Abstract
We investigated how somatic changes in HNSCC interact with environmental and host risk factors and whether they influence the risk of HNSCC occurrence and outcome. 180-paired samples diagnosed as HNSCC in two high incidence regions of Europe and South America underwent targeted sequencing (14 genes) and evaluation of copy number alterations (SCNAs). TP53, PIK3CA, NOTCH1, TP63 and CDKN2A were the most frequently mutated genes. Cases were characterized by a low copy number burden with recurrent focal amplification in 11q13.3 and deletion in 15q22. Cases with low SCNAs showed an improved overall survival. We found significant correlations with decreased overall survival between focal amplified regions 4p16, 10q22 and 22q11, and losses in 12p12, 15q14 and 15q22. The mutational landscape in our cases showed an association to both environmental exposures and clinical characteristics. We confirmed that somatic copy number alterations are an important predictor of HNSCC overall survival.
Introduction
Head and neck squamous cell carcinomas (HNSCC) constitute a heterogeneous group of cancers, which include cancers arising at the oral cavity, nasopharynx, oropharynx, hypopharynx, and larynx. Collectively, these cancers are the seventh most common malignancy diagnosed worldwide [1], with areas of high incidence including Mediterranean Europe and South America [2]. Despite current therapeutic approaches, the prognosis is quite poor, with a 5-year survival ranging from approximately 25% to 60%, according to cancer subsite [3].
Cigarette smoking and alcohol abuse are the major risk factors, consistently associated with the incidence of head and neck cancers [4]. Additionally, human papillomavirus (HPV) infection is strongly associated with oropharyngeal cancer risk and prognosis, alongside a small number of other HNSCC [5]. Recent studies have highlighted the association between numerous differential genomic features and these exposures as well as clinical factors, providing insights for potentially improving prognostic risk stratification for HNSCC[6, 7]. The Cancer Genome Atlas TCGA has conducted the largest comprehensive genomic study of 528 HNSCC cases, consisting of an integrative analysis of multi-genomic data including somatic mutations, gene expression, methylation and miRNAs expression in a clinically and pathologically characterized dataset. The complete data analysis of a subset of 279 patients has allowed the description of the landscape of somatic genomic alterations and the identification of the principal molecular pathways involved in HNSCC development. Particularly, HNSCC are characterized by mutation of TP53, whole genome duplications and multiple recurrent chromosomal gains and losses associated to increased genomic disruption affecting cell cycle checkpoints and PI3K-AKT signaling[8, 9]. Increased rates of somatic copy number alterations (SCNAs) across the tumour genome are associated with poor prognosis and therefore it becomes important to identify SCNAs that might be functionally driving progression and outcome. In addition, genomic studies have revealed how differential genomic patterns among cases could identify various subgroups of tumours showing specific associations with histological subtypes, smoking, HPV status and overall survival[6, 10]
The principal objective of this study was to investigate whether somatic genetic changes identified in two large comprehensive case series in Europe and South America could influence the risk of HNSCC occurrence and outcome from those areas. A second objective was to investigate how somatic changes interact with environmental and host risk factors including HPV infection, alcohol and smoking. We selected 180 paired samples diagnosed as HNSCC from three multicentre studies representative of high incidence regions in Europe (ARCAGE study), Brazil (GENCAPO study) and Argentina (LA study); from which both tumour and blood samples were available in the IARC biorepository, along with complete epidemiological data.
Materials and methods
Study population and risk factor data collection
A total of 240 HNSCC cases were selected from three multicentre studies: two conducted in South America (LA study) between 1998 and 2002, and (GENCAPO study) between 1998 and 2008; and one completed in Europe (ARCAGE study) between 2002 and 2005. Selection of cases was based on availability for biological samples along with complete epidemiological and clinical data. However, no treatment information was obtained from most of these cases as this variable was not included in the original protocols. Extensive details of the three-large multicentre case-control studies are included elsewhere [11–13]. Briefly, all subjects underwent personal interviews to collect information on lifestyle exposures and hospital records were reviewed to obtain clinical and pathological information. All cases had biological samples collected at diagnosis and before any treatment [11–13]. Centralized HPV testing was completed for the three participating studies determined on serology testing as described before [14]. HPV positivity was defined based on HPV16 E6 status, which has been shown to be a highly sensitive and specific marker of HPV16-related oropharyngeal tumours [15–17]. Immunohistochemical evaluation of P16INK4a expression and HPV DNA genotyping were also completed for a subset of samples using protocols previously described [12, 14], and these data were also used to confirm HPV status.
Informed consent was obtained from all participants in the three studies, and the analysis was approved by the Ethical Review Committee of the International Agency for Research on Cancer. All experiments were performed in accordance with relevant guidelines and regulations.
Targeted sequencing
A customized gene panel of 14 genes (GeneRead DNAseq Custom Panels, Qiagen®) was used for targeted sequencing of tumour-blood pair cases. Gene selection was based on an independent analysis of TCGA data on HNSCC using MutsigCV algorithm complemented with the list of the most frequently mutated genes reported in the literature. Briefly, 20ng of DNA were used in multiplex PCR reactions using Qiagen® recommended protocol. For library preparation, 100 ng of multiplex pools and the NEBNext End Repair Module (New England Biolabs, Ipswich, MA, USA) following manufacturer’s instructions. Individual barcodes (designed in-house and produced by Eurofins MWG Operon, Ebersberg, Germany) were ligated to each multiplex pool for sequencing. Both tumour and blood samples were sequenced at an average depth of 250X and 50X respectively using the PGM/PROTON™ Systems (Life Technologies, Carlsbad, CA, USA); sequences used for mutational calling had on target sequencing of 85%, and uniformity of 80–85%
Mutational calling
Identification of somatic variants was performed using a recently developed statistical model called Needlestack[18] based on the idea that analysing several samples together can help estimate the distribution of sequencing errors to accurately identify variants. At each position and for each candidate variant, we model sequencing errors using a robust Negative-Binomial regression with a linear link and a zero intercept [19]. We calculate for each sample a p-value for being a variant (outlier from the regression) that we further transform into q-values to account for multiple testing. Needlestack has a detection limit of variant allelic fractions between 0.05% and 0.5% depending on the error rate at the base change considered (ranging from 0.001% to >10% at homopolymers) and the sequencing depth. Needlestack is free and open-source and is available publicly as a beta version under https://github.com/IARCbioinfo/needlestack. A detailed description of the Needlestack variant caller has been previously published [20, 21]. Variant calls were annotated using ANNOVAR [22] and indels, nonsense, splicing, or missense variants were only kept for subsequent analyses if reported in COSMIC-76 and/or classified as deleterious, disease causing or damaging in at least one of the five variant classification databases (SIFT, Polyphen, MutationTaster, MutationAssessor, FATHMM, LR) (S3 Table).
