Abstract
Restraint and cold stress increase both corticosterone and glycemia, which lead to oxidative damages in hepatic tissue. This study assessed the effect of royal jelly (RJ) supplementation on the corticosterone level, glycemia, plasma enzymes and hepatic antioxidant system in restraint and cold stressed rats. Wistar rats were allocated into no-stress, stress, no-stress supplemented with RJ and stress supplemented with RJ groups. Initially, RJ (200mg/Kg) was administered for fourteen days and stressed groups were submitted to chronic stress from the seventh day. The results showed that RJ supplementation decreases corticosterone levels and improves glycemia control after stress induction. RJ supplementation also decreased the body weight, AST, ALP and GGT. Moreover, RJ improved total antioxidant capacity, SOD activity and reduced GSH, GR and lipoperoxidation in the liver. Thus, RJ supplementation reestablished the corticosterone levels and the hepatic antioxidant system in stressed rats, indicating an adaptogenic and hepatoprotective potential of RJ.
Introduction
The adaptive response to stress is characterized by psychophysiological adaptations of an organism to restore homeostasis [1]. It is well documented that chronic restraint and cold stress effectively mimics physical and psychological stress [2], elevate metabolic rate and also increase production of reactive oxygen species (ROS). Physical and psychological stress activates the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS), increasing plasma glucocorticoid levels [3]. Increased corticosterone levels were observed in stress responses using the stress models, such as restraint and cold [2], immobilization [4], cold [5], cold water immersion [5], electric foot shock [6] and social isolation stress [7]. Corticosterone increases gluconeogenesis and hepatic glycogenolysis in rats, resulting in an increase in the availability of metabolic substrates [8].
A metabolic byproduct of stress-induced increase in energy production is the formation of ROS [9] e.g. hydrogen peroxide (H2O2), hydroxyl radicals (HO·) and superoxide anion radicals (O2–·), which cause lipid peroxidation. In addition, the increased corticosterone levels trigger ROS production and promote redox imbalance in different tissues of the body [10, 11]. Oxidative stress also associated with physical stress induces changes in antioxidant defense systems [11]. Therefore, to neutralize reactive oxygen species, the body uses mainly enzymatic and non-enzymatic antioxidant defense system [12].
Several studies have investigated whether nutraceutical supplementation results in enhancement of the antioxidant defense system. In relation to stress management, nutraceutical supplements have been used as an adaptogenic agent to support the body’s adaptation in stressful situations [13]. Thus, royal jelly (RJ) could be used as a nutraceutical product. RJ is secreted by the mandibular and hypopharyngeal glands of worker bees (Apis mellifera L.). Royal Jelly composition includes major royal jelly proteins, free amino acids, sugars, vitamins (B1, B2, B6, folic acid, pantothenic acid, nicotinic acid, and biotin) and lipids such as 10-hydroxy-2-decenoic acid (HDA-10). The RJ has several biological properties including anti-inflammatory [14], vasodilator and hypotensive [15], antimicrobial [16], immunomodulatory [17], hypocholesterolemic [18] and antioxidant [19] activities.
In a previous study from our research group, we demonstrated a neuroprotective effect of royal jelly supplementation and a reduction of corticosterone levels in a stress condition [20]. With these interesting results, the interest arose to evaluate the effect of the royal jelly supplementation on the liver, which is a central organ of metabolism and is related to the synthesis of cholesterol, a precursor of corticosterone. As well as, to investigate the effect of RJ supplementation on a non-stressful situation. As glucocorticoids have direct and indirect modulatory roles in oxidative stress [9], our hypothesis was that RJ could decrease corticosterone levels, even in the absence of stress, and oxidative stress in liver tissue. Thus, the aim of the study was to evaluate the adaptogenic and antioxidant effect of RJ supplementation in rats submitted to chronic stress induced by restraint and cold.
Materials and methods
Samples
RJ was imported from China and provided by Apiário Girassol Ltda. (Uberlândia—MG, Brazil). The centesimal analysis of the royal jelly was carried out by the Laboratory of Bromatology and Animal Nutrition of the Federal University of Uberlândia. The royal jelly has 67% of humidity and 33% of dry matter, of these, 14.19% are crude protein, 2.01% are lipids, 0.87% are ash and 15.93% are carbohydrates. The RJ was stored at -20°C until use. Daily, 200 mg/kg b.w. of RJ samples were prepared for use in supplementation.
