Abstract
Rates of type 2 diabetes mellitus (T2DM) are rising rapidly across the globe and the impact of this devastating disease threatens to plague the 21st century. While some contributing factors are well-recognized (e.g. sedentary lifestyles and caloric excess), others diabetes-promoting risk factors are less established or poorly appreciated. The latter category includes environmental exposures to diabetogenic contaminants. Herein we review some of the latest concepts and mechanisms by which environmental exposures may contribute to rising rates of T2DM with a particular focus on mechanisms involving mitochondrial dysfunction and imbalances in reactive oxygen species (ROS). Furthermore, while the pathogenesis of diabetes includes impairments in insulin sensitivity as well as insulin secretion, we will specifically delve into the links between environmental exposures to toxicants such as arsenic and disruptions in insulin release from pancreatic β-cells. Since β-cell death or dysfunction lies at the heart of both T2DM as well as type 1 diabetes mellitus (T1DM), environmental endocrine disrupting chemicals (EDCs) that disrupt the production or regulated release of the glucose-lowering hormone insulin are likely contributors to diabetes risk. Importantly, understanding the contribution of toxicants to diabetes risk as well as improved understanding of their mechanisms of action offer unique opportunities to modulate diabetes risk via targeted therapeutics or public policy interventions to reduce and remediate exposures.
Keywords: Arsenic, Selenium, Oxidative Stress, Type 2 Diabetes, Endocrine Disrupting Chemicals
Introduction
Projected to afflict 642 million individuals globally by 2040 [31], diabetes contributes to significant morbidity and mortality. In the U.S. diabetes is the leading cause of adult blindness, non-traumatic amputations, and kidney failure as well as a potent contributor to cardiovascular disease and an important driver of societal medical costs [32–34]. Thus, identification and remediation of factors that promote diabetes pathogenesis, including environmental toxicants, have the potential to alleviate significant human suffering.
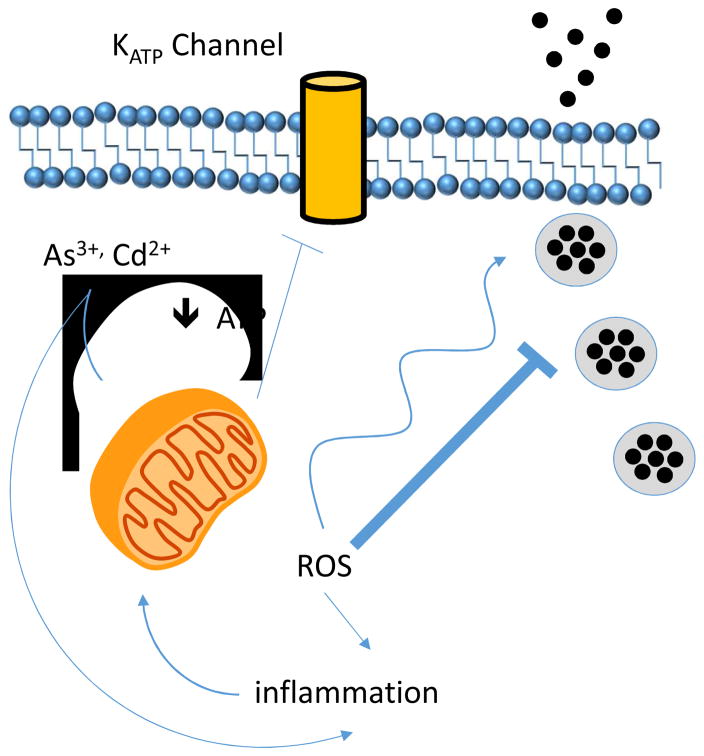
Diabetes is a complex metabolic disease that is characterized by impairments in the secretion or action of the glucose-lowering hormone insulin. While increasing evidence implicates a variety of toxicants in the induction of insulin resistance [39–41], little is known about the mechanisms underlying these biological effects and still less is understood regarding how environmental toxicants disrupt insulin release, a pathological process central to the development of both type 2 diabetes mellitus (T2DM) (relative insulin deficiency) and type 1 diabetes (T1DM) (absolute deficiency). Pancreatic β-cells located in the islets of Langerhans synthesize insulin in a process that is regulated by multiple transcription factors and proceeds through a series of biosynthetic steps that include processing in the Golgi and endoplasmic reticulum as well as within maturing insulin granules. To maintain glucose levels within a tight physiological range, β-cells in the pancreatic islets of Langerhans couple insulin secretion to circulating glucose levels. Glucose enters β-cells via glucose transporters and is metabolized, first by glycolysis (with glucokinase being the rate-limiting step) and then mitochondrial oxidation, which accounts for the fate of nearly all glucose entering β-cells. Catabolism of glucose generates ATP and raises the intracellular ATP/ADP ratio, which promotes the closure of ATP-dependent potassium (KATP) channels. Closure of KATP channels induces β-cell depolarization and opening of voltage-gated Ca2+ channels. This rise in cellular Ca2+ levels then promotes insulin granule exocytosis, resulting in a rise in circulating insulin levels. Released insulin then binds to its receptor on the surface of insulin-responsive tissues (e.g. adipose, muscle, and liver) where it activates an intracellular signal transduction cascade that shifts cellular metabolism from catabolic process to anabolic processes, including glucose uptake and clearance from the circulation. Thus, pathological processes that impair insulin synthesis, release, or signaling promote hyperglycemia and the development of diabetes.
