Abstract
Insecticides based on botanical sources have taken on increased attention due to differing modes of action from current insecticides in use and the view that they may be environmentally friendly. Thymoquinone, a component in the essential oil of incense cedar heartwood, has been shown to have insecticidal action against adult mosquitoes. This study evaluated relative toxicities of thymoquinone, selected derivatives of thymoquinone, hydroquinone, and arbutin to determine if any had similar or better activity. The intrinsic toxicities of hydroquinone and thymohydroquinone were not significantly different from thymoquinone, while libocedrol and arbutin were significantly less toxic.
Keywords: Culex quinquefasciatus, thymoquinone, botanical insecticide
Essential oils extracted from plants that repel insects were the principal means that people used for protecting themselves or their domestic animals prior to World War I. World War II opened the Modern Era of chemical control with the introduction of a new concept of insect control, synthetic organic insecticides, the first of which was DDT (Novak and Gerberg 2005). Many of today’s pesticides are designed using naturally derived chemistries. For example, pyrethroid insecticides are modeled after pyrethrins, which are natural, plant-derived poisons that have been used as insecticides for hundreds of years. Insect growth regulators mimic hormones that affect insect growth, but they have little effect on nontarget animals. These products and similar ones using bacteria, viruses, or other natural pest control agents are called “biorational” pesticides (Ware and Whitacre 2004).
Biorational pesticides are becoming increasingly important as vector mosquitoes develop resistance to the few synthetic insecticides registered as existing broad-spectrum pesticides are being dropped from the market. Resistance of insects to insecticides is not a new phenomenon. At least 457 populations of Culex pipiens L. and Culex quinquefasciatus Say have been reported as resistant to over 40 different active ingredients according to the Arthropod Pesticide Resistance Database (APRD) maintained by Michigan State University (http://www.pesticideresistance.com; accessed 11 November 2016).
Currently, four major types of botanical products are used for insect control including rotenone, neem, pyrethrum, and essential oils (Isman 2006). Essential oils have been suggested as an alternative source of materials for arthropod control due to their broad source of bioactive constituents. The essential oil of incense cedar heartwood (Calocedrus decurrens (Torr.)) has been shown to have significant activity against adult Aedes aegypti L. (Dolan et al. 2007). GC-MS analysis of that oil showed thymoquinone to be the major component along with a significant amount of carvacrol (Veluthoor et al. 2011). Lesser amounts of thymohydroquinone (a reduced form of thymoquinone) and libocedrol (a dimeric diterpene derivative) were also found. Thymoquinone, isolated from wild bergamot, Monarda fistulosa L., has been previously reported to have insecticidal activity against Ae. aegypti larvae (Johnson et al. 1998). In a more recent study, thymoquinone has been shown to have biological activity against four strains of Anopheles gambiae Giles mosquitos, exhibiting a different mode of action than what is found in currently used commercial mosquito control products (McAllister and Adams 2010).
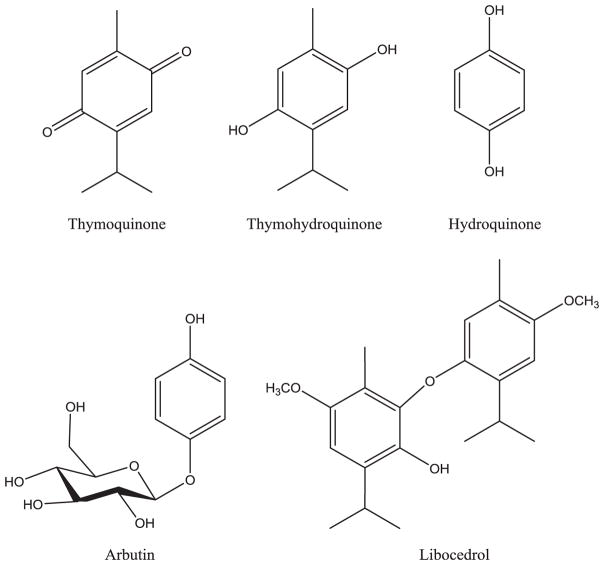
It was observed that mortality produced by thymoquinone was not immediate, suggesting some metabolism might be necessary for the toxic effect to be achieved (McAllister and Adams 2010). The purpose of this study was to compare the toxicities of thymoquinone, arbutin, thymohydroquinone, hydroquinone, and libocedrol (Fig. 1) against Cx. quinquefasciatus to determine if any were more toxic than thymoquinone and thus have greater potential as new insecticides.
Fig. 1.