Filtering of VCF calls was done using a threshold of 0.5% allelic fraction, minimal read depth of 100X and minimal phred-scaled q-value of 30. Removal of germline variants was additionally confirmed by comparison of corresponding paired blood sequences; all filtered variants were manually curated by inspection of BAM files using the Integrative Genomics Viewer (IGV) 2.3 (Broad Institute, Cambridge, MA, USA).
Internal technical validation of both the sequencing procedure and the mutational calling was done by including 10% of samples as technical replicates in each library preparation. Additionally, an independent library preparation including a random selection of 20 tumour samples was sequenced and analysed independently and results were 100% concordant. All cases from the GENCAPO study had been previously sequenced for TP53 mutations using Sanger sequencing, which we used to further validate our mutational calls and compare them with a different calling method (GeneRead Panel Variant Calling analysis tool from Qiagen®) (S1 Fig).
Somatic copy number alterations (SCNAs)
DNA from each tumour was hybridized to Illumina HumanCytoSNP-12v2.1 arrays using standard manufacturer’s protocol. Formalin-fixed paraffin-embedded (FFPE) samples underwent a quality control assay using the Illumina FFPE QC Kit, samples were selected based on a ΔCq below or equal to 2 and then restored using the Infinium HD FFPE Restore Protocol. We included 10% of technical and biological replicates for quality control and validation. Microarray data are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-4863. The R package crlmm [23] was used for pre-processing, genotyping and calculation of circular binary segmentation to estimate the normalized copy number. Germline copy number alterations were removed using the Database of Genomic Variants [24]. Identification of significant amplified or deleted regions was performed by using GISTIC 2.0 [25] using 99% confidence level and q-value threshold 0.25. Focal amplification or deletion for all the 14 genes sequenced was determined only using the GISTIC copy number value 2 or -2 respectively as the true value. OncoPrinter and MutationMapper tools were used for visualization of mutational data [26, 27]. Integrative cluster analysis of mutation and copy number data was performed using the R package iClusterPlus [28].
Statistical analysis
Mutual exclusion and co-occurrence test for mutations (including both single nucleotide variants and copy number alterations) found in the 14 genes evaluated, were based on weighted permutations assessing the deviation of the observed coverage compared to expected obtained by permuting events [29]. Fisher exact test was used to determine the relationship of clinical characteristics in the 3 studies. For each patient, time at risk was calculated from cancer diagnosis to death or end of follow up (Last Follow up date: 30/01/2013 for the ARCAGE study, 30/06/2009 for GENCAPO and 30/06/2006 for the LA study). Follow-up was censored at 5 years, given that most cancer related events occur before that time. The Kaplan-Meier estimator was used to estimate the distribution of the 5-year survival. Multivariate Cox proportional hazard models were used to estimate HRs and their corresponding p values for all candidate risk factors and genomic biomarkers. Age, subsite, stage, nodal status (defined by pathological nodal stage), smoking and alcohol status were used as covariates. A correction for multiple-hypothesis testing was employed using the method of Benjamini and Hochberg [30] Log-rank test was used to compare the different survival distributions.
Results
Epidemiological description of the three studies
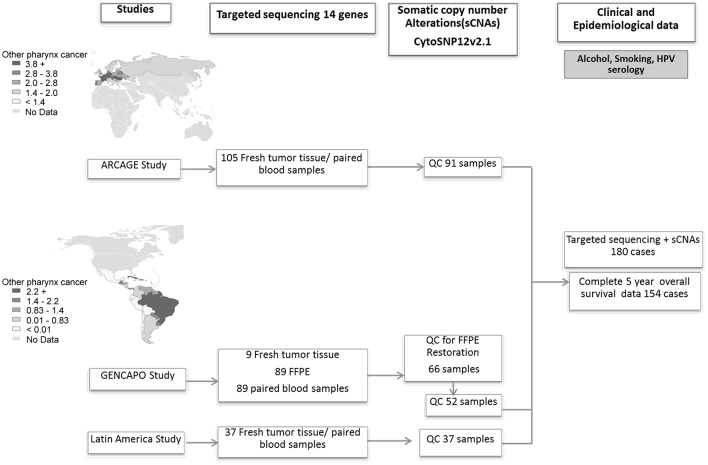
A total of 180 cases had complete sequencing and copy number information (Fig 1). Clinical and pathologic characteristics of cases in the three studies are described in Table 1. Consistent with previous reports the majority of the cases were males (82%), current smokers (67%) and current drinkers (70%). Mean age at diagnosis was 59 years (range 18–88 years). Thirty-three- percent of all cases were diagnosed with oral cavity cancer, 25% with oropharyngeal cancer, 18% with laryngeal cancer, 7% with hypopharyngeal cancer and 16% with overlapping topographies. Seventy-percent of all cases presented advanced disease (stages III-IV). The majority of non-smokers (80%) and oropharyngeal cases (67%) were part of the European study (ARCAGE). Fifteen cases out of 180 (8%) were classified as HPV16 positive, 73% of which were oropharyngeal cases.
Fig 1. Workflow of processing and analysis of HNSCC samples from the three different studies.
QC for copy number evaluation: Quality control of samples based on signal to noise ratio>5.0. Maps show estimated age-standardized incidence rates for HNSCC (other pharynx sites) in Europe and South America. [31].
Table 1. Clinical and epidemiological description of 180 HNSCC cases from the three studies.
