Abstract
Human central nervous system myelin development extends well into the fourth decade of life, and this protracted period underscores the potential for experience to modulate myelination. The concept of myelin plasticity implies adaptability in myelin structure and function in response to experiences during development and beyond. Mounting evidence supports this concept of neuronal activity-regulated changes in myelin-forming cells, including oligodendrocyte precursor cell proliferation, oligodendrogenesis and modulation of myelin microstructure. In healthy individuals, myelin plasticity in associative white matter structures of the brain is implicated in learning and motor function in both rodents and humans. Activity-dependent changes in myelin-forming cells may influence the function of neural networks that depend on the convergence of numerous neural signals on both a temporal and spatial scale. However, dysregulation of myelin plasticity can disadvantageously alter myelin microstructure and result in aberrant circuit function or contribute to pathological cell proliferation. Emerging roles for myelin plasticity in normal neurological function and in disease are discussed.
Keywords: myelin, plasticity, adaptive myelination, oligodendrocyte, oligodendrocyte precursor cell (OPC)
Introduction
The importance of myelin physiology to neurological function has long been appreciated. For much of the relatively young history of neuroscience, the focus on myelination and myelin-forming cells has centered on development and regeneration of white matter tracts. The protracted nature of human myelin development, with myelination of the prefrontal cortex extending into the fourth decade of life (Lebel et al., 2012; Yakovlev, 1967) highlights the complex regulation of human myelination and the potential for maldevelopment to contribute to disease.
Oligodendrocytes, the myelin-producing cells of the central nervous system (CNS), wrap concentric sheaths of myelin around axons in order to facilitate saltatory neurotransmission. As well, oligodendrocytes provide important trophic support to the axon (Chrast et al., 2011; Funfschilling et al., 2012; Hirrlinger and Nave, 2014). Oligodendrocyte precursor cells (OPCs) maintain a dynamic ability to respond and alter their physiological state to match the needs of the developing brain. But how does this robust population of persistent precursor cells act after the developmental need for myelin generation is met? And what role, if any, does de novo myelination or myelin remodeling play in neurological function and dysfunction in adulthood? The regulation of OPC proliferation, differentiation and myelin remodeling outside of the developmental window has now come into sharp focus. One of the crucial modulators of myelin-producing cells throughout a lifetime may be the very cell that oligodendrocyte lineage cells are meant to support: the neuron.
Myelin remodeling continues throughout life and can be influenced by experience. Adult-born oligodendrocytes in the CNS of rodents contribute to already highly myelinated structures such as the optic nerve (Young et al., 2013), as well as white matter fibers associated with the acquisition of new, complex behaviors (McKenzie et al., 2014; Schneider et al., 2016). The new oligodendrocytes that contribute to de novo skill learning in adulthood do so in rapid fashion, with the onset of oligodendrogenesis occurring within hours of the experience (Xiao et al., 2016). Blocking adult oligodendrogenesis has also been shown to affect nodes of Ranvier and associated paranodal architecture and motor function within mice (Schneider et al., 2016). Like rodents, ongoing myelin changes occur throughout adulthood in non-human primates (Bowley et al., 2010; Peters, 2002; Peters et al., 2008). In humans the extent to which these myelin changes are mediated by new or existing oligodendrocytes remains an open question (Yeung et al., 2014). Regardless of the source of new myelin, these studies highlight that ongoing myelin remodeling occurs throughout adulthood and may function to sustain or alter the function of neural circuits as regulated by the activity of that circuit.
The concept of experience-dependent myelin remodeling during both postnatal development and adulthood has been explored in several vertebrate systems. Provocative studies indicate that acquisition of new, complex behaviors such as skilled reaching in rats (Sampaio-Baptista et al., 2013) or piano playing (Bengtsson et al., 2005) and juggling (Scholz et al., 2009) in humans, are associated with enhancement of white matter microstructure in neural circuits related to the attained skill. Conversely, experiential deprivation such as social isolation results in changes to prefrontal cortex myelin in young and adult rodents (Liu et al., 2012a; Makinodan et al., 2012) and to corpus callosum volume in non-human primates (Sanchez et al., 1998). Together, these intriguing studies suggest the ability of circuit activity to alter white matter parameters in healthy subjects.
But how are experiences translated into dynamic myelin changes? One potential mediator of experience-dependent myelin remodeling, referred to as adaptive myelination when the myelin change positively influences neurological function, is neuronal activity itself (for reviews on adaptive myelination see (Baraban et al., 2016; Bergles and Richardson, 2015; Fields, 2015; Mount and Monje, 2017; Purger et al., 2016)). The role of neurons in altering oligodendrocyte lineage cell dynamics was first described in models of decreased neuronal activity that found reduced OPC proliferation in response to blockade of action potential propagation (Barres and Raff, 1993). Subsequently, others demonstrated electrical impulses in vitro can affect myelination both directly (Demerens et al., 1996; Wake et al., 2011) and indirectly via astrocyte-derived growth factor secretion (Ishibashi et al., 2006). In vivo models suggest that not only does increased neuronal activity mediated by optogenetic stimulation of the premotor circuit in mice enhance OPC proliferation, differentiation, and myelin thickness within the activated circuit (Gibson et al., 2014), but in zebrafish models vesicular glutamate release also increases the number of myelin sheaths produced by an individual oligodendrocyte and influences the selection of axons to undergo myelination (Hines et al., 2015; Mensch et al., 2015).
Neural network function depends on the convergence of multiple neural signals on a temporal and spatial scale that allows for proper signal integration and propagation within the circuit. Myelin variations can enable precise synchronization of action potentials from various excitatory and inhibitory inputs to facilitate proper neurological function, such as has been described for the synchronous integration of spatially disparate signals from both ears (as reviewed in (Seidl, 2014)). Myelin sheath microstructure is thus imperative to this process, as the speed of these converging signals depends on the integrity of the axonal-myelin relationship, the geometric parameters of the myelin sheath such as sheath thickness and internode length, and the relationship between myelinated regions and unmyelinated nodes of Ranvier along a single axon (Hartline and Colman, 2007; Tasaki, 1939). Any disruption to or dysregulation of myelin plasticity may have a drastic impact on neural network functioning, as myelin alterations can either promote or disrupt the synchrony of signals and thus influence neural coherence. As a result, competing or cooperative neural pathways may become temporally incompatible (de Hoz and Simons, 2015; Pajevic et al., 2014). Thus, the dysregulation of these robust processes of myelin plasticity could in theory become maladaptive and lead to disease states of the nervous system.