Thymoquinone and related structures tested on Culex quinquefasciatus Sebring mosquitoes.
Materials and Methods
The susceptible Cx. quinquefasciatus Sebring strain was used. This strain was originally collected from Sebring, FL, in 1988 (Vanlandingham et al. 2008), and has been colonized at the Centers for Disease Control and Prevention (Fort Collins, CO) since 2004. Adult mosquitoes were maintained at 27.5 °C and 70–80% relative humidity on a photoperiod of 14:10 (L:D) h in environmental chambers. Adult mosquitoes were exposed to compounds at 5–7 d posteclosion.
The compounds tested were thymoquinone, arbutin, thymohydroquinone, hydroquinone, and libocedrol. Hydroquinone and arbutin were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO). Thymoquinone and its derivatives thymohydroquinone and libocedrol were isolated from the steam distillation of incense cedar essential oil. Each compound was serially diluted for a total of five concentrations, producing a range of 3–99% mortality. Twenty-six female Cx. quinquefasciatus mosquitoes, 6–7 d old, were exposed to CO2 for 5 s then placed on a chill table (BioQuip Products, Rancho Dominguez, CA) maintained between −1 to −7°C. Each female was treated with 0.2 μl of the compound or acetone, which served as the control, on the dorsal thorax using a syringe and repeating dispenser (Hamilton, Reno, NV). Assays were replicated four times. After all individuals were treated for a particular concentration, they were weighed and an average weight per mosquito was calculated. Mosquitoes were maintained on 5% sucrose for 24 h using the same conditions as the colonies. Mortality was recorded after 24 h.
Arbutin is water soluble and did not adhere to the mosquitoes. Several different solvents including acetone, ethanol, and a detergent, tween-20, were combined with the compound. Each female was treated with 0.2 μl of arbutin and the selected solvent and detergent, but no droplet would form that was absorbed by the cuticle. It was determined that the only way to test arbutin was to feed it via a sucrose solution. Sucrose was removed from the mosquitoes 12 h before the assay. Twenty-five mosquitoes were anesthetized with CO2, so they could be weighed before placing in individual cups. Five different concentrations of arbutin were dissolved in 3 ml 5% sucrose solution and provided to 6–7-d-old females for a 24-h period before mortality was recorded. Dose mortality (LD) data were evaluated using probit analysis generated using SAS software. (Copyright 2002–2010 by SAS Institute Inc., Cary, NC).
Results
The toxicities on adult Cx. quinquefasciatus are presented in Table 1. The LD50 for thymoquinone was 7.51 μg/mg (CI 7.13–7.88) and hydroquinone was 7.79 μg/mg (CI 7.16–8.43). While these values fall within the confidence interval (CI) of each other, the LD95 of these two compounds did not overlap and their slopes are different. Although three of the four related compounds exhibited some level of insecticidal activity, on the basis of both LD50 and LD95 values, only hydroquinone was similar in toxicity to thymoquinone. Hydroquinone was the most toxic metabolite to Cx. quinquefasciatus, followed by thymohydroquinone, libocedrol, and arbutin. Arbutin proved ineffective, as not enough of the compound could be dissolved in the sucrose solution to induce mortality without forming a paste.
Table 1.
Lethal doses of compounds tested on Culex quinquefasciatus Sebring mosquitoes
| Chemical | N | Slope | LD50 (95% CI) μg/mg | LD95 (95% CI) μg/mg | χ2 |
|---|---|---|---|---|---|
| Thymoquinone | 625 | 0.86 | 7.51 (7.13–7.88) | 12.77 (11.96–13.87) | 8.11 |
| Hydroquinone | 503 | 0.52 | 7.79 (7.16–8.43) | 16.50 (14.94–18.76) | 10.3 |
| Thymohydroquinone | 384 | 0.26 | 9.57 (7.05–12.08) | 27.25 (21.72–40.12) | 15.69 |
| Libocedrol | 493 | 0.24 | 16.03 (14.66–17.94) | 34.77 (31.22–40.01) | 13.55 |
| Arbutin | – | – | – | – | – |
Discussion
The essential oil of incense cedar heartwood, C. decurrens, was previously found to be toxic to three medically important arthropods, Ae. aegypti mosquitoes, Ixodes scapularis (Say) ticks, and Xenopsylla cheopis (Rothchild) fleas (Dolan et al. 2007). Thymoquinone and carvacrol, which are the major components of this oil, are viable compounds for vector control and commercial development (McAllister and Adams 2010). The related compounds of thymoquinone that were evaluated in this study were not as effective as thymoquinone. This suggests that the quinone moiety of thymoquinone is key to its toxicity and derivatization significantly reduces toxicity. Hydroquinone could have the potential for controlling vector mosquitoes, as the LD50 only varied 0.28 μg/mg from thymoquinone.