| STUDY | ||||||||
|---|---|---|---|---|---|---|---|---|
| ARCAGE (Czech Republic, Italy, Greece) |
GENCAPO (Brazil) |
LA (Argentina) |
Total | |||||
| Sex* | n | % | n | % | n | % | n | % |
| Female | 26 | 28.57 | 2 | 3.85 | 5 | 13.51 | 33 | 18.33 |
| Male | 65 | 71.43 | 50 | 96.15 | 32 | 86.49 | 147 | 81.67 |
| Age group | ||||||||
| 18 to 50 | 18 | 19.78 | 9 | 17.31 | 7 | 18.92 | 34 | 18.89 |
| 51 to 60 | 25 | 27.47 | 28 | 53.85 | 14 | 37.84 | 67 | 37.22 |
| 61 to 70 | 28 | 30.77 | 10 | 19.23 | 9 | 24.32 | 47 | 26.11 |
| >70 | 20 | 21.98 | 5 | 9.62 | 7 | 18.92 | 32 | 17.78 |
| Subsite* | ||||||||
| Oral cavity | 32 | 35.16 | 14 | 26.92 | 13 | 35.14 | 59 | 32.78 |
| Oropharynx | 30 | 32.97 | 10 | 19.23 | 5 | 13.51 | 45 | 25 |
| Hypopharynx | 2 | 2.2 | 11 | 21.15 | 0 | 0 | 13 | 7.22 |
| Larynx | 17 | 18.68 | 3 | 5.77 | 12 | 32.43 | 32 | 17.78 |
| Overlapping | 10 | 10.99 | 12 | 23.08 | 7 | 18.92 | 29 | 16.11 |
| No information | 0 | 0 | 2 | 3.85 | 0 | 0 | 2 | 1.11 |
| Stage* | ||||||||
| T1 | 8 | 8.79 | 1 | 1.92 | 0 | 0 | 9 | 5 |
| T2 | 25 | 27.47 | 9 | 17.31 | 0 | 0 | 34 | 18.89 |
| T3 | 16 | 17.58 | 17 | 32.69 | 3 | 8.11 | 36 | 20 |
| T4 | 40 | 43.96 | 19 | 36.54 | 34 | 91.89 | 93 | 51.67 |
| No information | 2 | 2.2 | 6 | 11.54 | 0 | 0 | 8 | 4.44 |
| Nodal Status* | ||||||||
| N0 | 50 | 54.95 | 14 | 26.92 | 3 | 8.11 | 67 | 37.22 |
| N+ | 38 | 41.76 | 27 | 51.92 | 8 | 21.62 | 73 | 40.56 |
| No information | 3 | 3.30 | 11 | 21.11 | 26 | 70.27 | 40 | 22.22 |
| Smoking* | ||||||||
| Non-smoker | 18 | 19.78 | 1 | 1.92 | 3 | 8.11 | 22 | 12.22 |
| Former smoker | 13 | 14.29 | 14 | 26.92 | 6 | 16.22 | 33 | 18.33 |
| Current smoker | 60 | 65.93 | 32 | 61.54 | 28 | 75.68 | 120 | 66.67 |
| No information | 0 | 0 | 5 | 9.62 | 0 | 0 | 5 | 2.78 |
| Alcohol* | ||||||||
| Non-drinker | 7 | 7.69 | 2 | 3.85 | 6 | 16.22 | 15 | 8.33 |
| Former drinker | 11 | 12.09 | 18 | 34.62 | 4 | 10.81 | 33 | 18.33 |
| Current drinker | 73 | 80.22 | 26 | 50 | 27 | 72.97 | 126 | 70 |
| No information | 0 | 0 | 6 | 11.54 | 0 | 0 | 6 | 3.33 |
| HPV Status (HPV16E6 serology) | ||||||||
| Negative | 82 | 90.11 | 47 | 90.38 | 34 | 91.89 | 163 | 91.57 |
| Positive | 9 | 9.89 | 3 | 5.77 | 3 | 8.11 | 15 | 8.43 |
| No information | 0 | 0 | 2 | 3.85 | 0 | 0 | 2 | 1.11 |
*p value<0.05
Mutational profile of the 14 gene panel in cases
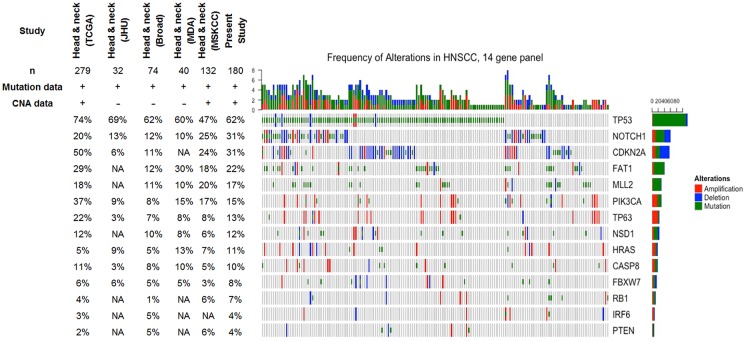
Ninety four-percent of all sequenced cases had at least one alteration (single nucleotide variants (SNVs) or amplification/deletion) in any of the 14 genes selected (Fig 2) (S3 Table). The overall frequency of alterations for the 14 genes was similar to previous publications with a higher enrichment of alterations in the TP53, NOTCH1 and CDKN2A genes [10, 32–34]. Among the 10 cases without alteration in the 14 genes, 4 corresponded to HPV positive cases (S4 Fig).
Fig 2. OncoPrint diagram of mutational frequencies and types of alterations of the 14 genes sequenced.
Only altered samples are shown. Rows are sorted based on the frequency of the alterations in all samples and columns are sorted to visualize the mutual exclusivity across genes. Frequency of mutations for the following Head and Neck cancer publications are shown: Head & Neck (TCGA)[10], Head & Neck (JHU)[39], Head & Neck (Broad)[32], Head & Neck (MDA)[40], Head & Neck (MSKCC)[41]. NA: Not available.
TP53, FAT1, MLL2 and NOTCH1 were the genes more frequently altered by single nucleotide variants (SNVs) (Fig 2). As previously described [35, 36], TP53 mutation was mostly prevalent in HPV negative tumours (only three out of 15 HPV16 positive tumours harboured a TP53 mutation, and all three cases were current smokers) (S4 Fig). TP53 mutations clustered predominantly in DNA binding domains, particularly in hotspot codons 175, 248, 249, 273 and 282 (S3 Fig). Forty-four-percent of all mutations were classified as disruptive mutations according to the definition by Poeta and colleagues[37]. Fifty-five-percent of all TP53 SNVs were missense mutations and from those 64% were classified as high-risk mutations based on the evolutionary action score EAP53[38]. FAT1, MLL2 and NOTCH1 mutations (missense and truncating mutations) were distributed along the gene coding region and did not show mutational enrichment of specific protein domains (S3 Fig).
Mutual exclusive alterations were identified between genes with recognized activity in the same signalling pathway, suggesting overlapping functional consequences of those mutations. This included TP53 and PIK3CA (p<0.001), both involved in cell cycle control and survival, and NOTCH1 and TP63 (p = 0.003) genes, which play important functions of squamous cell differentiation (S2 Fig).
Significant co-occurring alterations were found principally in the TP63 and PIK3CA genes (p<0.001), both genes located on a frequently amplified region (3q) along with concomitant alterations in HRAS and NOTCH1 genes (p<0.001).