Myelin Plasticity in Disease
Cancer
The neuronal regulation of normal oligodendrocyte precursor cell proliferation that is important to the process of myelin plasticity suggests that similar mechanisms could play a role in the aberrant proliferation of brain cancers that molecularly resemble OPCs. High-grade gliomas such as glioblastoma (GBM) and diffuse intrinsic pontine glioma (DIPG), the most lethal forms of brain cancer in adults and in children, respectively, are thought to originate from OPCs or earlier stem cells (Alcantara Llaguno et al., 2011; Alcantara Llaguno and Parada, 2016; Alcantara Llaguno et al., 2015; Galvao et al., 2014; Liu et al., 2011; Monje and Dietrich, 2011; Nagaraja et al., 2017; Tate et al., 2015). Oligodendroglioma, another important glioma type chiefly affecting adults, is similarly thought to arise from oligodendroglial lineage precursors (Persson et al., 2010; Sugiarto et al., 2011). Recently, the role of neuronal activity as a mediator of the glioma microenvironment was studied using a patient-derived orthotopic xenograft model. Pediatric cortical glioblastoma cells were xenografted into the deep layers of the premotor cortex of Thy1∷ChR2 mice and layer V output neurons of this circuit were then optogenetically stimulated. Elevated neuronal activity of these premotor pyramidal neurons resulted in increased glioma proliferation and growth within the activated circuit. Neuronal-activity regulated secretion of brain derived neurotrophic factor (BDNF) and neuroligin-3 (NLGN3) mediates the proliferative effect of not only pGBM, but also other classes of high-grade gliomas such as DIPG, adult glioblastoma and anaplastic oligodendroglioma. NLGN3 binding to the glioma cell promotes PI3K-mTOR pathway activity as well as a feed-forward stimulation of glioma cell NLGN3 expression, linking the importance of PI3K pathway activity in normal precursor cell progression with aberrant glioma cell proliferation. Accordingly, elevated NLGN3 expression in human GBM is associated with decreased survival (Venkatesh et al., 2015). While the role of NLGN3 in normal oligodendroglial lineage cells and myelination remains to be fully elucidated, in vitro evidence suggests a role for neuroligin-neurexin binding at the axo-glial synapse (Proctor et al., 2015), and BDNF has well established roles in white matter development and regeneration (discussed in detail below). These data suggest that in the context of glioma, mechanisms of OPC proliferation may be hijacked by the tumor and utilized to enhance glioma growth. In this way, neuronal activity regulates both normal and neoplastic glial cell behavior.
The detrimental effect of neuronal-activity induced proliferation of glioma cells may be compounded by the fact that gliomas themselves can increase the excitability of the surrounding neural circuits, in part due to an increase in glutamate secretion by glioma cells (Buckingham et al., 2011; Campbell et al., 2012). Other populations known to influence neuronal excitability, such as astrocytes, may also contribute to activity-dependent influences on glioma progression and invasion. Recently, subpopulations of astrocytes exhibiting distinct molecular profiles have been identified in both mice and humans (John Lin et al., 2017). A specific subpopulation of astrocytes promotes synaptogenesis, and the malignant counterpart of synaptogenesis-inducing astrocytoma cells emerges in correlation with the onset of seizures in a mouse model of glioblastoma (John Lin et al., 2017). These data further support the bi-directionality of influence between neuronal activity and glioma progression (John Lin et al., 2017). While the reciprocal importance of neural circuit activity and brain tumor progression has been established, the role of neuronal activity in glioma initiation remains enigmatic. A more in-depth discussion of the role of neuronal activity in cancer pathogenesis can be found in (Venkatesh, 2017).
What do these mechanisms mediating malignant neuron-glial interactions teach us about molecular mediators of healthy plasticity in the oligodendroglial lineage cell population? Does neuroligin-3 play a role in healthy neuron-OPC interactions? Does BDNF, crucial in myelin development (Peckham et al., 2016; Wong et al., 2014; Wong et al., 2013; Xiao et al., 2010) and remyelination (Fulmer et al., 2014; Vondran et al., 2010; VonDran et al., 2011), play a role in activity-regulated myelin plasticity? The parallel importance of the PI3K/AKT/mTOR pathway in both normal myelination (Bercury et al., 2014; Flores et al., 2008; Goebbels et al., 2010; Goebbels et al., 2017; Guardiola-Diaz et al., 2012; Harrington et al., 2010; Hu et al., 2013; Lebrun-Julien et al., 2014; Tyler et al., 2009) and in glioma suggests that further mechanistic parallels will come to light.
Psychiatric Disorders
Bipolar Disorder
A molecule called neuregulin creates a possible link between the concept of adaptive myelination to bipolar disease. An intriguing study by Lundgaard and colleagues posits that two distinct modes of myelination exist, a theory that may reconcile apparently conflicting evidence in the field related to the role of glutamatergic signaling in myelination (Lundgaard et al., 2013). One mode is independent of neuronal activity, such as can occur on innert nanofibers (Lee et al., 2013; Lee et al., 2012), and the second is an activity-dependent mode that requires glutamate release and NMDA activation. Once oligodendrocyte lineage cells are exposed to neuregulin (NRG), an EGF-like signaling molecule, a switch from the passive default state of myelination to an activity-responsive state occurs, with a rapid increase in NMDA receptor response and increased myelination in vitro (Lundgaard et al., 2013). While NRG is a critical regulator of PNS myelination and Schwann cell development, the myelin forming cells of the PNS (Brinkmann et al., 2008; Chen et al., 2006; Michailov et al., 2004; Stassart et al., 2013; Taveggia et al., 2005; Torii et al., 2014), its role in CNS myelination is less clear. Mice in which Nrg1 is conditionally inactivated at various developmental time points exhibit no differences in CNS myelination, though over-expression of NRG1 increases myelin density in the cortex (Brinkmann et al., 2008). Regional differences exist in mice haploinsufficient for NRG1-III: myelin sheaths and overall myelin proteins are decreased in the brain, however the optic nerve and spinal cord are fully myelinated (Taveggia et al., 2008). Conditionally ablating ErbB3, the receptor for NRG, in PLP+ oligodendrocytes during a critical period of development in prefrontal cortex myelination results in reduced myelin gene expression and thinner myelin sheaths, mimicking the phenotype seen in socially isolated animals (Makinodan et al., 2012). This idea that the role of NRG in myelination may potentially exhibit temporal and spatial specificity fits well into the concept proposed by Lundgaard et al. in which NRG acts as a switch between activity-independent and activity-dependent myelination (Lundgaard et al., 2013).