Reactive Oxygen Species (ROS) and Pathways of Diabetes Pathogenesis
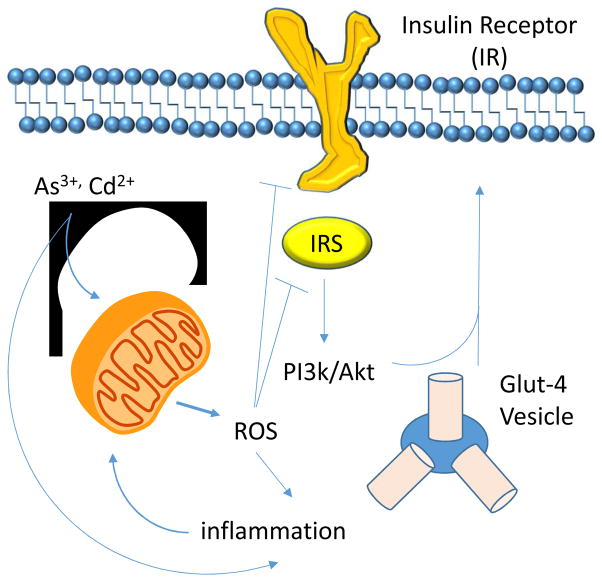
The terminology “reactive oxygen species” or “ROS” refer to a broad family of related chemical species that share little in common. In fact, most ROS are indeed reactive, which by extension suggests they are short-lived species. In vivo, most biologically relevant ROS will exist for amounts of time that range from nanoseconds to less than a few seconds. Chemically, however, ROS differ widely [1]. Some are oxidants such as hydrogen peroxide (H2O2), peroxynitrite (ONOO−/ONOOH), and free radicals derived from these species [2, 3]. Others, such as superoxide radical (O2•−), are contextual oxidants behaving either as a reductant or an oxidant depending on certain variables (such as pH, electronic structure of the reactant, and concentration) [1]. Here, we will focus on discussing the role of O2•− and its decomposition product H2O2 as major contributors to oxidative stress originating from changes in mitochondrial function caused by environmental toxicants such as arsenic (As) and other diabetogenic heavy metals. In fact both O2 •− and H2O2 have been linked to insulin resistance and diabetes development either because of their activities as modulators of signaling or because of damaging oxidative reactions [4–6]. Oxidative stress is a powerful force compromising the viability of insulin-producing pancreatic β-cell. It has also been shown to change the capacity of target cells to sense insulin or react to it appropriately [7, 8]. For instance it has been demonstrated that ROS can initiate pro-oxidative reactions that change how the insulin receptor and insulin receptor substrates (IRS1/2) propagates signaling upon insulin binding as well as how downstream players are engaged in response to it [9–12]. For example, modification of IRS proteins by oxidative modifications disrupts activation of this molecule in response to insulin in a manner that resembles aberrant signaling seen in diabetic patients. Hence it appears that mechanisms initiating, extending, or amplifying oxidative stress can have a meaningful impact on the risk of developing insulin resistance and diabetes. Furthermore, evidence suggests that ROS modulate insulin secretion from pancreatic β-cells. Thus, ROS likely modulates diabetes risk by altering both insulin production and action. In this regard some risk factors leading to diabetes, in part via increased ROS production, are well-established (e.g. caloric excess, obesity, physical inactivity); however, others are less appreciated (e.g. environmental exposures). Here, we will focus on the latest advances towards a better understanding of the role of heavy metals, and As in particular, on β-cell function and consequential diabetes risk.
Arsenic, Selenium, and Mitochondrial ROS
As is an environmental contaminant endemic in certain regions of the US, China, Taiwan, South America, Africa, India, and Bangladesh. Though it is a well-established human carcinogen, more recent studies provide significant evidence that As is also a risk factor for type 2 diabetes (T2DM) [13–15]. As is known to be a potent inducer of oxidative stress, which is partially due to its activity as a disruptor of the mitochondrial electron transport chain [16, 17]. Studies on the mechanisms by which As promotes an increase in ROS production in mitochondria indicate that As dampens the expression of sirtuin-3 a mitochondrial deacetylase responsible for activating several of the ETC complexes as well as the superoxide dismutase activity of SOD2 [18]. Acetylation of ETC complexes and SOD2 have been connected with an increase in mitochondrial ROS, namely O2•− and a switch in cellular metabolism to a reliance on glycolysis for ATP production [19, 20]. This metabolic shift, which is driven by both a reduction in mitochondrial-derived ATP and increased ROS, may contribute to an increase in reactive carbonyls such as methylglyoxal and acetoacetate, which have been proposed to be themselves important contributors to diabetes development. Hence, it appears that As may have a direct impact on mitochondrial metabolism, cellular respiration, and ATP synthesis.