Somatic copy number alterations (SCNAs)
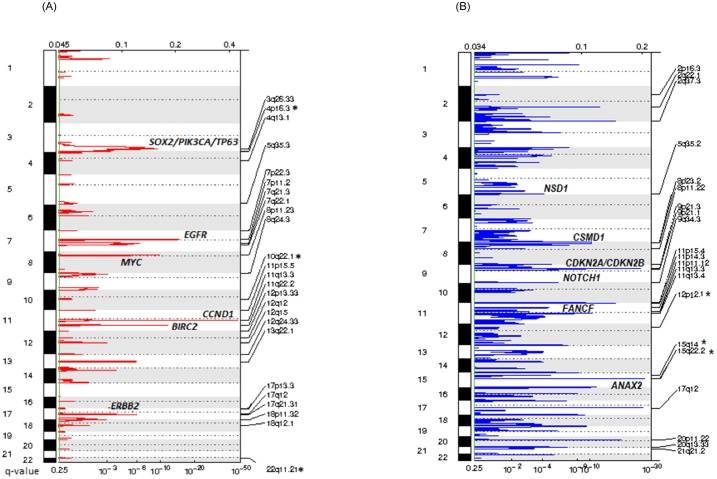
Overall, cases were characterized by low chromosomal instability represented by a low copy number burden (mean 23 alterations included amplifications and deletions) compared to the TCGA dataset [10]. We found a total of 47 significantly recurrent amplified regions and 69 deleted regions (q-value<0.1) (Fig 3 and S1 and S3 Tables). The most recurrent focal amplified region was 11q13.3 including the CCND1 and FGF3 genes amplified in 40% of samples (60/66 with smoking history), consistent with a region preferentially amplified on smoking related tumours [10, 42]. In addition, we identified regions harbouring oncogenes frequently activated in HNSCC as previously described [10, 32, 33, 39, 40]: 11q22 (BIRC2), 3q26 (SOX2, PIK3CA), 3q28 (TP63), 7p11 (EGFR), 17q12 (ERBB2), along with amplification of regions 8p11, 13q22 and 7q22.
Fig 3. Diagram of significant focal copy number alterations.
FDR (Top) and q-values of the alterations are shown in each panel. (A) Copy number gains (B) Copy number losses. Selected associated genes in some regions are shown. (*) Regions significantly associated with overall survival.
The most frequently deleted region was 15q22, including the locus of the ANXA2 gene that has been previously found to be downregulated in both head and neck dysplasia and HNSCC [43, 44]. Additionally, recurrent focal deletions were present in cases, particularly at three regions on chromosome 11 (11p15-p15.5, 11q13-q13.3 and 11q23-q24) previously identified as being of frequent microsatellite instability and/or loss of heterozygosity (MSI/LOH) in HNSCC. We also identified deletions in regions of commonly described transcription regulators and tumour suppressor genes in HNSCCs [10, 45]: 5q35.2 (NSD1), 20p11 (NKX2-2), 8p22.2 (CSMD1), 9q34.3 (NOTCH1); together with loss of 9p21.3 containing the CDKN2A gene which was found almost exclusively in HPV negative tumours (deletion in 1 out of 15 HPV positive cases) (S4 Fig).
Comparison of copy number alterations based on HPV16 status showed a lower proportion of significantly altered regions in HPV positive cases. In particular, the 11q24.3 region (containing the ATM and APLP2 genes) was differentially lost in HPV positive cases (S4 Fig). Additional losses in the 6p region, close to the HLA class I genes loci, were also identified in HPV positive cases.
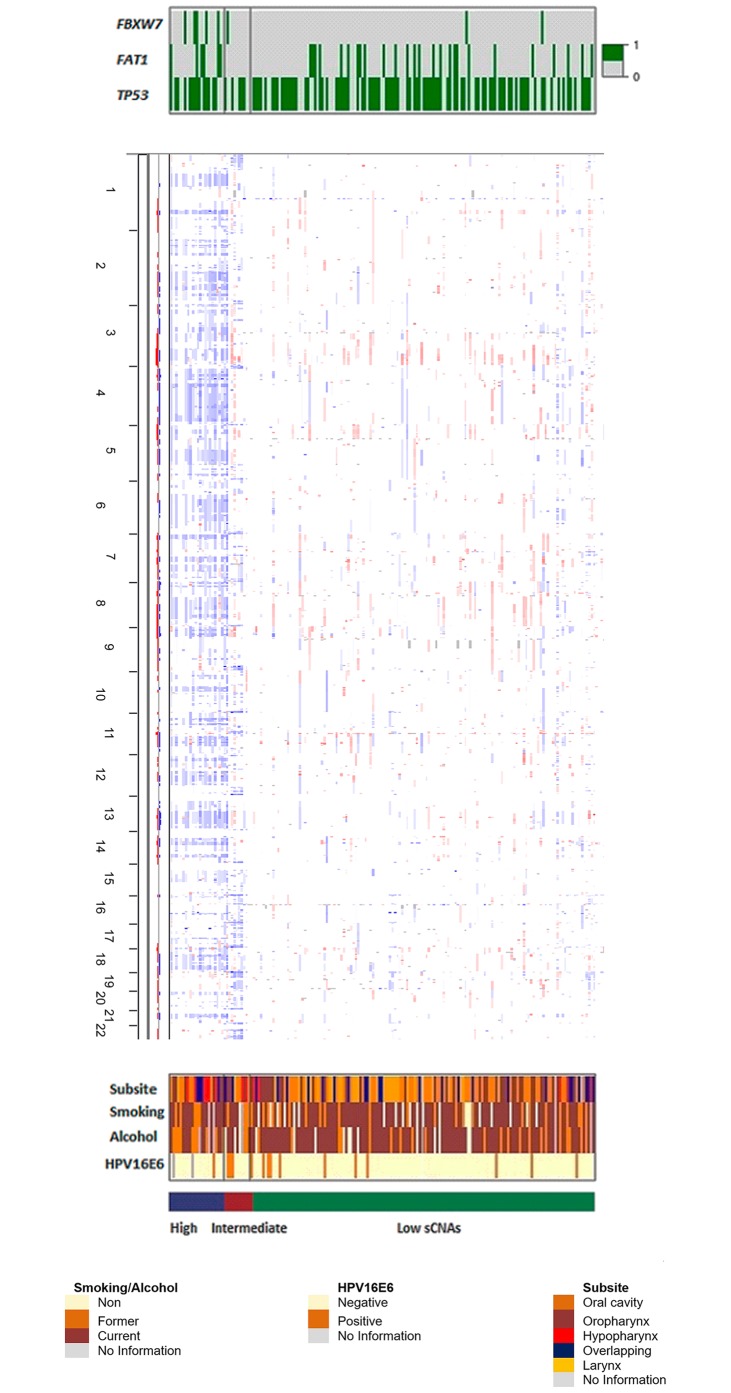
Integrated analysis
Integrative cluster analysis of both mutational and copy number data identified three distinct clusters with major genomic features including TP53, FAT1 and FBXW7 SNVs and low, intermediate and high genomic instability. The FBXW7 gene was significantly mutated in both groups with high and intermediate SCNAs (Fig 4). Eighty-percent of total cases were clustered in the low SCNAs group (mean copy number events = 19). The intermediate SCNAs group (mean copy number events = 39) had only advanced cases (11) and the high SCNAs group (mean copy number events = 43) clustered only cases from Brazil with history of alcohol and smoking exposure (23 cases).
Fig 4. Integrative cluster analysis plot.
Cases are grouped by mutation and SCNA status. Top panel: only significant clustering genes are shown (0 = non-mutated, 1 = mutated), middle panel: SCNAs. Amplified (red) and deleted (blue) chromosomal regions. Altered regions are arranged vertically and sorted by genomic locus, with chromosome 1 at the top of the panel and chromosome 22 at the bottom, lower panel: colour coded clinical and epidemiological characteristics.