Interestingly, numerous groups have identified changes in NRG-ErbB signaling in people with bipolar disease. Many NRG1 polymorphisms are associated with increased risk of bipolar disease; the 5′ risk-associated genetic variants of NRG1 are correlated with decreased white matter density and integrity of the prefrontal cortex (McIntosh et al., 2009; Mechelli et al., 2009). Using qPCR and microarray analyses of gene expression, Tkachev et al. found altered expression of Olig2, a pan-oligodendroglial marker, and ErbB3 in subjects with bipolar disease (Tkachev et al., 2003). Altered ErbB signaling could change NMDA expression in oligodendrocytes (Karadottir et al., 2005). Thus, changes to activity-dependent myelination mediated by NRG signaling could prove important to the pathophysiology associated with psychiatric disorders such as bipolar disorder.
Asynchrony between neural signals, potentially due to either insufficient or exuberant myelination, may underlie the circuit dysfunction seen in many psychiatric disorders. However, discerning the relative contributions of aberrant myelination and axon integrity are an ongoing challenge to the study of neuropsychiatric diseases in which aberrant white matter has been implicated. This concept is further complicated by the reciprocal interaction between neuronal activity and myelination. For example, determining if myelin alterations are causative to or a consequence of neuronal activity remains enigmatic. This is especially true in relation to bipolor disorder in which altered neuronal activity may cause the changes in myelin content associated with this disease or may be a consequence of pre-existing aberrant myelination. Advanced neuroimaging techniques such as diffusion tensor imaging (DTI) demonstrate white matter abnormalities in people with psychiatric disease (White et al., 2008). However, it is not possible to discern the relative contribution of disordered myelin versus disordered axonal integrity using DTI. Using magnetization transfer ratio (MTR) in concert with diffusion tensor spectroscopy (DTS) one can begin to separate the contributions of myelin and axon integrity, respectively, to white matter structure. In adult patients with bipolar disorder, MTR, the proxy measure for myelin, was decreased while no change in n-acetylaspartate (NAA) diffusion in axons as detected with DTS was found, implicating myelin dysregulation irrespective of changes to axonal structure in bipolar disorder (Lewandowski et al., 2015). Similarly, white matter abnormalities and frontotemporal dysfunction are seen in adolescent subjects with or at risk for bipolar disorder based on genetic predispositions (de Zwarte et al., 2014). Further underscoring the role for myelin anomalies in bipolar disorder, increases in the total volume of white matter hyperintensities as measured by T2 weighted MRI are seen in patients with bipolar disorder compared to healthy controls and are correlated with familiality of the disease (Tighe et al., 2012). These prominent changes in white matter raise the question of potential alterations to neuron-oligodendroglial cell interactions in bipolar disorder. It is possible that abnormal activity-regulated myelination, “maladaptive myelination”, could contribute to bipolar disorder pathology, although whether alterations to neuronal circuit dynamics precede or are a consequence of maladaptive myelination remains to be determined. For a comprehensive review of the putative role for myelin plasticity in bipolar disorder see (Bellani et al., 2016). More research focusing on the hypothesized role of myelin plasticity in the onset and progression of bipolar disorder, especially related to neuronal activity-mediated NRG signaling, are imperative to understanding the origins and pathophysiology of this affective disorder.
Schizophrenia
While patients with bipolar disorder exhibit changes in myelin but not axonal geometry (Lewandowski et al., 2015), aberrations in both parameters are evident in patients with schizophrenia (Du and Ongur, 2013). Changes not only in myelin but also axon structure may contribute to altered circuit dynamics and thus lead to the more severe cognitive dysfunction associated with schizophrenia. Like bipolar disorder, numerous studies of subjects with schizophrenia have demonstrated multiple alterations (single nucleotide polymorphisms, SNPs) in genes associated with myelin pathways, including neuregulin 1 (NRG1) and myelin oligodendrocyte glycoprotein (MOG) as well as white matter abnormalities in neural circuit underpinnings of emotional function (i.e. limbic structures including the cingulum, fornix, parahippocampal gyrus; (Cannon et al., 2012; Yu et al., 2014)). Association studies using SNPs, though, do not necessarily translate to functional molecular effects and this data is often too complicated to interpret fully. Pathway analysis, however, can help clarify how networks of these single genes combine to elicit molecular and cellular effects. A study investigating the role of glial cells in schizophrenia used the largest schizophrenia genomewide association study (GWAS) to date and found that roughly 25% of the genes were oligodendrocyte-associated genes. After filtering out neuronal genes, overlapping glial genes and assessing functional gene sets using gene ontology (GO), the study found highly significant associations for groups of oligodendrocyte genes to risk of schizophrenia (Goudriaan et al., 2014). In particular, gene sets of oligodendrocyte lipid metabolism, including genes critical in myelin sheath development, and gene transcription of oligodendrocytes were found highly associated with schizophrenia risk (Goudriaan et al., 2014). Other candidate genes involved in schizophrenia include ST8SIA2, which has a role in polysialylation. ST8SIA2 knock out mouse models exhibit delayed myelination and decreased myelin content, including thinner sheaths, irregularly shaped axons, and increased white matter lesions in adulthood. ST8SIA2 knock out mice also exhibit increases in the number of immature oligodendrocytes in the cortex and the corpus callosum that may imply a blockade of OPC differentiation to myelinating oligodendrocytes (Szewczyk et al., 2017).
Despite the complexity and variability associated with gene polymorphisms in schizophrenia, altered white matter integrity have been consistently identified in DTI analyses of schizophrenic subjects. White matter abnormalities are diffuse in subjects with schizophrenia, with globally decreased levels of fractional anisotropy (FA) and increased radial diffusivity (RD) (Chavarria-Siles et al., 2016; Reid et al., 2016), proxy measures for myelin integrity. Deficits are evident diffusely in frontomedial white matter tracts (Drakesmith et al., 2016), orbitofrontal cortex, posterior parietal cortex (Green et al., 2016), arcuate fasciculus, cingulum bundle, and inferior longitudinal fasciculus (Oestreich et al., 2016; Seitz et al., 2016). The apparently more global disruptions to white matter in schizophrenia compared to other neuropsychiatric disorders are consistent with the more severe and complex nature of dysfunction in schizophrenia. Mouse models of schizophrenia, including the conditional knockout of nicastrin, a subunit of γ-secretase, and NMDAR antagonist MK-801 models, both exhibit myelin deficits ranging from hypomyelination to increased sheath splitting and segmental demyelination (Dries et al., 2016; Xiu et al., 2015). Do these myelin abnormalities reflect an inappropriate response to neuronal activity, or is inappropriate neuronal activity reflected in the myelin abnormalities? While no animal model fully recapitulates the behavioral phenotype of schizophrenia, use of these models to better understand the progression of white matter abnormalities, especially related to the response of oligodendroglial cells to active neurons is imperative to discern the underlying mechanisms driving white matter contributions to atypical circuit structure and function in schizophrenia.