Thymoquinone has been indicated as having other biological activities of interest for human use. Thymoquinone is the bioactive constituent of the oil of black cumin (Nigella sativa, L.), which has been used since antiquity in the Middle East for treatment of many human diseases (Padhye et al. 2008). This compound has been shown to have antioxidant, anti-inflammatory, and promising anticancer activities in both in vitro and in vivo studies (Gali-Mahtasib et al. 2006, Sutton et al. 2014). It has also been studied as an immune enhancer in Aedes caspius (Pallas) to control disease agents in a novel way (Ahmed et al. 2010). Hydroquinone is commonly used as a skin whitening agent in cosmetics (Tse 2010).
The prospective of using the same application strategies as current commercial insecticides with the compounds evaluated is probably unlikely since they are less toxic than thymoquinone. Many essential oils are known to exhibit repellent, antifeeding, and insecticidal activities against various arthropod species (Isman 2006). The compounds tested here may serve better as natural repellants than insecticides, and should be further evaluated. The benefit of researching and developing natural products based on plant essential oils may result in relatively low-cost production, be environmentally friendly as compared to commercial insecticides, have public favorability, and have different modes of action. However, additional biological activity needs to be considered before insecticide development.
Acknowledgments
We wish to thank the deployed war fighter protection program for funding this project.
Footnotes
The views of the authors do not necessarily reflect the position of the Centers for Disease Control and Prevention or the Department of Wood Science and Engineering, Oregon State University.
References Cited
- Ahmed AM, Al-Olayan EM, Aboul-Soud MAM, Al-Khedhairy AA. The immune enhancer, thymoquinone, and the hope of utilizing the immune system of Aedes caspius against disease agents. Afr J Biotechnol. 2010;9:3183–3195. [Google Scholar]
- Dolan MC, Dietrich G, Panella NA, Montenieri JA, Karchesy JJ. Biocidal activity of three wood essential oils against Ixodes scapularis (Acari: Ixodidae), Xenopsylla cheopis (Siphonaptera: Pulicidae), and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2007;100:662–625. doi: 10.1603/0022-0493(2007)100[622:baotwe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gali-Mahtasib H, Roessner A, Schneider-Stock R. Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol. 2006;8:1249–1253. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Rogers LL, Alkire ML, McCloud TG, McLaughlin JL. Bioactive monoterpenes from Monarda fistulosa (Lamiaceae) Nat Prod Lett. 1998;11:241–250. [Google Scholar]
- McAllister JC, Adams MF. Mode of action for natural products isolated from essential oils of two trees is different from available mosquito adulticides. J Med Entomol. 2010;41:1123–1126. doi: 10.1603/me10098. [DOI] [PubMed] [Google Scholar]
- Novak RJ, Gerberg EJ. Natural-based repellent products: efficacy for military and general public uses. J Am Mosq Control. 2005;21:7–11. doi: 10.2987/8756-971X(2005)21[7:NRPEFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity – the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–509. [PMC free article] [PubMed] [Google Scholar]
- Sutton KM, Greenshields AL, Hoskin DW. Thymoquinone, A bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr Cancer. 2014;66:408–418. doi: 10.1080/01635581.2013.878739. [DOI] [PubMed] [Google Scholar]
- Tse TW. Hydroquinone for skin lightening: safety profile, duration of use and when should we stop? J Dermatol Treat. 2010;21:272–275. doi: 10.3109/09546630903341945. [DOI] [PubMed] [Google Scholar]
- Vanlandingham DL, McGee CE, Klingler KA, Galbraith SE, Barrett ADT, Higgs S. Short report: comparison of oral infectious dose of West Nile virus isolates representing three distinct genotypes in Culex quinquefasciatus. Am J Trop Med Hyg. 2008;79:951–954. [PMC free article] [PubMed] [Google Scholar]
- Veluthoor S, Kelsey RG, Gonzalez-Hernandez MP, Panella N, Dolan M, Karchesy J. Composition of the heartwood essential oil of incense cedar (Calocedrus decurrens Torr.) Holzforschung. 2011;65:333–336. [Google Scholar]
- Ware GW, Whitacre DM. The pesticide book. 6. MeisterPro Information Resources; Willoughby, OH: 2004. [Google Scholar]