Survival analysis
Survival data was available for 154 cases (Fig 1). Age and nodal status were the only clinical or demographic variables significantly associated to overall survival (p = 0.01) (Fig 5 and S2 Table). Multivariate analysis including each of the 14 genes sequenced showed no association with overall survival. Further analysis of TP53 mutational status showed no association between mutation type (either disruptive/non-disruptive or EAP53 score of missense mutations) and overall survival (S2 Table).
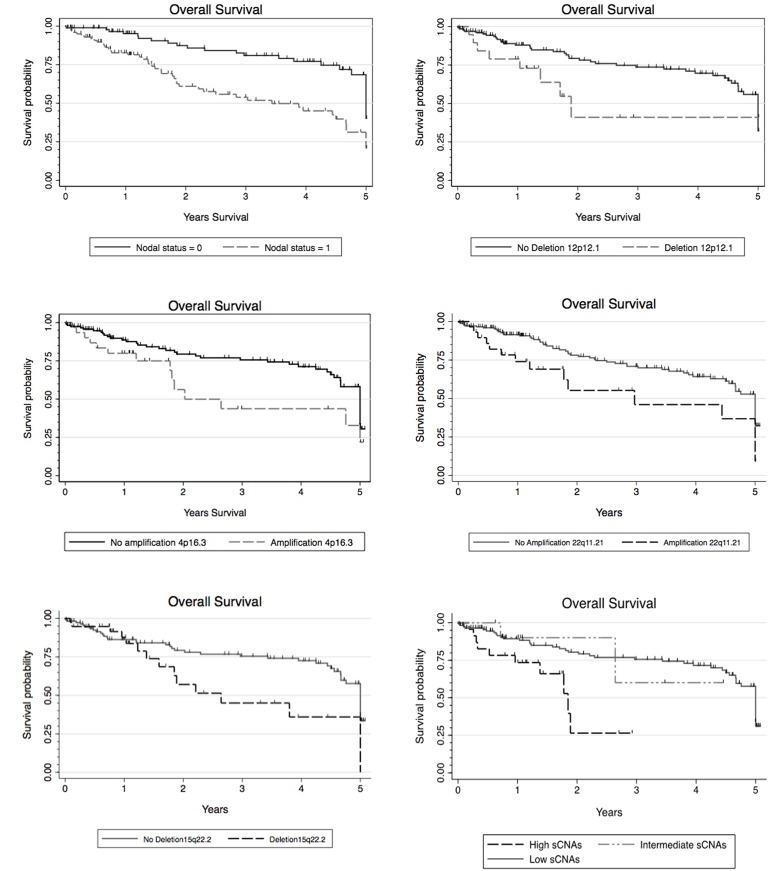
Fig 5. Kaplan-Meier curves showing overall survival outcome for nodal status, significant focal copy number alterations in 22q11.2,15q22 and 12p12 regions associated to smoking and advanced stage, amplification in 4p16.3 and for the three SCNAs clusters.
Analysis of the most frequently focal SCNAs showed significant associations between the amplified regions 4p16, 10q22 and 22q11 and a reduction in overall survival. We found additional associations between losses in regions 12p12, 15q14 and15q22 and decreased overall survival (Fig 5 and S2 Table). Although individual candidate genes in these regions were difficult to identify due to the large number of enclosed genes (>20), we identify some genes that have been previously altered in HNSCC (S2 Table) and have been included in our discussion below.
Our integrative clustering approach based on copy number events was also associated with improved overall survival for cases clustered in the low copy number group (p = 0.01) (Fig 5 and S2 Table).
Discussion
Head and neck carcinomas show common genomic features determined by SNVs and copy number events in driver genes and cellular pathways associated to the common histology of squamous cell types. However, there is broader genomic heterogeneity due to the variability in anatomic subsite location and the interaction of multiple risk factors such as alcohol and tobacco exposure as well as HPV infection.
Even though we limited our sequencing study to 14 genes, our results showed that most of the mutations described in these genes are representative of the mutational profile of head and neck cancer cases (mutations in 94% of cases). Additionally, the mutational frequency in all 14 genes was comparable to the frequencies observed in previous publications from the largest sequencing projects of Head and Neck cancer cases. In future studies, inclusion of some additional genes such as AJUBA, HLA-A/B, NFE2L2, KRAS, FGFR2/3 and TRAF3 could improve mutation detection and better capture the mutational landscape of HPV positive tumours, as well as favour the understanding of additional cellular and molecular mechanisms involved in tumour development such as the oxidative stress pathway.
The predominance of low SCNAs in our cases confirms previous studies that differentiate subsets of head and neck tumours (described as M-mutational class tumours) characterized predominantly by mutations rather than chromosomal instability events [8]. A subclass of these low SCNAs group is enriched with alterations in the PIK3CA-AKT and p53-mediated apoptosis pathways, in agreement with the number of alterations in TP53, CDKN2A and PIK3CA we observed in our cases.
Eight percent of all cases were HPV 16 positive and 73% corresponded to oropharyngeal tumours. The reduced number of oropharyngeal tumours in the study (25%) and the predominance of older cases, current smokers and drinkers, characteristics preferentially associated to non-related HPV HNSCC[46], might account for the low number of HPV positive cases. In addition, half of our study cases were from Brazil and Argentina which could contribute to the low percentage of HPV positive HNSCC, as it has been previously described in South America [12, 47]. Despite of the limited number of HPV positive cases in our series, we established that HPV positive tumours remain a distinct subset characterized by lower somatic copy number events and differential mutation patterns [36, 48, 49]. Loss of the 11q24.3 region which contains both ATM and APLP2 genes, is a frequent alteration in HPV positive cases[48]. Moreover, the APLP2 gene is related to tumour immunology as it regulates surface expression of the MHC class I molecules[50, 51]. These results suggest that alterations related to immunological responses might differentiate infection related HNSCC tumours. Further characterization should however, be performed for this group particularly to address the associations between genomic alterations and smoking and alcohol exposure and a differentiated analysis by histological subsite.
Our results confirmed that somatic copy number alterations are an important predictor of overall survival. We have described an improved overall survival for those cases with low SCNAs. These results are in agreement with recent observations showing the direct association between low copy number events, intratumour heterogeneity and clonality with genomic instability and how the joint effect of these factors might influence survival [10, 52–54]. Recently, Andor and colleagues analysed clonality across 12 cancer types from the TCGA dataset, including head and neck cancer cases, and showed that intratumour heterogeneity levels above or below an intermediate measure of clonality were associated with significantly reduced risk of mortality. Moreover, they used copy number alteration abundance as a surrogate measure of genomic instability and found that when SCNAs were present either in a low or a high fraction of the tumours, cases had an improved survival [53]. The high SCNAs group in our study showed the lowest overall survival and clustered only samples from Brazil, all characterized by higher stage and history of both smoking and alcohol exposure. These results give additional evidence to support the rise in mortality due to this malignancy in this country [55].