Multiple Sclerosis
The importance of neuron-OPC signaling in myelin development and plasticity suggests that this interaction may be important for regeneration as well, including in demyelinating diseases such as multiple sclerosis (MS). Recently, using a model of ethidium bromide demyelination in the cerebellum, Káradóttir and colleagues found that inhibiting neuronal activity within the demyelinated lesion, specifically via blockade of AMPA receptors or axonal vesicular release, decreases remyelination with a concomitant increase in OPC density, suggesting a blockade of differentiation into re-myelinating oligodendrocytes in the absence of neuronal activity. OPCs in the lesioned area receive de novo synapses from demyelinated axons and respond to neuronal activity with increased differentiation via AMPA/kainate receptor signaling. When neuronal activity is blocked with the voltage-gated sodium channel blocker TTX, OPCs remained in their proliferative state (Gautier et al., 2015). While pharmacological blockade of activity is not without caveats, these data implicate the role of neuronal activity, specifically glutamatergic signaling, in remyelination processes. The potential for dysregulated interactions between precursor cells and neurons within MS lesions may underlie the inability to fully remyelinate lesions. Future studies utilizing specific means to modulate neuronal activity, such as optogenetic or chemogenetic approaches, may further discern the role of activity-regulated responses of OPC lineage cells in remyelination.
The functional role of neuron to OPC synapses (Bergles et al., 2000) remains enigmatic (Etxeberria et al., 2010; Sahel et al., 2015). While deletion of the NR1 NMDA receptor subunit in oligodendrocytes in the EAE model of MS had no effect on either the severity or progression of the disease (Guo et al., 2012), NMDA signaling may still play an important role in myelin regeneration. Blocking NMDA signaling with the antagonist MK- 801 was found to delay the onset of remyelination, as well as reduce myelin thickness after recovery in a cuprizone model of demyelination, supporting the concept that remyelination may, in part, be mediated through glutamatergic signaling (Li et al., 2013).
The role of neuronal activity-dependent influences on myelin-forming cells within demyelinated lesions may in part be complicated by compensatory mechanisms mediating remyelination. As previously discussed, in addition to neuregulin, Lundgaard and colleagues proposed that brain derived neurotrophic factor (BDNF) may also contribute to the switch between activity-independent and activity-dependent myelination (Lundgaard et al., 2013). BDNF, a protein robustly regulated by activity, was first identified in culture to promote activity-dependent survival of cortical neurons (Ghosh et al., 1994). BDNF is also a critical regulator of developmental myelination via signaling through its membrane receptor TrkB (Xiao et al., 2010). BDNF promotes oligodendrocyte maturation in vitro (Du et al., 2006; Lundgaard et al., 2013; Miyamoto et al., 2015; Van't Veer et al., 2009) and in vivo (Cellerino and Kohler, 1997; Fulmer et al., 2014; Vondran et al., 2010; Xiao et al., 2010), as well as promotes developmental myelination in vivo (Wong et al., 2013).
Given the importance of BDNF in normal myelination, BDNF-TrkB signaling has emerged as a promising mechanism to improve remyelination after injury. In cuprizone models of demyelination, BDNF protein expression is reduced in lesions. BDNF+/- mice exhibit impaired remyelination, with reduced myelin protein expression and increased OPC density that may reflect impaired differentiation (Tsiperson et al., 2015; VonDran et al., 2011). Interestingly, administering genetically engineered stem cells that overproduce BDNF before inducing the EAE model of MS in mice delayed the onset of the disease, as well as decreased demyelination, resulting in a less severe clinical phenotype (Makar et al., 2009). Similarly, administering the MS drug glatiramer acetate to EAE mice resulted in increased levels of BDNF throughout the brain; this increase was correlated with a decrease in the severity of symptoms (Aharoni et al., 2005). Triggering astrocytic release of BDNF via activating metabotropic glutamate receptors increases myelin proteins in the corpus callosum (Fulmer et al., 2014). These data indicate that BDNF may be harnessed from multiple cell types to enhance remyelination. Studies in humans indicate that BDNF levels are decreased in the blood of MS patients, and that increases in serum BDNF concentrations occur in patients following recovering from the relapsing phase of relapsing-remitting MS (Azoulay et al., 2005; Frota et al., 2009). This supports the idea that BDNF may be important in aiding recovery in patients following relapses, possibly through activity-dependent mechanisms, and that activating this signaling system in patients may be a promising therapeutic target to promote recovery in demyelinating diseases.
Epilepsy
Not all neuronal activity is equal in its influence on myelin-forming cells. Low frequency (0.1 Hz) electrical stimulation of mouse dorsal root ganglia in vitro inhibits normal myelination by the peripheral nervous system myelin forming cells, Schwann cells, while higher frequencies (1 Hz) do not (Stevens et al., 1998). In the CNS, acute optogenetically-induced motor seizures do not result in an increase in OPC proliferation, while optogenetically-induced neuronal activity in the premotor circuit that results in complex motor output rather than seizures does increase OPC proliferation (Gibson et al., 2014). These data suggest that the frequency or pattern of firing, or perhaps the functional outcome of neuronal activity, may influence neuron-OPC interactions and the response of oligodendroglial cells through mechanisms that are yet to be determined.
Does chronic aberrant neuronal activity, such as that seen in epilepsy, influence the behavior of myelin forming cells? MRI/DTI analyses in patients with various forms of epilepsy suggest white matter abnormalities. The most common type of epilepsy in adults, as well as one of the most extensively studied, is temporal lobe epilepsy (TLE). DTI in patients with TLE demonstrates decreased FA and/or mean diffusivity (MD) predominately on the side ipsilateral to the seizures, specifically within the hippocampus and temporal lobe (Gross et al., 2006; Riley et al., 2010; Rodriguez-Cruces and Concha, 2015; Widjaja et al., 2011); in more severe cases of temporal lobe epilepsy (i.e. mesial temporal sclerosis), DTI changes are noted in contralateral white matter tracts as well, suggesting large circuits may be affected (Liu et al., 2012b). A case study involving an adult patient who experienced chronic epilepsia partialis continua starting as a juvenile exhibited cortical dysplasia and subcortical dysmyelination on neuroimaging (Misawa et al., 2004), although it is hard to know if the dysmyelination is the result of maldevelopment or ongoing aberrant activity. Such white matter abnormalities correlate with cognitive dysfunction in working memory and language (Hermann et al., 2007; McDonald et al., 2008; Riley et al., 2010). Histological assessment of mature oligodendrocytes in the brains of adult patients with TLE and pediatric patients with intractable epilepsy indicates an increase in oligodendrogenesis in white matter regions associated with aberrant neuronal activity (Sakuma et al., 2014; Stefanits et al., 2012). In rodent models of chemically-induced epileptogenesis, histological analyses show a decrement in myelin proteins and oligodendrocytes concomitant with a transient increase in OPC density, depending on the phase of epileptogenesis (Luo et al., 2015). The exact underlying cause of these oligodendroglial aberrancies remains unknown, and could be caused by axonal damage or loss due to seizure activity. An intriguing alternative hypothesis posits that changes in white matter integrity are a result of more subtle changes in myelin microstructure due to persistent hyperactivity of the neural circuit.