Selenium is a naturally occurring micronutrient essential for the function of seleno-enzymes that rely on a selenocysteine moiety as the active amino acid. Because of its high affinity for As, dietary selenium has been proposed as a countermeasure to manage the detrimental health effects of As on populations living in areas where the risk of exposure is high (to be further discussed below) [21–23]. Interestingly, one major component of the mitochondrial antioxidant system involves a selenoprotein called glutathione-peroxidase 1 (GPx-1). GPx-1 catalyzes the reduction of H2O2 originating from O2•-decomposition [24]. In this reaction, one molecule of glutathione (GSH) is consumed to reduce one H2O2 equivalent to H2O making the reaction catalyzed by GPx-1 an essential component of the chain of antioxidant reactions that convert potentially harmful ROS to inoffensive H2O. We contend that an additional mechanism by which As may amplify oxidative stress is via inactivation of GPx-1. This concept is supported by findings indicating that cells exposed to inorganic As exhibit reduced GPx-1 activity in the absence of changes in intracellular GSH; this suggests that As may directly impair the capacity of GPx-1 to detoxify H2O2 [25].
It is noteworthy however, that an increase in the production of ROS by mitochondria can and often does become a self-propelling process since ROS damage mitochondrial components ranging from complexes of the ETC to membranes and mitochondrial DNA. Thus, ROS and mitochondrial dysfunction are intimately linked and interrelated. Most data currently available seem to support the notion that these injuries contribute to the amplification of mtROS production and oxidative stress in cells.
Oxidative Stress, Inflammation, and Diabetes
It is challenging to establish the order of events connecting diabetes, inflammation, and oxidative stress since these processes happen in parallel, share common mechanisms, and are propelled or amplified by each other. Thus, disentangling these processes from one another is extremely difficult. Nevertheless, exposure to environmental toxicants, and heavy metals in particular, notably induce oxidative stress, either because of their effects in disrupting mitochondrial function or because of redox cycling reactions that convert the antioxidant pool into a potential endogenous source of ROS. Either directly through signaling effects or via damaging cells and tissues, ROS are pro-inflammatory agents chemically produced in the interaction of metals with most biomolecules. Hence, predisposition to or activation of inflammation are potential mechanisms connecting metal exposures to increased diabetes risk. In fact, significant research is available indicating that the oxidation of critical thiol residues in numerous proteins involved in the activation or resolution of inflammation is, to a significant extent, involved with the initiation, extension or amplification of inflammation [26, 27] that in turn promotes many cellular and molecular changes leading to diabetes. Inflammatory and oxidative damage to β-cells is a major cause of β-cell dysfunction and eventual β-cell death, which shuts down the capacity of the pancreas to produce and secrete the glucose-lowering hormone insulin. Nevertheless, β-cells are just one target in the complex array of events underlying insulin resistance, impaired insulin secretion, and the ultimate evolution of diabetes. For instance, inflammatory damage to caveolin-1 (Cav-1, a scaffold protein on the membrane of endothelial cells) causes its depletion [28, 29]. Since Cav-1 is the major non-lipid component of caveolae, loss of Cav-1 drastically diminishes the ability of endothelial cells to transport insulin from circulation into tissue limiting insulin access and signaling in target organs [30]. Just like the pancreas and the vasculature, the liver is another major target of inflammation and oxidative stress. Changes to the metabolism of sugars and lipids in the liver as well as to mechanisms of insulin sensing promote systemic metabolic adaptations leading to immune cell activation and the amplification of inflammation. Hence, whether exacerbating oxidative and inflammatory damage initiated by other organic causes or acting as direct inflammatory agents, heavy metals and As are likely to increase the risk of insulin resistance and ultimately diabetes via activation of inflammatory pathways in a number of organ systems regulating glucose homeostasis.
Arsenic as an Environmental Diabetogen
While some controversy persists in the literature due to differences in exposure levels, measures of glucose regulation, and statistical methodology, there is strong epidemiological data linking arsenic exposure to diabetes risk. Indeed, in studies of high drinking water exposures (i.e. levels exceeding 150 μg/L) that also provocatively examined glucose regulation, arsenic exposure was associated with relative risks of diabetes of 2.1–10.05 (95% confidence interval: 1.3–77.9) [35]. Similarly, studies that have used quality assessments of glycemia have associated lower levels of exposure with diabetes risk as well [14, 36, 37]. In a more recent meta-analysis in which diabetes was confirmed by biochemical testing or from medical charts, arsenic was associated with a relative risk of diabetes of 1.71 (95% confidence interval: 1.32–2.23) [38]. These data support and are consistent with the findings of the National Toxicology Program, which in 2012 found that the evidence supported an arsenic-diabetes association [35]. Less is known, however, about the mechanisms by which arsenic exposure increases diabetes risk.
As discussed above, As is known to disrupt mitochondrial function, leading to a shift in energy metabolism away from oxidative phosphorylation and toward glycolysis. Because the function of pancreatic β-cells is to tightly couple insulin secretion to glucose levels (sensed as a rise in ATP levels resulting from glycolysis and oxidative phosphorylation), toxicants that impair ATP production by poisoning mitochondrial function are likely to impair insulin release. The consequence of this impairment is insufficient insulin responses to rising glucose levels, consequential chronic hyperglycemia, and ultimately diabetes.