Animals
Wistar rats (207–250g) were obtained and kept in the Center for Bioterism and Experimentation at the Federal University of Uberlândia, Uberlândia, Brazil. Animals were kept in controlled conditions (22 ± 1 °C, humidity 60% ± 5 and 12-hour light-dark cycles– 6:00/18:00 h lights on/off) with a standard diet and water ad libitum. Body weight was measured at the beginning and end of the study, while water and food intake per animal were measured daily. All experimental procedures were approved and conducted by the Brazilian Society of Laboratory Animal Science and the Ethics Committee for Animal Research of the Federal University of Uberlândia, Brazil (CEUA No. 047/14).
Induction of stress by restraint and cold
Animals were randomly allocated into four groups (n = 10/group): no stress (NS); no stress supplemented with royal jelly (NSRJ); stress (S); and stress supplemented with royal jelly (SRJ). The animals were exposed to stress by restraint and cold, using the method of Paula-Freire et al. [21]. These method effectively mimics a condition of physical and psychological stress [2]. The restraint stress was carried out using individual acrylic hemicylindrical plastic tubes (4.5 cm diameter, 12 cm long) for 2 hours daily in the morning (8:00 h– 10:00 h.). The cold stress was carried at 10°C for 2 hours daily in the afternoon (16:00 h– 18:00 h).
Supplementation with RJ began seven days before the stress sessions with subsequent supplementation for seven more days during the period of stress induction [21]. RJ was administered by oral gavage 45 minutes before the stress session. The NS and S rats received a placebo (water) and the NS and NSRJ rats were not exposed to stressors.
On the fourteenth day, animals were exposed to the two stressors simultaneously for 2 hours [21] until euthanasia. Glucose levels were measured before and after this last session of stress (Sb—stress before; SRJb—stress supplemented with RJ before stress session; Sa—stress after; SRJa—stress supplemented with RJ after stress session) by puncturing the tail vein, using reactive strips (Accu-Chek Performa, Roche Diagnostic Systems, Basel, Switzerland).
The NS, NSRJ, S, SRJ rats were anaesthetized with ketamine (90 mg/kg) and xylazine (20 mg/kg), in accordance the methods of Arnold and Langhans, 2010 [22]. All groups were subjected to the same manipulation procedure. Plasma samples were used to assess corticosterone levels and hepatic enzyme activities, whereas liver tissues were used for oxidative stress analysis.
Determination of corticosterone levels by radioimmunoassay
Blood samples were collected (08:00 h– 11:00 h in heparinized plastic tubes and centrifuged at 1200g at 4°C for 15 min.) after the last session of stress via cardiac puncture in the right ventricle. Plasma was separated and frozen at -20°C until the assay. Radioimmunoassay (RIA) used H3-corticosterone from NEN Life Science Products (Boston, USA) and a standard reference specific antibody from Sigma (St. Louis, MO, USA). Corticosterone was used to measure tritiated recovery [23]. The intra-assay error was 4.5% and the minimum detectable dose was 0.08 ng/ml.
Hepatic enzymes activities in plasma
Aspartate transaminase (AST), alanine transaminase (ALT), γ-glutamyl transferase (GGT) and alkaline phosphatase (ALP) were measured at the Laboratory of Clinical Analyses, School of Veterinary Medicine, Federal University of Uberlândia, using an automatic analyzer (Cobas Mira, Roche Diagnostic Systems, Basel—Switzerland), by using commercial kits (Labtest Diagnóstica, Lagoa Santa—Brazil).
Sample collection and tissue preparation
The liver tissues were quickly removed, separated in lobes, washed (NaCl 0.9% buffer) and immersed in liquid nitrogen. Then, the same part of liver tissues were thawed and homogenized in phosphate buffer (1:10 w/v, pH 7.4). The homogenates were centrifuged at 800 x g for 15 min at 4°C, and the total protein concentration in the supernatant samples was measured, according to the Bradford assay [24].
Oxidative stress marker analysis
Thiobarbituric acid reactive substances (TBARS)
Lipid peroxidation was measured by the reaction between malondialdehyde in the liver samples (MDA) and thiobarbituric acid (0.67% TBA). Organic-phase fluorescence was evaluated at 515 nm (excitation) and at 553 nm (emission). A MDA standard curve allowed the quantification of the compound in the samples by linear regression [25]. TBARS levels were calculated as nmol TBARS/mg of protein.
Total antioxidant capacity (FRAP)
Total antioxidant capacity was evaluated by the capacity of the samples to reduce Fe+3 to Fe+2, which was then chelated by TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine) in order to form the deep-blue colored Fe+2-TPTZ complex [25]. This complex was measured in a spectrophotometer at 593 nm.