The mutational profile described in our series of cases showed a clear association to both environmental exposures and clinical characteristics including associations with overall survival. We found that both mutational and focal copy number alterations were correlated with genetic alterations previously described for smoking related head and neck cancers as well as for biomarkers of late stage tumours [10, 32]. Alterations exclusively found in cases with history of both smoking and alcohol consumption included 5q35.3 amplification and 11p14.3 deletion. This last region is of interest as it encloses the FANCF gene, involved in the Fanconi anemia pathway and commonly associated to squamous cell carcinoma susceptibility. In addition, FANCF inactivation has been previously related to chromosomal instability on sporadic HNSCC [56].
Additionally, focal copy number alterations were found to be significant prognostic markers: 22q11.2 amplification and deletions in 15q22 and 12p12 regions have been associated to smoking related tumours and advanced stage. The 22q11 region contains the CRKL gene, which has been characterized as an oncogene in lung SCC [57] and as a promoter of cell growth, motility and adhesion during HNSCC tumorigenesis [58]. Decreased survival in cases with loss of 12p12.1 region, locus of the PIK3C2G gene, showed a HR of 3.0 95% CI [1.2; 7.77]. Advanced stage HNSCC tumours have shown mutations in more than one PI3K pathway molecule: PIK3CA, PTEN and described alterations in PI3C2G [59, 60]. Moreover, the 15q22 region, locus of the ANXA2 gene, has been previously shown to be associated with poorly differentiated tumours in advanced cases. Decreased ANXA expression has not however, been formerly shown to be an independent prognostic factor for disease-specific survival in HNSCC [43, 44]. We report for the first time an association between decreased overall survival and amplification of the region 4p16.3, locus of the FGFR3 gene. High expression levels of FGFR3 contribute of tumour initiation and early-stage progression in HNSCC[61]. More importantly, preclinical studies have demonstrated that FGFR inhibition reduced cell proliferation and increased cell apoptosis in head and neck cancer in vitro and in vivo[62], highlighting the potential prognostic and therapeutic role of FGFR3 in HNSCC.
Most studies on HNSCC have documented a decreased overall survival associated to TP53 mutations[6, 37, 63]. Our study, however, did not find any association between the mutational status of the 14 genes sequenced and overall survival. A specific analysis based on TP53 mutation type (disruptive vrs nondisruptive or EAP53 score of missense mutations) showed no association to overall survival, either. In agreement to our results, Kim and colleagues, found that patients diagnosed with oral squamous cell carcinoma of the gingivo-buccal region (GBSCC) from the Indian Team project of the International Cancer Genome Consortium (ICGC), did not showed an association between TP53 mutation status and overall survival [64]. Similar to the epidemiological and clinical characteristics of our study cases, GBSCC patients from the ICGC study were most exposed to tobacco and/or alcohol, presented advanced stage (III/IV) and half of the cases had confirmed nodal metastasis [33].
One of the main limitations of our study is the reduced number of HNSCC cases with early stage tumours. It would be important to further characterize the genomic alterations in early stages of head and neck cancer cases in order to identify biomarkers for early detection and prognostic stratification especially for the high-risk groups in regions of increase incidence. In addition, our survival analysis was limited due to the lack of complete treatment information for most cases. Treatment regimens have an important association with Head and Neck cancer overall survival and should be included in future analysis specially those involving multicentre studies[65].
In summary, we have identified HNSCC cases with low SCNAs that differentiate as a subset of head and neck cancers driven predominantly by gene mutations and focal alterations rather than chromosomal instability events and are characterized by an improved overall survival. The mutational landscape described in our series of cases showed a clear association to both environmental exposures (alcohol and smoking consumption and HPV infection) and clinical characteristics. Further studies integrating genomic, clinical and epidemiological data, especially in high-risk populations, are necessary to better identify high-risk stratification and characterize prognosis of head and neck cancer cases.
Supporting information
(A) Venn diagram of number of TP53 mutations detected in the Gencapo Series. Example: TP53Asn239Asp mutation previously detected by Sanger sequencing (B) Plots of mutational calling showing an example of independent libraries sequenced from the same case.
(PDF)
(A) Mutually exclusive alterations between the 14 genes sequenced (Significance p-value of mutual exclusivity derived from the Z-score) (B) Co-occurrence of alterations (Significance p-value of co-occurrence derived from the Z-Score). Z score based on deviation of the observed mutations compared to expected, obtained by permuting events.
(PDF)
Mutation colours represent: Green: Missense Mutations; red: Truncating Mutations (Nonsense, Nonstop, Frameshift deletion, Frameshift insertion, Splice site), black: Inframe Mutations (Inframe deletion, Inframe insertion). Circles colored with purple indicate residues that are affected by different mutation types at the same proportion.
(PDF)
(A) Mutational frequencies of the 14 genes sequenced in 15 HPV16E6 positive cases. (B) Comparison of Significant Focal copy number losses between HPV positive and HPV negative cases. (*) Regions significantly associated with overall survival.
(PDF)
In red regions significantly associated with overall survival and head and neck cancer related genes.
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors wish to acknowledge Dr Javier Oliver for his valuable collaboration in the standardization of the targeted sequencing assays and Valérie Gaborieau for her contribution in database curation and data homogenization from the three studies. The authors acknowledge all patients who donated their biological specimens and the contribution of GENCAPO (Brazilian Head and Neck Genome Project) for clinical samples and for clinical and pathological data collection (complete list of members and affiliations presented at http://www.gencapo.famerp.br).