Importantly, several pathways modulate insulin biosynthesis and secretion, and disruptions of these pathways may underlie how environmental toxicants like arsenic promote diabetes risk. Of particular interest is evidence that ROS modulate β-cell function in a complex fashion. Due to the high demand for oxidative metabolism, β-cells generate significant amounts of diverse ROS yet express lower levels of antioxidants compared to other tissues [42]; moreover, pathways implicated in metabolic deterioration such as insulin resistance augment ROS generation in β-cells via increased free fatty acid delivery and metabolism [43]. Importantly, oxidative stress has been implicated in β-cell dysfunction directly [44]. Noteworthy, however, there is also intriguing evidence that some ROS can augment insulin secretion. In isolated mouse islets and the INS-1 cell line, exogenous H2O2 stimulated insulin secretion, an effect mimicked by supplementation with diethyl maleate, which raises intracellular H2O2 levels [45]. In contrast, glucose-stimulated insulin secretion (GSIS) was antagonized by cell permeable catalase or the antioxidant N-acetyl cysteine [45]. In an alternative model using isolated rat islets, mitochondria-derived ROS was essential for insulin release, an effect mimicked by disruption of mitochondrial complexes and inhibited by the addition of antioxidants [46]. Collectively, this suggests that reductions in H2O2 attenuate insulin secretion, an effect predicted to promote hyperglycemia. Importantly, this argues that the tight regulation of ROS production as well as the regulation of steady-state, basal levels strongly impact insulin release, indicating how critical it is to maintain a balanced cellular redox state. Exposure to pro-oxidant toxicants like arsenic are likely to disrupt this balance and thereby impair regulated insulin release.
Selenium, Diabetes, and Modulation of Arsenic Risk
Selenoproteins exist at the core of pathways regulating cellular ROS. These proteins include enzymes such as GPx-1 (discussed above). Genetic models of GPx-1 disruption point toward a significant role for selenoproteins in glucose regulation. For example, global deletion of the antioxidant selenoprotein GPx-1 coupled with a high fat diet reduces pancreatic insulin content and impairs glucose-stimulated insulin secretion [47], while global GPx-1 overexpression promotes insulin hypersecretion and expanded β-cell mass [48, 49]. While not a selenoprotein, catalase engages in similar redox reactions in cells facilitating elimination of H2O2. Genetic manipulation of catalase expression yields effects similar to those observed with GPx-1. Specifically, catalase overexpression confers protection from streptozotocin (STZ)-induced diabetes [50], while catalase knockout models are sensitized to alloxan-induced (but not STZ-induced) diabetes [51]. Both STZ and alloxan are inducers of oxidative stress. Collectively, these data suggest that modulation of selenoproteins or related pathways that alter ROS handling have profound implications on diabetes risk. Furthermore, these data suggest that the toxicity of ROS modulators like As is likely to be influenced by the functional status of these pathways.
Indeed, the relationship between selenium and As has a long history. In 1938, Moxon showed that rats could be rescued from selenium toxicity with the administration of As [52]. Since that time, it has been shown that these two metalloids have complex biological interactions. Indeed, selenium facilitates As elimination [53]. Furthermore, higher blood selenium levels are associated with lower As levels [54], reduced As-associated premalignant skin lesions [55], and better motor function in As-exposed children [56]. While some of these effects are undoubtedly a consequence of chemical interactions between As and Se, it is also likely that selenoproteins play an essential role in those relationships since expression of several selenoproteins is regulated by selenium availability.
Selenium and diabetes have a complex relationship. Selenium supplementation trials have yielded beneficial [57], neutral [58, 59], and adverse [60] effects on glucose homeostasis. The apparent inconsistency of selenium supplementation trials should not, however, be surprising given the complex role of ROS in β-cell function. Indeed, there is marked divergence in the biological effects of ROS on insulin secretion depending on whether the system is acutely or chronically manipulated. For example, acute increases in H2O2 augment insulin release while acute decreases in H2O2 impair insulin release in β-cell models [45]. Conversely, chronic overexpression of pathways that reduce H2O2 (e.g. overexpression of GPx-1 or catalase) promote insulin hypersecretion and protection from β-cell death [48–50], while chronic depletion of pathways predicted to increase H2O2 (e.g. GPx-1 or catalase knockout models) impair insulin release or promote toxin-induced β-cell death [47, 51]. This strongly suggests that β-cells require fine-tuning of ROS generation in order to dynamically regulate insulin secretion while also surviving an oxidative environment. The dynamic nature of these relationships is underscored by data from a recent meta-analysis that demonstrated a non-monotonic relationship between selenium and diabetes [61].