A second common form of epilepsy is childhood absence epilepsy (CAE), an idiopathic epilepsy characterized by spike wave discharges at 3-4Hz frequency, and associated with a broad range of cognitive and behavioral deficits (Caplan et al., 2008). In patients with CAE, white matter abnormalities, including regions with both increases and decreases in FA values, are seen throughout the basal ganglia-thalamocortical circuit (Yang et al., 2012), as well as decreased white matter volume in the basal forebrain (Chan et al., 2006) and decreased FA in the genu of the corpus callosum (Liang et al., 2016). These data suggest that overt alterations to circuit function at the level of white matter content may contribute to circuit dysregulation. It is important to note that increases in myelin content in some areas of a circuit may be just as maladaptive as decreases given the necessity for proper myelin structure and resultant conduction velocities tuned to the dynamics of that circuit. However, similar to studies involving patients with TLE, discerning neuroimaging changes in white matter volume due to dysmyelination or loss of neurons due to excitotoxic seizure activity is complicated. Monogenic rodent models of absence epilepsy, such as the tottering and stargazing mice, which exhibit specific mutations in voltage-gated calcium channels, show little to no change in white matter tracts, however few studies have investigated white matter microstructure specifically (Isaacs and Abbott, 1992; Zhang et al., 2004). Moreover, absence epilepsy is rarely driven by a single mutation, and multigenic models of rat epilepsy have been shown to recapitulate some of the observed DTI anomalies. A study using Wistar albino Glaxo rats of Rijswijk (WAG/Rij), a multigenic rat model of absence epilepsy, showed that after the onset of seizures, these rats exhibit a localized decrease in FA in the corpus callosum. This was associated with an increase in perpendicular diffusivity, suggesting a reduction in myelin integrity in white matter pathways (Chahboune et al., 2009). Inducing seizures in rodents with pentylenetetrazol (PTZ), a GABA receptor antagonist, results in decreased myelin basic protein (MBP) expression but no changes in OPC density in the hippocampus and cortex; however, white matter tracts were not assessed in that study (You et al., 2011). Myelinated fiber volume, myelin sheath thickness, and MBP expression in the hippocampus are reduced in a rat lithium-pilocarpine seizure model, with smaller diameter axons more susceptible to injury (Ye et al., 2013).
While these data suggest that epileptic activity may decrease myelin content, a case study involving early postnatal epilepsy due to hemimegalencephaly describes a contradictory finding; researchers found an acceleration in developmental myelination in the hemimegalencephalic, seizing cerebral hemisphere, but not the anatomically and electrophysiologically normal contralateral hemisphere (Goldsberry et al., 2011). The various correlations between seizures and either increased or decreased myelination indicate that numerous variables could influence the effects of epileptic neuronal activity on myelin-forming cells, including developmental stage, location of epileptic foci, neuronal firing pattern, and the frequency and duration of aberrant seizure activity. While intriguing associations between recurrent seizure activity, DTI abnormalities and limited histological information exist, the relationship between seizure activity and its effect on myelin structure and plasticity remains incompletely characterized.
Conclusions
It is becoming increasingly clear that myelin development and remodeling throughout adulthood is a continually adaptive and dynamic process, modulated by neuronal activity and critical for normal brain function. The molecular mechanism(s) contributing to neuronal activity-induced myelination remain incompletely understood and could include neurotransmitter or neurotrophic factor-mediated processes. Though co-culture systems, integrating oligodendrocytes and neurons in vitro, have greatly increased our level of understanding of neuronal-glial communication, more work in vivo is necessary to elucidate how this complex array of activity-regulated signaling molecules converge to regulate myelin-forming cells. This level of understanding would shed light on possible contribution of dysregulated or dysfunctional myelin plasticity in disease. There are many important questions still unanswered. Given the plasticity evident in the healthy brain, why do demyelinated lesions lose the ability to remyelinate in MS? Can these mechanisms be harnessed to improve neurological recovery after injury? How can we mitigate the putatively aberrant myelination seen in diseases such as epilepsy, schizophrenia, and bipolar disorder? How do cancers like high-grade gliomas hijack mechanisms of myelin plasticity to promote glioma growth and potentially gliomagenesis, and can neuron-glioma interactions be disrupted or redirected? As technologies for interrogating cell-cell communication and mapping neural circuits advance, these questions and more will come to light.
Figure 1. Myelin plasticity in health and disease.
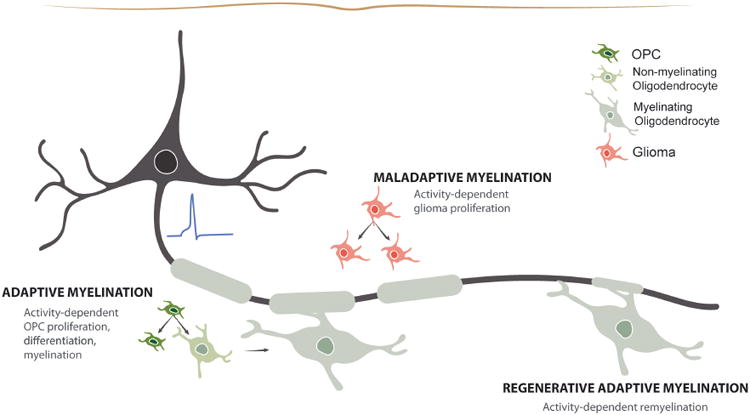
In the healthy brain, neuronal activity can influence OPC proliferation, differentiation, and myelination to facilitate adaptive myelin changes in active circuits. However, in diseases such as high-grade glioma, these mechanisms of plasticity may be hijacked to promote cancer progression, becoming maladaptive. In disease of white matter degeneration, the mechanisms of adaptive myelination may be compromised resulting in persistent demyelination or may be able to be harnessed to regenerate lost myelin.