This work was undertaken during the tenure of a Postdoctoral Fellowship to Dr Sandra Perdomo from The International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions—People—Co-funding of regional, national and international programmes (COFUND).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been funded by the International Agency for Research on Cancer (IARC). The GENCAPO study was supported by grants from FAPESP (São Paulo Research Foundation) (04/12054-9 and 10/51168-0), which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 2014. http://globocan.iarc.fr/. [DOI] [PubMed]
- 2.Forman D BF, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay J. Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon: IARC; 2013. [DOI] [PubMed] [Google Scholar]
- 3.Gatta G, Botta L, Sanchez MJ, Anderson LA, Pierannunzio D, Licitra L, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer. 2015. doi: 10.1016/j.ejca.2015.07.043 . [DOI] [PubMed] [Google Scholar]
- 4.Humans IWGotEoCRt. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt E):1–538. . [PMC free article] [PubMed] [Google Scholar]
- 5.Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst. 2016;108(6). doi: 10.1093/jnci/djv403 . [DOI] [PubMed] [Google Scholar]
- 6.Gross AM, Orosco RK, Shen JP, Egloff AM, Carter H, Hofree M, et al. Multi-tiered genomic analysis of head and neck cancer ties TP53 mutation to 3p loss. Nat Genet. 2014;46(9):939–43. Epub 2014/08/05. doi: 10.1038/ng.3051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy RB, Bhat AR, James BL, Govindan SV, Mathew R, Dr R, et al. Meta-Analyses of Microarray Datasets Identifies ANO1 and FADD as Prognostic Markers of Head and Neck Cancer. PLoS One. 2016;11(1):e0147409 doi: 10.1371/journal.pone.0147409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–33. doi: 10.1038/ng.2762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 2013;45(10):1134–40. doi: 10.1038/ng.2760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. doi: 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagiou P, Georgila C, Minaki P, Ahrens W, Pohlabeln H, Benhamou S, et al. Alcohol-related cancers and genetic susceptibility in Europe: the ARCAGE project: study samples and data collection. Eur J Cancer Prev. 2009;18(1):76–84. Epub 2008/10/03. doi: 10.1097/CEJ.0b013e32830c8dca . [DOI] [PubMed] [Google Scholar]
- 12.López RVM, Levi JE, Eluf-Neto J, Koifman RJ, Koifman S, Curado MP, et al. Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes & Control. 2014;25(4):461–71. doi: 10.1007/s10552-014-0348-8 [DOI] [PubMed] [Google Scholar]
- 13.Szymańska K, Hung RJ, Wünsch-Filho V, Eluf-Neto J, Curado MP, Koifman S, et al. Alcohol and tobacco, and the risk of cancers of the upper aerodigestive tract in Latin America: a case–control study. Cancer Causes & Control. 2011;22(7):1037–46. doi: 10.1007/s10552-011-9779-7 [DOI] [PubMed] [Google Scholar]
- 14.Anantharaman D, Gheit T, Waterboer T, Abedi-Ardekani B, Carreira C, McKay-Chopin S, et al. Human Papillomavirus Infections and Upper Aero-Digestive Tract Cancers: The ARCAGE Study. JNCI Journal of the National Cancer Institute. 2013;105(8):536–45. doi: 10.1093/jnci/djt053 [DOI] [PubMed] [Google Scholar]
- 15.Reuschenbach M, Waterboer T, Wallin KL, Einenkel J, Dillner J, Hamsikova E, et al. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer. 2008;123(11):2626–31. doi: 10.1002/ijc.23837 . [DOI] [PubMed] [Google Scholar]
- 16.Herrero R. Human Papillomavirus and Oral Cancer: The International Agency for Research on Cancer Multicenter Study. CancerSpectrum Knowledge Environment. 2003;95(23):1772–83. doi: 10.1093/jnci/djg107 [DOI] [PubMed] [Google Scholar]
- 17.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–15. doi: 10.1200/JCO.2012.47.2738 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh JE, Ohta T, Satomi K, Foll M, Durand G, McKay J, et al. Alterations in the NF2/LATS1/LATS2/YAP Pathway in Schwannomas. J Neuropathol Exp Neurol. 2015;74(10):952–9. doi: 10.1097/NEN.0000000000000238 . [DOI] [PubMed] [Google Scholar]
- 19.Aeberhard WH, Cantoni E, Heritier S. Robust inference in the negative binomial regression model with an application to falls data. Biometrics. 2014;70(4):920–31. doi: 10.1111/biom.12212 . [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Cuesta L, Perdomo S, Avogbe PH, Leblay N, Delhomme TM, Gaborieau V, et al. Identification of Circulating Tumor DNA for the Early Detection of Small-cell Lung Cancer. EBioMedicine. 2016. doi: 10.1016/j.ebiom.2016.06.032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Calvez-Kelm F, Foll M, Wozniak MB, Delhomme TM, Durand G, Chopard P, et al. KRAS mutations in blood circulating cell-free DNA: a pancreatic cancer case-control. Oncotarget. 2016;7(48):78827–40. doi: 10.18632/oncotarget.12386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010. p. e164 doi: 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scharpf RB, Irizarry RA, Ritchie ME, Carvalho B, Ruczinski I. Using the R Package crlmm for Genotyping and Copy Number Estimation. J Stat Softw. 2011;40(12):1–32. . [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW. The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42(Database issue):D986–92. doi: 10.1093/nar/gkt958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12(4):R41 doi: 10.1186/gb-2011-12-4-r41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1 doi: 10.1126/scisignal.2004088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo Q, Wang S, Seshan VE, Olshen AB, Schultz N, Sander C, et al. Pattern discovery and cancer gene identification in integrated cancer genomic data. Proc Natl Acad Sci U S A. 2013;110(11):4245–50. doi: 10.1073/pnas.1208949110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One. 2011;6(5):e19541 doi: 10.1371/journal.pone.0019541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, and Hochberg Yosef. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1). Epub 300. [Google Scholar]
- 31.Ervik M.FL, Ferlay J., Mery L., Soerjomataram I., Bray F.. Cancer Today Lyon, France: International Agency for Research on Cancer; 2016. [cited 2016 10 May]. http://gco.iarc.fr/today. [Google Scholar]
- 32.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science. 2011;333(6046):1157–60. doi: 10.1126/science.1208130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.India Project Team of the International Cancer Genome C. Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat Commun. 2013;4:2873 doi: 10.1038/ncomms3873 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris LG, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, et al. The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.1790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai M, Clifford GM, le Calvez F, Castellsague X, Snijders PJ, Pawlita M, et al. Human papillomavirus type 16 and TP53 mutation in oral cancer: matched analysis of the IARC multicenter study. Cancer Res. 2004;64(2):468–71. Epub 2004/01/28. . [DOI] [PubMed] [Google Scholar]
- 36.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–41. doi: 10.1158/1078-0432.CCR-13-3310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–61. doi: 10.1056/NEJMoa073770 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neskey DM, Osman AA, Ow TJ, Katsonis P, McDonald T, Hicks SC, et al. Evolutionary Action Score of TP53 Identifies High-Risk Mutations Associated with Decreased Survival and Increased Distant Metastases in Head and Neck Cancer. Cancer Res. 2015;75(7):1527–36. doi: 10.1158/0008-5472.CAN-14-2735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science. 2011;333(6046):1154–7. doi: 10.1126/science.1206923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3(7):770–81. doi: 10.1158/2159-8290.CD-12-0537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedberg ML, Goh G, Chiosea SI, Bauman JE, Freilino ML, Zeng Y, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest. 2016;126(1):169–80. doi: 10.1172/JCI82066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.!! INVALID CITATION!!! {Pattle, 2017 #4150;Pattle, 2017 #4150}.