Furthermore, β-cells likely need to shift their ROS defense mechanisms in the face of changes in the oxidative status of the environment. This may result from underlying pathophysiological processes that increase the ROS burden, such as glucolipotoxicity stemming from systemic insulin resistance [43], or exposure to environmental toxicants like As that induce ROS. Indeed, evidence suggests that exogenous antioxidants may antagonize the deleterious effects of toxicants on insulin secretion. For example, N-acetyl cysteine was shown to mitigate the deleterious effects of As on insulin secretion [62], an effect observed in other models of As toxicity [63] and with other toxicants such as bisphenol A [64]. However, how chronic delivery of antioxidants under states of persistent oxidative stress impact the dynamic changes in ROS species that augment insulin secretion require further study. Similarly, how these antioxidants modulate mitochondrial function may further illuminate the integrated relationship between ROS, cellular metabolism, and β-cell health and function.
Broader Implications for Metabolic Toxicity
While the current discussion suggests that arsenic is associated with oxidative stress, β-cell dysfunction, and diabetes risk, other environmental toxicants are likely to promote diabetes pathogenesis via similar processes. Indeed, several compounds have been shown to increase oxidative stress in cell lines and animal models. These compounds include cadmium [65], lead [66], mercury [67–69], bisphenol A [64], diethylhexyl phthalate [70, 71], perfluorooctanoic acid [72], and tributyl tin [73]. Expression of proteins regulating oxidative stress was also shown to be modulated by polychlorinated biphenyl exposure [74]. In addition, several compounds have been specifically associated with reduced expression of antioxidant defense proteins, including cadmium [75], mercury [69], and diethylhexyl phthalate [70], while perfluorooctanoic acid was shown to upregulate expression of antioxidant enzymes [72]. Consequently, exposures to various organic and inorganic toxicants, in addition to As, are likely to disrupt β-cell function via perturbations in normal ROS balance.
Furthermore, in addition to As, other toxicants have been shown to adversely affect mitochondrial function and cellular energy metabolism. Disruption in ATP production or mitochondrial structure, function, or gene expression has also been reported for cadmium [65], mercury [67], bisphenol A [76, 77], octylphenol [77], nonylphenol [77], and triphenyl tin [78]. Furthermore, the dichlorodiphenyltrichloroethane (DDT) metabolite dichlorodiphenyldichloroethylene (DDE) has been shown to downregulate expression of glycolysis-associated proteins [79], which may also impair ATP production and glucose-insulin coupling.
Collectively, these data suggest that alterations in ROS production and handling as well as disruptions in cellular energetics that control ROS generation are potential shared mechanisms by which diverse toxicants disrupt β-cell function and potentially promote diabetes risk. Because humans are exposed to a wide array of toxicants in various combinations, the possibility that multiple environmental contaminants induce similar cellular stresses on insulin-secreting β-cells provides a conceptual framework to understand how diverse exposures may lead to a common disease phenotype (i.e. diabetes) as well as how subtoxic exposures to multiple compounds could additively or synergistically promote disease development.
Conclusion
Diabetes poses a grave threat to human health. Appreciating the environmental drivers of diabetes risk offers unique opportunities beyond typical interventions built around diet and lifestyle to stem the tide of this devastating epidemic. While exposure reduction strategies and environmental remediation must be a core focus of efforts to address toxicant-induced disease risk, understanding the molecular physiology by which diabetogenic chemicals like As impair insulin release as well as action have the potential to identify populations susceptible to diabetes development as well as targeted interventions to treat patients exposed to diabetogenic chemicals. It may also elucidate different mechanisms leading to the onset and progression of diabetes that can be specifically targeted to protect specific populations in order to reduce the risk of developing this devastating disease.
Figure 1.
Schematic representation of possible effects of environmental toxicants (e.g As3+, Cd2+) on insulin sensing pathways that may promote diabetogenesis.
Figure 2.
Schematic representation of possible effects of environmental toxicants (e.g As3+, Cd2+) on insulin secretion that may promote diabetogenesis.
HIGHLIGHTS.