Acknowledgments
The authors gratefully acknowledge support from the National Institute of Neurological Disorders and Stroke (NINDS R01NS092597 to M.M.), the California Institute for Regenerative Medicine (CIRM RN3-06510 to M.M.) and the Stanford Bio-X Genentech Fellowship (A.G.).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Llaguno SR, Chen Y, McKay RM, Parada LF. Stem cells in brain tumor development. Curr Top Dev Biol. 2011;94:15–44. doi: 10.1016/B978-0-12-380916-2.00002-4. [DOI] [PubMed] [Google Scholar]
- Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. British journal of cancer. 2016;115:1445–1450. doi: 10.1038/bjc.2016.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Llaguno SR, Wang Z, Sun D, Chen J, Xu J, Kim E, Hatanpaa KJ, Raisanen JM, Burns DK, Johnson JE, Parada LF. Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes. Cancer cell. 2015;28:429–440. doi: 10.1016/j.ccell.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. Journal of neuroimmunology. 2005;167:215–218. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Baraban M, Mensch S, Lyons DA. Adaptive myelination from fish to man. Brain research. 2016;1641:149–161. doi: 10.1016/j.brainres.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Bellani M, Boschello F, Delvecchio G, Dusi N, Altamura CA, Ruggeri M, Brambilla P. DTI and Myelin Plasticity in Bipolar Disorder: Integrating Neuroimaging and Neuropathological Findings. Frontiers in psychiatry. 2016;7:21. doi: 10.3389/fpsyt.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, Macklin WB. Conditional ablation of raptor or rictor has differential impact on oligodendrocyte differentiation and CNS myelination. J Neurosci. 2014;34:4466–4480. doi: 10.1523/JNEUROSCI.4314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Richardson WD. Oligodendrocyte Development and Plasticity. Cold Spring Harbor perspectives in biology. 2015;8:a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Cabral H, Rosene DL, Peters A. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. The Journal of comparative neurology. 2010;518:3046–3064. doi: 10.1002/cne.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17:1269–1274. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Buckingham SC, Sontheimer H. Human glioma cells induce hyperexcitability in cortical networks. Epilepsia. 2012;53:1360–1370. doi: 10.1111/j.1528-1167.2012.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Walshe M, Dempster E, Collier DA, Marshall N, Bramon E, Murray RM, McDonald C. The association of white matter volume in psychotic disorders with genotypic variation in NRG1, MOG and CNP: a voxel-based analysis in affected individuals and their unaffected relatives. Translational psychiatry. 2012;2:e167. doi: 10.1038/tp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R, Shields WD. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Kohler K. Brain-derived neurotrophic factor/neurotrophin-4 receptor TrkB is localized on ganglion cells and dopaminergic amacrine cells in the vertebrate retina. The Journal of comparative neurology. 1997;386:149–160. [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML, Crunelli V, Hyder F, Blumenfeld H. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. NeuroImage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF, Jackson GD. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47:399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Chavarria-Siles I, White T, de Leeuw C, Goudriaan A, Lips E, Ehrlich S, Turner JA, Calhoun VD, Gollub RL, Magnotta VA, Ho BC, Smit AB, Verheijen MH, Posthuma D. Myelination-related genes are associated with decreased white matter integrity in schizophrenia. Eur J Hum Genet. 2016;24:381–386. doi: 10.1038/ejhg.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrast R, Saher G, Nave KA, Verheijen MH. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. Journal of lipid research. 2011;52:419–434. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoz L, Simons M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. BioEssays: news and reviews in molecular, cellular and developmental biology. 2015;37:60–69. doi: 10.1002/bies.201400127. [DOI] [PubMed] [Google Scholar]
- de Zwarte SM, Johnston JA, Cox Lippard ET, Blumberg HP. Frontotemporal White Matter in Adolescents with, and at-Risk for, Bipolar Disorder. Journal of clinical medicine. 2014;3:233–254. doi: 10.3390/jcm3010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith M, Dutt A, Fonville L, Zammit S, Reichenberg A, Evans CJ, Lewis G, Jones DK, David AS. Mediation of Developmental Risk Factors for Psychosis by White Matter Microstructure in Young Adults With Psychotic Experiences. JAMA psychiatry. 2016;73:396–406. doi: 10.1001/jamapsychiatry.2015.3375. [DOI] [PubMed] [Google Scholar]
- Dries DR, Zhu Y, Brooks MM, Forero DA, Adachi M, Cenik B, West JM, Han YH, Yu C, Arbella J, Nordin A, Adolfsson R, Del-Favero J, Lu QR, Callaerts P, Birnbaum SG, Yu G. Loss of Nicastrin from Oligodendrocytes Results in Hypomyelination and Schizophrenia with Compulsive Behavior. The Journal of biological chemistry. 2016;291:11647–11656. doi: 10.1074/jbc.M116.715078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Ongur D. Probing myelin and axon abnormalities separately in psychiatric disorders using MRI techniques. Frontiers in integrative neuroscience. 2013;7:24. doi: 10.3389/fnint.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci. 2006;31:366–375. doi: 10.1016/j.mcn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13:287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nature reviews. Neuroscience. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frota ER, Rodrigues DH, Donadi EA, Brum DG, Maciel DR, Teixeira AL. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neuroscience letters. 2009;460:130–132. doi: 10.1016/j.neulet.2009.05.057. [DOI] [PubMed] [Google Scholar]
- Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci. 2014;34:8186–8196. doi: 10.1523/JNEUROSCI.4267-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao RP, Kasina A, McNeill RS, Harbin JE, Foreman O, Verhaak RG, Nishiyama A, Miller CR, Zong H. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc Natl Acad Sci U S A. 2014;111:E4214–4223. doi: 10.1073/pnas.1414389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier HO, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Lao-Peregrin C, Reynolds R, Franklin RJ, Karadottir RT. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nature communications. 2015;6:8518. doi: 10.1038/ncomms9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, Karram K, Renier N, Tessier-Lavigne M, Rossner MJ, Karadottir RT, Nave KA. A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat Neurosci. 2017;20:10–15. doi: 10.1038/nn.4425. [DOI] [PubMed] [Google Scholar]