- 43.Rodrigo JP, Lequerica-Fernández P, Rosado P, Allonca E, García-Pedrero JM, de Vicente JC. Clinical significance of annexin A2 downregulation in oral squamous cell carcinoma. Head & Neck. 2011;33(12):1708–14. doi: 10.1002/hed.21661 [DOI] [PubMed] [Google Scholar]
- 44.Pena-Alonso E, Rodrigo JP, Parra IC, Pedrero JM, Meana MV, Nieto CS, et al. Annexin A2 localizes to the basal epithelial layer and is down-regulated in dysplasia and head and neck squamous cell carcinoma. Cancer Lett. 2008;263(1):89–98. doi: 10.1016/j.canlet.2007.12.029 . [DOI] [PubMed] [Google Scholar]
- 45.Imai FL, Uzawa K, Miyakawa A, Shiiba M, Tanzawa H. A detailed deletion map of chromosome 20 in human oral squamous cell carcinoma. Int J Mol Med. 2001;7(1):43–7. . [DOI] [PubMed] [Google Scholar]
- 46.Taberna M, Mena M, Pavon MA, Alemany L, Gillison ML, Mesia R. Human papillomavirus related oropharyngeal cancer. Ann Oncol. 2017. doi: 10.1093/annonc/mdx304 . [DOI] [PubMed] [Google Scholar]
- 47.Anantharaman D, Abedi-Ardekani B, Beachler DC, Gheit T, Olshan AF, Wisniewski K, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140(9):1968–75. doi: 10.1002/ijc.30608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes DN, Van Waes C, Seiwert TY. Genetic Landscape of Human Papillomavirus-Associated Head and Neck Cancer and Comparison to Tobacco-Related Tumors. J Clin Oncol. 2015;33(29):3227–34. doi: 10.1200/JCO.2015.62.1086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–81. doi: 10.1158/1078-0432.CCR-14-2481 . [DOI] [PubMed] [Google Scholar]
- 50.Peters HL, Tuli A, Sharma M, Naslavsky N, Caplan S, MacDonald RG, et al. Regulation of major histocompatibility complex class I molecule expression on cancer cells by amyloid precursor-like protein 2. Immunol Res. 2011;51(1):39–44. doi: 10.1007/s12026-011-8238-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feenstra M, Veltkamp M, van Kuik J, Wiertsema S, Slootweg P, van den Tweel J, et al. HLA class I expression and chromosomal deletions at 6p and 15q in head and neck squamous cell carcinomas. Tissue Antigens. 1999;54(3):235–45. doi: 10.1034/j.1399-0039.1999.540304.x [DOI] [PubMed] [Google Scholar]
- 52.Mroz EA, Tward AD, Hammon RJ, Ren Y, Rocco JW. Intra-tumor genetic heterogeneity and mortality in head and neck cancer: analysis of data from the Cancer Genome Atlas. PLoS Med. 2015;12(2):e1001786 doi: 10.1371/journal.pmed.1001786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, et al. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. 2015. doi: 10.1038/nm.3984 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smeets SJ, Brakenhoff RH, Ylstra B, van Wieringen WN, van de Wiel MA, Leemans CR, et al. Genetic classification of oral and oropharyngeal carcinomas identifies subgroups with a different prognosis. Cell Oncol. 2009;31(4):291–300. doi: 10.3233/CLO-2009-0471 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatenoud L, Bertuccio P, Bosetti C, Malvezzi M, Levi F, Negri E, et al. Trends in mortality from major cancers in the Americas: 1980–2010. Annals of Oncology. 2014;25(9):1843–53. doi: 10.1093/annonc/mdu206 [DOI] [PubMed] [Google Scholar]
- 56.Stoepker C, Ameziane N, van der Lelij P, Kooi IE, Oostra AB, Rooimans MA, et al. Defects in the Fanconi Anemia Pathway and Chromatid Cohesion in Head and Neck Cancer. Cancer Res. 2015;75(17):3543–53. doi: 10.1158/0008-5472.CAN-15-0528 . [DOI] [PubMed] [Google Scholar]
- 57.Kim YH, Kwei KA, Girard L, Salari K, Kao J, Pacyna-Gengelbach M, et al. Genomic and functional analysis identifies CRKL as an oncogene amplified in lung cancer. Oncogene. 2010;29(10):1421–30. doi: 10.1038/onc.2009.437 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanagi H, Wang L, Nishihara H, Kimura T, Tanino M, Yanagi T, et al. CRKL plays a pivotal role in tumorigenesis of head and neck squamous cell carcinoma through the regulation of cell adhesion. Biochem Biophys Res Commun. 2012;418(1):104–9. doi: 10.1016/j.bbrc.2011.12.142 . [DOI] [PubMed] [Google Scholar]
- 59.Lui VWY, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent Mutation of the PI3K Pathway in Head and Neck Cancer Defines Predictive Biomarkers. Cancer Discovery. 2013;3(7):761–9. doi: 10.1158/2159-8290.CD-13-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giudice FS, Squarize CH. The determinants of head and neck cancer: Unmasking the PI3K pathway mutations. J Carcinog Mutagen. 2013;Suppl 5 doi: 10.4172/2157-2518.S5-003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vairaktaris E, Ragos V, Yapijakis C, Derka S, Vassiliou S, Nkenke E, et al. FGFR-2 and -3 play an important role in initial stages of oral oncogenesis. Anticancer Res. 2006;26(6B):4217–21. . [PubMed] [Google Scholar]
- 62.Sweeny L, Liu Z, Lancaster W, Hart J, Hartman YE, Rosenthal EL. Inhibition of fibroblasts reduced head and neck cancer growth by targeting fibroblast growth factor receptor. Laryngoscope. 2012;122(7):1539–44. Epub 2012/03/27. doi: 10.1002/lary.23266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou G, Liu Z, Myers JN. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J Cell Biochem. 2016;117(12):2682–92. doi: 10.1002/jcb.25592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim KT, Kim BS, Kim JH. Association between FAT1 mutation and overall survival in patients with human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E2021–9. doi: 10.1002/hed.24372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994–1001. doi: 10.1634/theoncologist.2009-0289 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Venn diagram of number of TP53 mutations detected in the Gencapo Series. Example: TP53Asn239Asp mutation previously detected by Sanger sequencing (B) Plots of mutational calling showing an example of independent libraries sequenced from the same case.
(PDF)
(A) Mutually exclusive alterations between the 14 genes sequenced (Significance p-value of mutual exclusivity derived from the Z-score) (B) Co-occurrence of alterations (Significance p-value of co-occurrence derived from the Z-Score). Z score based on deviation of the observed mutations compared to expected, obtained by permuting events.
(PDF)
Mutation colours represent: Green: Missense Mutations; red: Truncating Mutations (Nonsense, Nonstop, Frameshift deletion, Frameshift insertion, Splice site), black: Inframe Mutations (Inframe deletion, Inframe insertion). Circles colored with purple indicate residues that are affected by different mutation types at the same proportion.
(PDF)
(A) Mutational frequencies of the 14 genes sequenced in 15 HPV16E6 positive cases. (B) Comparison of Significant Focal copy number losses between HPV positive and HPV negative cases. (*) Regions significantly associated with overall survival.
(PDF)
In red regions significantly associated with overall survival and head and neck cancer related genes.
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.