Arsenic is a diabetogenic metalloid of public health relevance
Arsenic interferes with mechanisms sensing glucose as well as regulating insulin secretion
Reactive Oxygen Species are involved in the mechanisms of As-induced diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 2.Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med. 2002;32:841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 3.Bonini MG, Consolaro ME, Hart PC, Mao M, de Abreu AL, Master AM. Redox control of enzymatic functions: The electronics of life’s circuitry. IUBMB Life. 2014 doi: 10.1002/iub.1258. [DOI] [PubMed] [Google Scholar]
- 4.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadler K. Oxidative stress in diabetes. Adv Exp Med Biol. 2012;771:272–287. doi: 10.1007/978-1-4614-5441-0_21. [DOI] [PubMed] [Google Scholar]
- 6.Bir SC, Kevil CG. Diabetic neutrophil mitochondrial dysfunction: an inflammatory situation? Free Radic Biol Med. 2011;50:1213–1214. doi: 10.1016/j.freeradbiomed.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoi W, Naito Y, Tokuda H, Tanimura Y, Oya-Ito T, Yoshikawa T. Exercise-induced muscle damage impairs insulin signaling pathway associated with IRS-1 oxidative modification. Physiol Res. 2012;61:81–88. doi: 10.33549/physiolres.932239. [DOI] [PubMed] [Google Scholar]
- 8.Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, Tanaka T, Maruyama M, Katahira H, Yoshimoto K, Itagaki E, Nagamatsu S. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: Possible protection of beta cells from oxidative stress. Metabolism. 2004;53:488–494. doi: 10.1016/j.metabol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Prasannarong M, Santos FR, Hooshmand P, Hooshmand P, Giovannini FJ, Henriksen EJ. The lipid peroxidation end-product and oxidant 4-hydroxynonenal induces insulin resistance in rat slow-twitch skeletal muscle. Arch Physiol Biochem. 2014;120:22–28. doi: 10.3109/13813455.2013.834937. [DOI] [PubMed] [Google Scholar]
- 10.Cerqueira FM, da Cunha FM, Caldeira da Silva CC, Chausse B, Romano RL, Garcia CC, Colepicolo P, Medeiros MH, Kowaltowski AJ. Long-term intermittent feeding, but not caloric restriction, leads to redox imbalance, insulin receptor nitration, and glucose intolerance. Free Radic Biol Med. 2011;51:1454–1460. doi: 10.1016/j.freeradbiomed.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Pilon G, Charbonneau A, White PJ, Dallaire P, Perreault M, Kapur S, Marette A. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO(−) induced tyrosine nitration of IRS-1 in skeletal muscle. PLoS One. 2010;5:e15912. doi: 10.1371/journal.pone.0015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 2010;59:861–871. doi: 10.2337/db09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Guo X, Wu B, Yu H, Zhang X, Li M. Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci Rep. 2014;4:6894. doi: 10.1038/srep06894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, Drobna Z, Loomis D, Styblo M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health. 2011;10:73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, Hsu KH, Chen CJ. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect. 2000;108:847–851. doi: 10.1289/ehp.00108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blajszczak C, Bonini MG. Mitochondria targeting by environmental stressors: Implications for redox cellular signaling. Toxicology. 2017 doi: 10.1016/j.tox.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh B, Kulawiec M, Owens KM, Singh A, Singh KK. Sustained Early Disruption of Mitochondrial Function Contributes to Arsenic-Induced Prostate Tumorigenesis. Biochemistry (Mosc) 2016;81:1089–1100. doi: 10.1134/S0006297916100072. [DOI] [PubMed] [Google Scholar]
- 18.Padmaja Divya S, Pratheeshkumar P, Son YO, Vinod Roy R, Andrew Hitron J, Kim D, Dai J, Wang L, Asha P, Huang B, Xu M, Luo J, Zhang Z. Arsenic Induces Insulin Resistance in Mouse Adipocytes and Myotubes Via Oxidative Stress-Regulated Mitochondrial Sirt3-FOXO3a Signaling Pathway. Toxicol Sci. 2015;146:290–300. doi: 10.1093/toxsci/kfv089. [DOI] [PubMed] [Google Scholar]
- 19.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman MC, Olivier AK, Jacobus JA, Mapuskar KA, Mao G, Martin SM, Riley DP, Gius D, Spitz DR. Superoxide mediates acute liver injury in irradiated mice lacking sirtuin 3. Antioxid Redox Signal. 2014;20:1423–1435. doi: 10.1089/ars.2012.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krohn RM, Raqib R, Akhtar E, Vandenberg A, Smits JE. A high-selenium lentil dietary intervention in Bangladesh to counteract arsenic toxicity: study protocol for a randomized controlled trial. Trials. 2016;17:218. doi: 10.1186/s13063-016-1344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poojan S, Kumar S, Verma V, Dhasmana A, Lohani M, Verma MK. Disruption of Skin Stem Cell Homeostasis following Transplacental Arsenicosis; Alleviation by Combined Intake of Selenium and Curcumin. PLoS One. 2015;10:e0142818. doi: 10.1371/journal.pone.0142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argos M, Rahman M, Parvez F, Dignam J, Islam T, Quasem I, KHS, THA, Hossain Z, IPT, Rakibuz-Zaman M, Sarwar G, La Porte P, Harjes J, Anton K, Kibriya MG, Jasmine F, Khan R, Kamal M, Shea CR, Yunus M, Baron JA, Ahsan H. Baseline comorbidities in a skin cancer prevention trial in Bangladesh. Eur J Clin Invest. 2013;43:579–588. doi: 10.1111/eci.