Superoxide dismutase (SOD) activity
SOD activity was measured by the inhibition autoxidative capacity of pyrogallol. The SOD activity was evaluated using a spectrophotometer at 420 nm. A calibration curve was constructed using SOD as standard. A 50% inhibition of autoxidation of pyrogallol was defined as one SOD unit [25].
Reduced glutathione (GSH)
The protein content of the samples was initially precipitated by metaphosphoric acid (MPA) at the ratio of 1:1 (homogenate/MPA). The samples were centrifuged at 7000xg for 10 minutes. The supernatant was collected and mixed with sodium phosphate buffer (100 mM, pH 8.0), containing EDTA (5mM) and ortho-phthaldialdehyde (1 mg/mL in methanol). The mixture was incubated in the dark at room temperature for 15 min and fluorescence was measured at 350 nm (excitation) and 420 nm (emission). A standard curve of GSH (0.001–0.1 mM) was used for linear regression [25].
Glutathione peroxidase (GPx) activity
To measure the glutathione peroxidase activity, the homogenate was incubated with GPx buffer (100 mM potassium phosphate containing 1 mM EDTA, pH7.7), sodium azide (40 mM), GSH (diluted in 5% metaphosphoric acid), GR (diluted in GPx buffer), NADPH (diluted with sodium bicarbonate 5%) and tert-butyl (0.5 mM). The reduction in NADPH concentration was evaluated for 10 minutes in a spectrophotometer, at 340 nm [25].
Glutathione reductase (GR) activity
GR activity was evaluated using oxidized glutathione (GSSG) and nicotinamide adenine dinucleotide phosphate (NADPH) as substrates. The activity of the enzyme was determined using sodium phosphate buffer (200 mM, pH 7.5), EDTA (6.3 mM), GSSG (1 mM), NADPH (1 mM) and the samples [25]. The consumption of NADPH was measured at 340 nm for 10 minutes. A GR unit is defined as one μmol of reduced GSSG per minute. The specific activity was calculated as U/mg of protein.
Glucose-6-phosphate dehydrogenase (G6PDH) activity
The activity of glucose-6-phosphate dehydrogenase was monitored by the production of NADPH with a consequent increase in absorbance at 340nm. The samples were incubated with Tris-HCl buffer (100mM, pH 7.5), magnesium chloride (MgCl2, 2 M), NADP+ (0.5 mM) and glucose-6-phosphate (1mM). The kinetic readings were monitored for ten minutes [25].
Statistical analyses
Data were used as independent variables (stress by restraint and cold) and as the dependent variables (body weight, water and food intake, biochemical parameters and oxidative stress). The data were analyzed using the one-way analysis of variance (ANOVA) followed by the Tukey Multiple Comparison as a post-hoc test. All analyses were performed using the software GraphPad Prism (GraphPad Prism version 6.00 for Windows; GraphPad Software, San Diego, CA, USA). Outliers were detected by performing Grubb’s test using an online GraphPad outlier calculator (http://graphpad.com/quickcalcs/Grubbs1.cfm). Only values of p < 0.05 were considered significant. Results were expressed as mean ± SEM.
Results
Table 1 shows the effect of RJ supplementation on body weight, water and food intake and hepatic enzyme activities in the plasma of restraint and cold stressed rats. S, NSRJ and SRJ decreased the body weight compared to NS (F3, 33 = 7.757, p < 0.05, F3, 33 = 7.757, p < 0.05; F3, 33 = 7.757, p < 0.001, respectively), and no change was observed between S and SRJ rats. No significant difference was observed in the water or food intake among the groups. In addition, NSRJ and SRJ decreased the AST levels compared to NS (F3, 29 = 17.64, p < 0.001; F3, 29 = 17.64, p < 0.01, respectively) and SRJ compared to S rats (F3, 29 = 17.64, p < 0.05), while ALT was not different among the groups. S rats had increased GGT compared to NS (F3, 27 = 14.17, p < 0.001) whereas SRJ rats had decreased GGT levels compared to S rats (F3, 27 = 14.17, p < 0.05). Furthermore, NSRJ and SRJ had decreased ALP levels compared to NS rats (F3, 31 = 5.203, p < 0.01; F3, 31 = 5.203, p < 0.05, respectively), whereas no change was observed in SRJ compared to S rats or in S compared to NS rats.