- Goldsberry G, Mitra D, MacDonald D, Patay Z. Accelerated myelination with motor system involvement in a neonate with immediate postnatal onset of seizures and hemimegalencephaly. Epilepsy & behavior: E&B. 2011;22:391–394. doi: 10.1016/j.yebeh.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, Smit AB, Posthuma D, Verheijen MH. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 2014;40:925–935. doi: 10.1093/schbul/sbt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE, Croft RJ, Maller JJ, Fitzgerald PB. White matter correlates of episodic memory encoding and retrieval in schizophrenia. Psychiatry research. 2016;254:188–198. doi: 10.1016/j.pscychresns.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60:476–486. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A, Miers L, Burns T, Itoh T, Shen H, Lee E, Sohn J, Pleasure D. Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J Neurosci. 2012;32:639–645. doi: 10.1523/JNEUROSCI.4073-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol. 2010;68:703–716. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Current biology: CB. 2007;17:R29–35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. Journal of the International Neuropsychological Society: JINS. 2007;13:12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger J, Nave KA. Adapting brain metabolism to myelination and long-range signal transduction. Glia. 2014;62:1749–1761. doi: 10.1002/glia.22737. [DOI] [PubMed] [Google Scholar]
- Hu X, Schlanger R, He W, Macklin WB, Yan R. Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression. FASEB J. 2013;27:1868–1873. doi: 10.1096/fj.12-224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Abbott LC. Development of the paramedian lobule of the cerebellum in wild-type and tottering mice. Developmental neuroscience. 1992;14:386–393. doi: 10.1159/000111687. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20:396–405. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bachmann L, Norrmen C, Trotzmuller M, Kofeler H, Ruegg MA, Hall MN, Suter U. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J Neurosci. 2014;34:8432–8448. doi: 10.1523/JNEUROSCI.1105-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chong SY, Tuck SJ, Corey JM, Chan JR. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nature protocols. 2013;8:771–782. doi: 10.1038/nprot.2013.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nature methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Ongur D, Sperry SH, Cohen BM, Sehovic S, Goldbach JR, Du F. Myelin vs axon abnormalities in white matter in bipolar disorder. Neuropsychopharmacology. 2015;40:1243–1249. doi: 10.1038/npp.2014.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia. 2013;61:732–749. doi: 10.1002/glia.22469. [DOI] [PubMed] [Google Scholar]
- Liang JS, Lee SP, Pulli B, Chen JW, Kao SC, Tsang YM, Hsieh KL. Microstructural Changes in Absence Seizure Children: A Diffusion Tensor Magnetic Resonance Imaging Study. Pediatrics and neonatology. 2016;57:318–325. doi: 10.1016/j.pedneo.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012a;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Concha L, Lebel C, Beaulieu C, Gross DW. Mesial temporal sclerosis is linked with more widespread white matter changes in temporal lobe epilepsy. NeuroImage. Clinical. 2012b;1:99–105. doi: 10.1016/j.nicl.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Charles FC, Attwell D, Karadottir RT. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS biology. 2013;11:e1001743. doi: 10.1371/journal.pbio.1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Hu Q, Zhang Q, Hong S, Tang X, Cheng L, Jiang L. Alterations in hippocampal myelin and oligodendrocyte precursor cells during epileptogenesis. Brain research. 2015;1627:154–164. doi: 10.1016/j.brainres.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Makar TK, Bever CT, Singh IS, Royal W, Sahu SN, Sura TP, Sultana S, Sura KT, Patel N, Dhib-Jalbut S, Trisler D. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. Journal of neuroimmunology. 2009;210:40–51. doi: 10.1016/j.jneuroim.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Hall J, Lymer GK, Sussmann JE, Lawrie SM. Genetic risk for white matter abnormalities in bipolar disorder. International review of psychiatry. 2009;21:387–393. doi: 10.1080/09540260902962180. [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Viding E, Pettersson-Yeo W, Tognin S, McGuire PK. Genetic variation in neuregulin1 is associated with differences in prefrontal engagement in children. Hum Brain Mapp. 2009;30:3934–3943. doi: 10.1002/hbm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Misawa S, Kuwabara S, Hirano S, Shibuya K, Arai K, Hattori T. Epilepsia partialis continua as an isolated manifestation of motor cortical dysplasia. Journal of the neurological sciences. 2004;225:157–160. doi: 10.1016/j.jns.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J Neurosci. 2015;35:14002–14008. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2011;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount CW, Monje M. Wrapped to Adapt: Experience-Dependent Myelination. Neuron. 2017;95:743–756. doi: 10.1016/j.neuron.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja S, Vitanza NA, Woo PJ, Taylor KR, Liu F, Zhang L, Li M, Meng W, Ponnuswami A, Sun W, Ma J, Hulleman E, Swigut T, Wysocka J, Tang Y, Monje M. Transcriptional Dependencies in Diffuse Intrinsic Pontine Glioma. Cancer cell. 2017 doi: 10.1016/j.ccell.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich LK, Pasternak O, Shenton ME, Kubicki M, Gong X, McCarthy-Jones S, Whitford TJ Australian Schizophrenia Research B. Abnormal white matter microstructure and increased extracellular free-water in the cingulum bundle associated with delusions in chronic schizophrenia. NeuroImage Clinical. 2016;12:405–414. doi: 10.1016/j.nicl.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2014;276:135–147. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham H, Giuffrida L, Wood R, Gonsalvez D, Ferner A, Kilpatrick TJ, Murray SS, Xiao J. Fyn is an intermediate kinase that BDNF utilizes to promote oligodendrocyte myelination. Glia. 2016;64:255–269. doi: 10.1002/glia.22927. [DOI] [PubMed] [Google Scholar]
- Persson AI, Petritsch C, Swartling FJ, Itsara M, Sim FJ, Auvergne R, Goldenberg DD, Vandenberg SR, Nguyen KN, Yakovenko S, Ayers-Ringler J, Nishiyama A, Stallcup WB, Berger MS, Bergers G, McKnight TR, Goldman SA, Weiss WA. Non-stem cell origin for oligodendroglioma. Cancer cell. 2010;18:669–682. doi: 10.1016/j.ccr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Progress in brain research. 2002;136:455–465. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Peters A, Verderosa A, Sethares C. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia. 2008;56:1151–1161. doi: 10.1002/glia.20686. [DOI] [PubMed] [Google Scholar]
- Proctor DT, Stotz SC, Scott LO, de la Hoz CL, Poon KW, Stys PK, Colicos MA. Axo-glial communication through neurexin-neuroligin signaling regulates myelination and oligodendrocyte differentiation. Glia. 2015 doi: 10.1002/glia.22875. [DOI] [PubMed] [Google Scholar]