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekoue DN, He C, Diamond AM, Bonini MG. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta. 2017;1858:628–632. doi: 10.1016/j.bbabio.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang TS, Shu YF, Liu YC, Jan KY, Huang H. Glutathione peroxidase and catalase modulate the genotoxicity of arsenite. Toxicology. 1997;121:229–237. doi: 10.1016/s0300-483x(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 26.Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, Saqib U, Deng J, Chatterjee S, Block ML, Vogel SM, Malik AB, Consolaro ME, Christman JW, Minshall RD, Gantner BN, Bonini MG. NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med. 2015;212:1725–1738. doi: 10.1084/jem.20140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- 28.Yang KC, Rutledge CA, Mao M, Bakhshi FR, Xie A, Liu H, Bonini MG, Patel HH, Minshall RD, Dudley SC., Jr Caveolin-1 modulates cardiac gap junction homeostasis and arrhythmogenecity by regulating cSrc tyrosine kinase. Circ Arrhythm Electrophysiol. 2014;7:701–710. doi: 10.1161/CIRCEP.113.001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, Sharma T, Elliott WM, Szulcek R, Bogaard HJ, Comhair S, Erzurum S, van Nieuw Amerongen GP, Bonini MG, Minshall RD. Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm Circ. 2013;3:816–830. doi: 10.1086/674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Wang AX, Barrett EJ. Caveolin-1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab. 2011;300:E134–144. doi: 10.1152/ajpendo.00498.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IDF. Diabetes Atlas. International Diabetes Federation; Brussels, Belgium: 2016. [Google Scholar]
- 32.A. American Diabetes. 9. Microvascular Complications and Foot Care. Diabetes care. 2016;39(Suppl 1):S72–80. doi: 10.2337/dc16-S012. [DOI] [PubMed] [Google Scholar]
- 33.A. American Diabetes. 8. Cardiovascular Disease and Risk Management. Diabetes care. 2016;39(Suppl 1):S60–71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120:1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coronado-Gonzalez JA, Del Razo LM, Garcia-Vargas G, Sanmiguel-Salazar F, Escobedo-de la Pena J. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104:383–389. doi: 10.1016/j.envres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Ettinger AS, Zota AR, Amarasiriwardena CJ, Hopkins MR, Schwartz J, Hu H, Wright RO. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect. 2009;117:1059–1064. doi: 10.1289/ehp0800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung TC, Huang JW, Guo HR. Association between Arsenic Exposure and Diabetes: A Meta-Analysis. Biomed Res Int. 2015;2015:368087. doi: 10.1155/2015/368087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sargis RM. The hijacking of cellular signaling and the diabetes epidemic: mechanisms of environmental disruption of insulin action and glucose homeostasis. Diabetes & metabolism journal. 2014;38:13–24. doi: 10.4093/dmj.2014.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mimoto MS, Nadal A, Sargis RM. Polluted Pathways: Mechanisms of Metabolic Disruption by Endocrine Disrupting Chemicals. Curr Environ Health Rep. 2017;4:208–222. doi: 10.1007/s40572-017-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2016 doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology. 1999;140:3422–3428. doi: 10.1210/endo.140.8.6908. [DOI] [PubMed] [Google Scholar]
- 44.Zraika S, Aston-Mourney K, Laybutt DR, Kebede M, Dunlop ME, Proietto J, Andrikopoulos S. The influence of genetic background on the induction of oxidative stress and impaired insulin secretion in mouse islets. Diabetologia. 2006;49:1254–1263. doi: 10.1007/s00125-006-0212-9. [DOI] [PubMed] [Google Scholar]
- 45.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 46.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merry TL, Tran M, Stathopoulos M, Wiede F, Fam BC, Dodd GT, Clarke I, Watt MJ, Andrikopoulos S, Tiganis T. High-fat-fed obese glutathione peroxidase 1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxid Redox Signal. 2014;20:2114–2129. doi: 10.1089/ars.2013.5428. [DOI] [PubMed] [Google Scholar]
- 48.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 50.Xu B, Moritz JT, Epstein PN. Overexpression of catalase provides partial protection to transgenic mouse beta cells. Free Radic Biol Med. 1999;27:830–837. doi: 10.1016/s0891-5849(99)00130-6. [DOI] [PubMed] [Google Scholar]
- 51.Kikumoto Y, Sugiyama H, Inoue T, Morinaga H, Takiue K, Kitagawa M, Fukuoka N, Saeki M, Maeshima Y, Wang DH, Ogino K, Masuoka N, Makino H. Sensitization to alloxan-induced diabetes and pancreatic cell apoptosis in acatalasemic mice. Biochim Biophys Acta. 2010;1802:240–246. doi: 10.1016/j.bbadis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Moxon AL. The Effect of Arsenic on the Toxicity of Seleniferous Grains. Science. 1938;88:81. doi: 10.1126/science.88.2273.81. [DOI] [PubMed] [Google Scholar]
- 53.Gailer J, George GN, Pickering IJ, Prince RC, Ringwald SC, Pemberton JE, Glass RS, Younis HS, DeYoung DW, Aposhian HV. A Metabolic Link between Arsenite and Selenite: The Seleno-bis(S-glutathionyl) Arsinium Ion. J Am Chem Soc. 2000;122:4637–4639. [Google Scholar]