Table 1. The effect of the stress-induction by restraint and cold and supplementation of royal jelly on body weight, water intake, food intake and hepatic enzymes activities in plasma.
| Parameters | NS | NSRJ | S | SRJ |
|---|---|---|---|---|
| Δ Body weight (g) | 51.11±2.54 | 38.97±2.04* | 39.63±4.19* | 32.22±2.14* |
| Water intake (ml) | 41.25±4.19 | 44.07±4.66 | 37.56±2.52 | 38.91±6.25 |
| Food intake (g) | 23.51±0.35 | 24.30±0.71 | 22.98±0.40 | 22.82±0.27 |
| AST (U/L) | 99.78±3.30 | 67.13±5.41* | 97±1.19 | 83.44±3.24*# |
| ALT (U/L) | 47.5±4.03 | 46.38±2.19 | 48.22±1.66 | 53.44±2.91 |
| GGT (U/L) | 8.71±1.42 | 7.02±0.70 | 22.49±3.25* | 14.16±1.59# |
| ALP (U/L) | 336.2±38.04 | 223±16.35* | 279±8.60 | 242.3±16.89* |
Note: Values are expressed as mean ± S.E.M (n = 10).
*p < 0. 05 vs NS rats;
#p < 0. 05 vs S rats.
No stress (NS), No stress supplemented with royal jelly (NSRJ), Stress(S) and Stress supplemented with royal jelly (SRJ); Aspartate transaminase (AST); Alanine transaminase (ALT); γ-glutamyl transferase (GGT) and Alkaline phosphatase (ALP).
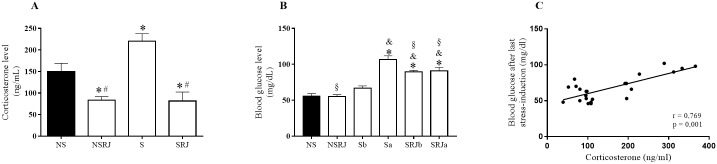
Stress biomarkers were evaluated in plasma samples of NS, NSRJ, S and SRJ rats. Plasma corticosterone levels increased in S compared to NS rats (F3,28 = 14.56, p < 0.05) whereas RJ supplementation (NSRJ and SRJ) decreased corticosterone levels compared to NS (F3,28 = 14.56, p < 0.05) and S rats (F3,28 = 14.56, p < 0.001) (Fig 1A). Furthermore, blood glucose levels did not differ in NSRJ and Sb compared to NS rats, whereas an increase was verified in Sa, SRJb and SRJa compared with both NS (F5, 47 = 32.55, p < 0.001) and between Sa and SRJa compared to Sb rats (F5, 47 = 32.55, p < 0.001; F5, 47 = 32.55, p < 0.01, respectively). When glycemia was compared among the stressed rats, SRJa (F5, 47 = 32.55, p < 0.05) and SRJb (F5, 47 = 32.55, p < 0.01) had decreased blood glucose levels compared with Sa rats, whereas no difference was observed between SRJb compared to Sb and SRJa rats (Fig 1B). In addition, Pearson correlation between corticosterone levels and glycemia after the last stress induction showed a strong positive correlation (r = 0.769, p = 0.001) (Fig 1C).
Fig 1. Biomarkers of chronic stress in liver tissue of rats stressed by restraint and cold.
Plasma corticosterone level after seven days of stress-induction (A). No stress (NS), No Stress supplemented with Royal Jelly (NSRJ), Stress (S) and Stress supplemented with Royal Jelly (SRJ). Blood glucose level before and after the last stress induction (B). No stress (NS), No Stress supplemented with Royal Jelly (NSRJ), Stress group before the last stress session (Sb), Stress group after the last stress session (Sa), Stress group supplemented with RJ before the last stress session (SRJb), Stress group supplemented with RJ after the last stress session (SRJa). Pearson correlation of mean values of corticosterone levels and blood glucose after the last stress-induction (C). Values are expressed as means ± SEM. *p < 0.05 vs NS, # p < 0.05 vs S, & p < 0.05 vs Sb, § p < 0.05 vs Sa (One-way ANOVA followed by Tukey test). Outliers were detected by performing Grubb’s test using an online GraphPad outlier calculator (http://graphpad.com/quickcalcs/Grubbs1.cfm).
