Le HS, Le VT, Christiane D, Tran TT, Menno de J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J World Health Organization International Avian Influenza Investigative T. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- Uyeki TM. Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin Infect Dis. 2009;49:279–290. doi: 10.1086/600035. [DOI] [PubMed] [Google Scholar]
- Webster RG, Govorkova EA. H5N1 influenza–continuing evolution and spread. N Engl J Med. 2006;355:2174–2177. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- WHO. Manual for the laboratory diagnosis and virological surveillance of influenza 2011 [Google Scholar]
- WHO. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO 2016a [Google Scholar]
- WHO. Human infection with avian influenza A(H5N6) virus – China 2016b [Google Scholar]
- Wood JM. Selection of influenza vaccine strains and developing pandemic vaccines. Vaccine. 2002;20(Suppl 5):B40–44. doi: 10.1016/s0264-410x(02)00509-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Carney PJ, Mishin VP, Guo Z, Chang JC, Wentworth DE, Gubareva LV, Stevens J. Molecular Characterizations of Surface Proteins Hemagglutinin and Neuraminidase from Recent H5Nx Avian Influenza Viruses. J Virol. 2016;90:5770–5784. doi: 10.1128/JVI.00180-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZF, Mok CK, Peiris JS, Zhong NS. Human Infection with a Novel Avian Influenza A(H5N6) Virus. N Engl J Med. 2015;373:487–489. doi: 10.1056/NEJMc1502983. [DOI] [PubMed] [Google Scholar]
- Zhong W, Liu F, Wilson JR, Holiday C, Li ZN, Bai Y, Tzeng WP, Stevens J, York IA, Levine MZ. Antibody-dependent cell-mediated cytotoxicity to Hemagglutinin of Influenza A viruses After Influenza vaccination in humans. open Forum. Infect Dis. 2016;3:ofw102. doi: 10.1093/ofid/ofw102. [DOI] [PMC free article] [PubMed] [Google Scholar]