- Purger D, Gibson EM, Monje M. Myelin plasticity in the central nervous system. Neuropharmacology. 2016;110:563–573. doi: 10.1016/j.neuropharm.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Reid MA, White DM, Kraguljac NV, Lahti AC. A combined diffusion tensor imaging and magnetic resonance spectroscopy study of patients with schizophrenia. Schizophrenia research. 2016;170:341–350. doi: 10.1016/j.schres.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–545. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cruces R, Concha L. White matter in temporal lobe epilepsy: clinico-pathological correlates of water diffusion abnormalities. Quantitative imaging in medicine and surgery. 2015;5:264–278. doi: 10.3978/j.issn.2223-4292.2015.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel A, Ortiz FC, Kerninon C, Maldonado PP, Angulo MC, Nait-Oumesmar B. Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Frontiers in cellular neuroscience. 2015;9:77. doi: 10.3389/fncel.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S, Halliday WC, Nomura R, Ochi A, Otsubo H. Increased population of oligodendroglia-like cells in pediatric intractable epilepsy. Neuroscience letters. 2014;566:188–193. doi: 10.1016/j.neulet.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, DeLuca GC, Miller KL, Taylor A, Thomas N, Kleim J, Sibson NR, Bannerman D, Johansen-Berg H. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci. 2013;33:19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Schneider S, Gruart A, Grade S, Zhang Y, Kroger S, Kirchhoff F, Eichele G, Delgado Garcia JM, Dimou L. Decrease in newly generated oligodendrocytes leads to motor dysfunctions and changed myelin structures that can be rescued by transplanted cells. Glia. 2016;64:2201–2218. doi: 10.1002/glia.23055. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH. Regulation of conduction time along axons. Neuroscience. 2014;276:126–134. doi: 10.1016/j.neuroscience.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz J, Zuo JX, Lyall AE, Makris N, Kikinis Z, Bouix S, Pasternak O, Fredman E, Duskin J, Goldstein JM, Petryshen TL, Mesholam-Gately RI, Wojcik J, McCarley RW, Seidman LJ, Shenton ME, Koerte IK, Kubicki M. Tractography Analysis of 5 White Matter Bundles and Their Clinical and Cognitive Correlates in Early-Course Schizophrenia. Schizophr Bull. 2016;42:762–771. doi: 10.1093/schbul/sbv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- Stefanits H, Czech T, Pataraia E, Baumgartner C, Derhaschnig N, Slana A, Kovacs GG. Prominent oligodendroglial response in surgical specimens of patients with temporal lobe epilepsy. Clinical neuropathology. 2012;31:409–417. doi: 10.5414/np300536. [DOI] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Hanecker P, Ayers-Ringler J, Phillips J, Siu J, Lim DA, Vandenberg S, Stallcup W, Berger MS, Bergers G, Weiss WA, Petritsch C. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer cell. 2011;20:328–340. doi: 10.1016/j.ccr.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk LM, Brozko N, Nagalski A, Rockle I, Werneburg S, Hildebrandt H, Wisniewska MB, Kuznicki J. ST8SIA2 promotes oligodendrocyte differentiation and the integrity of myelin and axons. Glia. 2017;65:34–49. doi: 10.1002/glia.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. American Journal of Physiology -- Legacy Content. 1939;127:211–227. [Google Scholar]
- Tate MC, Lindquist RA, Nguyen T, Sanai N, Barkovich AJ, Huang EJ, Rowitch DH, Alvarez-Buylla A. Postnatal growth of the human pons: a morphometric and immunohistochemical analysis. The Journal of comparative neurology. 2015;523:449–462. doi: 10.1002/cne.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe SK, Reading SA, Rivkin P, Caffo B, Schweizer B, Pearlson G, Potash JB, Depaulo JR, Bassett SS. Total white matter hyperintensity volume in bipolar disorder patients and their healthy relatives. Bipolar disorders. 2012;14:888–893. doi: 10.1111/bdi.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Torii T, Miyamoto Y, Takada S, Tsumura H, Arai M, Nakamura K, Ohbuchi K, Yamamoto M, Tanoue A, Yamauchi J. In vivo knockdown of ErbB3 in mice inhibits Schwann cell precursor migration. Biochemical and biophysical research communications. 2014;452:782–788. doi: 10.1016/j.bbrc.2014.08.156. [DOI] [PubMed] [Google Scholar]
- Tsiperson V, Huang Y, Bagayogo I, Song Y, VonDran MW, DiCicco-Bloom E, Dreyfus CF. Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN neuro. 2015;7 doi: 10.1177/1759091414566878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, Wood TL. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29:6367–6378. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. Journal of neuroscience research. 2009;87:69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh H, SM M. Neuronal Activity in Ontogeny and Oncology. Trends in Cancer. 2017;3:89–112. doi: 10.1016/j.trecan.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondran MW, Clinton-Luke P, Honeywell JZ, Dreyfus CF. BDNF+/- mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia. 2010;58:848–856. doi: 10.1002/glia.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31:14182–14190. doi: 10.1523/JNEUROSCI.6595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Topics in magnetic resonance imaging: TMRI. 2008;19:97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- Widjaja E, Geibprasert S, Otsubo H, Snead OC, 3rd, Mahmoodabadi SZ. Diffusion tensor imaging assessment of the epileptogenic zone in children with localization-related epilepsy. AJNR Am J Neuroradiol. 2011;32:1789–1794. doi: 10.3174/ajnr.A2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AW, Giuffrida L, Wood R, Peckham H, Gonsalvez D, Murray SS, Hughes RA, Xiao J. TDP6, a brain-derived neurotrophic factor-based trkB peptide mimetic, promotes oligodendrocyte myelination. Mol Cell Neurosci. 2014;63:132–140. doi: 10.1016/j.mcn.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J Neurosci. 2013;33:4947–4957. doi: 10.1523/JNEUROSCI.3990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neuro-Signals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu Y, Kong XR, Zhang L, Qiu X, Gao Y, Huang CX, Chao FL, Wang SR, Tang Y. The myelinated fiber loss in the corpus callosum of mouse model of schizophrenia induced by MK-801. Journal of psychiatric research. 2015;63:132–140. doi: 10.1016/j.jpsychires.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Yakovlev P. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific Publications; 1967. pp. 3–70. [Google Scholar]
- Yang T, Guo Z, Luo C, Li Q, Yan B, Liu L, Gong Q, Yao D, Zhou D. White matter impairment in the basal ganglia-thalamocortical circuit of drug-naive childhood absence epilepsy. Epilepsy research. 2012;99:267–273. doi: 10.1016/j.eplepsyres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Ye Y, Xiong J, Hu J, Kong M, Cheng L, Chen H, Li T, Jiang L. Altered hippocampal myelinated fiber integrity in a lithium-pilocarpine model of temporal lobe epilepsy: a histopathological and stereological investigation. Brain research. 2013;1522:76–87. doi: 10.1016/j.brainres.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisen J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- You Y, Bai H, Wang C, Chen LW, Liu B, Zhang H, Gao GD. Myelin damage of hippocampus and cerebral cortex in rat pentylenetetrazol model. Brain research. 2011;1381:208–216. doi: 10.1016/j.brainres.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Bi W, Liu C, Zhao Y, Zhang D, Yue W. A hypothesis-driven pathway analysis reveals myelin-related pathways that contribute to the risk of schizophrenia and bipolar disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2014;51:140–145. doi: 10.1016/j.pnpbp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Vilaythong AP, Yoshor D, Noebels JL. Elevated thalamic low-voltage-activated currents precede the onset of absence epilepsy in the SNAP25-deficient mouse mutant coloboma. J Neurosci. 2004;24:5239–5248. doi: 10.1523/JNEUROSCI.0992-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