- 54.George CM, Gamble M, Slavkovich V, Levy D, Ahmed A, Ahsan H, Graziano J. A cross-sectional study of the impact of blood selenium on blood and urinary arsenic concentrations in Bangladesh. Environ Health. 2013;12:52. doi: 10.1186/1476-069X-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Hall M, Graziano JH, Slavkovich V, van Geen A, Parvez F, Ahsan H. A prospective study of blood selenium levels and the risk of arsenic-related premalignant skin lesions. Cancer Epidemiol Biomarkers Prev. 2007;16:207–213. doi: 10.1158/1055-9965.EPI-06-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan K, Kline J, Ahsan H, Graziano JH. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119:1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alizadeh M, Safaeiyan A, Ostadrahimi A, Estakhri R, Daneghian S, Ghaffari A, Gargari BP. Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab. 2012;60:157–168. doi: 10.1159/000335470. [DOI] [PubMed] [Google Scholar]
- 58.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 61.Wang XL, Yang TB, Wei J, Lei GH, Zeng C. Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr J. 2016;15:48. doi: 10.1186/s12937-016-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu XX, Yao XF, Jiang LP, Geng CY, Zhong LF, Yang G, Zheng BL, Sun XC. Sodium arsenite induces ROS-dependent autophagic cell death in pancreatic beta-cells. Food Chem Toxicol. 2014;70:144–150. doi: 10.1016/j.fct.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Hill DS, Wlodarczyk BJ, Mitchell LE, Finnell RH. Arsenate-induced maternal glucose intolerance and neural tube defects in a mouse model. Toxicol Appl Pharmacol. 2009;239:29–36. doi: 10.1016/j.taap.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xin F, Jiang L, Liu X, Geng C, Wang W, Zhong L, Yang G, Chen M. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat Res Genet Toxicol Environ Mutagen. 2014;769:29–33. doi: 10.1016/j.mrgentox.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 65.Chang KC, Hsu CC, Liu SH, Su CC, Yen CC, Lee MJ, Chen KL, Ho TJ, Hung DZ, Wu CC, Lu TH, Su YC, Chen YW, Huang CF. Cadmium induces apoptosis in pancreatic beta-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PloS one. 2013;8:e54374. doi: 10.1371/journal.pone.0054374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mostafalou S, Baeeri M, Bahadar H, Soltany-Rezaee-Rad M, Gholami M, Abdollahi M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ Toxicol Pharmacol. 2015;39:16–26. doi: 10.1016/j.etap.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem Res Toxicol. 2006;19:1080–1085. doi: 10.1021/tx0600705. [DOI] [PubMed] [Google Scholar]
- 68.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes. 2006;55:1614–1624. doi: 10.2337/db06-0029. [DOI] [PubMed] [Google Scholar]
- 69.Chen KL, Liu SH, Su CC, Yen CC, Yang CY, Lee KI, Tang FC, Chen YW, Lu TH, Su YC, Huang CF. Mercuric compounds induce pancreatic islets dysfunction and apoptosis in vivo. Int J Mol Sci. 2012;13:12349–12366. doi: 10.3390/ijms131012349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.She Y, Jiang L, Zheng L, Zuo H, Chen M, Sun X, Li Q, Geng C, Yang G, Jiang L, Liu X. The role of oxidative stress in DNA damage in pancreatic beta cells induced by di-(2-ethylhexyl) phthalate. Chem Biol Interact. 2017;265:8–15. doi: 10.1016/j.cbi.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, Chen Y, Fang C, Ye T, Dong S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19:581–594. doi: 10.1111/jcmm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamendulis LM, Qiangen W, Sandusky GE, Hocevar BA. Perfluorooctanoic acid exposure triggers oxidative stress in the mouse pancreas. Toxicology Reports. 2014;1:513–521. doi: 10.1016/j.toxrep.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen YW, Lan KC, Tsai JR, Weng TI, Yang CY, Liu SH. Tributyltin exposure at noncytotoxic doses dysregulates pancreatic beta-cell function in vitro and in vivo. Arch Toxicol. 2017 doi: 10.1007/s00204-017-1940-y. [DOI] [PubMed] [Google Scholar]
- 74.Loiola RA, Dos Anjos FM, Shimada AL, Cruz WS, Drewes CC, Rodrigues SF, Cardozo KH, Carvalho VM, Pinto E, Farsky SH. Long-term in vivo polychlorinated biphenyl 126 exposure induces oxidative stress and alters proteomic profile on islets of Langerhans. Sci Rep. 2016;6:27882. doi: 10.1038/srep27882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bashir N, Manoharan V, Miltonprabu S. Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J Nutr Biochem. 2016;32:128–141. doi: 10.1016/j.jnutbio.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013;4:e460. doi: 10.1038/cddis.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, Wei Z, Xu B, Shen J, Li W, Xu S. Low-level phenolic estrogen pollutants impair islet morphology and beta-cell function in isolated rat islets. The Journal of endocrinology. 2012;215:303–311. doi: 10.1530/JOE-12-0219. [DOI] [PubMed] [Google Scholar]
- 78.Miura Y, Hori Y, Kimura S, Hachiya H, Sakurai Y, Inoue K, Sawada T, Kubota K. Triphenyltin impairs insulin secretion by decreasing glucose-induced NADP(H) and ATP production in hamster pancreatic beta-cells. Toxicology. 2012;299:165–171. doi: 10.1016/j.tox.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 79.Pavlikova N, Smetana P, Halada P, Kovar J. Effect of prolonged exposure to sublethal concentrations of DDT and DDE on protein expression in human pancreatic beta cells. Environ Res. 2015;142:257–263. doi: 10.1016/j.envres.2015.06.046. [DOI] [PubMed] [Google Scholar]