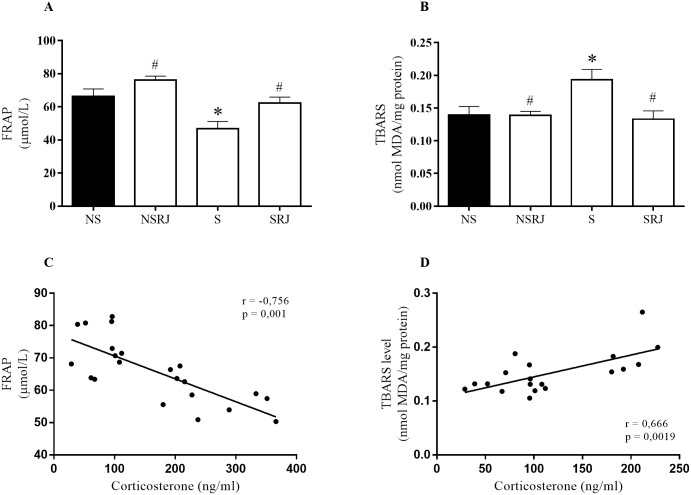
Fig 2 shows the oxidative stress status in liver tissue. Stressed rats (S) decreased the total antioxidative capacity (FRAP) (F3, 23 = 11.65, p < 0.01) and increased lipid peroxidation (TBARS) (F3, 21 = 5.027, p < 0.05) compared to NS rats. SRJ rats increased FRAP (F3, 23 = 11.65, p < 0.05) and decreased malondialdehyde levels (F3, 21 = 5.027, p < 0.05) compared to S rats (Fig 2A and 2B). Pearson correlation between corticosterone and FRAP showed a negative correlation (r = -0.756, p = 0.001), whereas a positive correlation (r = 0.666, p = 0.0019) was observed between corticosterone and lipid peroxidation (Fig 2C and 2D).
Fig 2. Biomarkers of oxidative stress in liver tissue of rats stressed by restraint and cold.
Total antioxidant capacity by FRAP method (A). Lipid Peroxidation by TBARS method (B). No stress (NS), No Stress supplemented with Royal Jelly (NSRJ), Stress (S) and Stress supplemented with Royal Jelly (SRJ). Values are expressed as mean±SEM, * p < 0.05 vs. NS, # p < 0.05 vs. S (One-way ANOVA followed by Tukey test). Pearson correlation of FRAP and TBARS (panels C and D) and means values of corticosterone levels. Outliers were detected by performing Grubb’s test using an online GraphPad outlier calculator (http://graphpad.com/quickcalcs/Grubbs1.cfm).
SOD enzyme activity in liver tissues is shown in Fig 3. The S rats displayed a decreased SOD activity compared to NS rats (F3, 40 = 18.18, p < 0.05), whereas RJ supplementation increased the SOD activity in SRJ groups compared to S rats (F3, 40 = 18.18, p < 0.001). Furthermore, NSRJ rats increased the SOD compared to NS (F3, 40 = 18.18, p < 0.05) (Fig 3).
Fig 3. Superoxide dismutase activity in liver tissue of rats stressed by immobilization and cold.
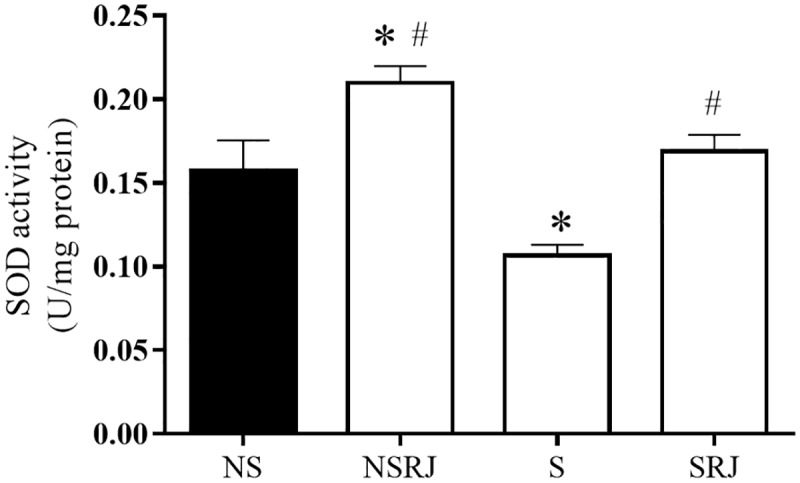
No stress (NS), No Stress supplemented with Royal Jelly (NSRJ), Stress (S) and Stress supplemented with Royal Jelly (SRJ). Values are expressed as mean±SEM, * p < 0.05 vs. NS, # p < 0.05 vs. S (One-way ANOVA followed by Tukey test). Outliers were detected by performing Grubb’s test using an online GraphPad outlier calculator (http://graphpad.com/quickcalcs/Grubbs1.cfm).
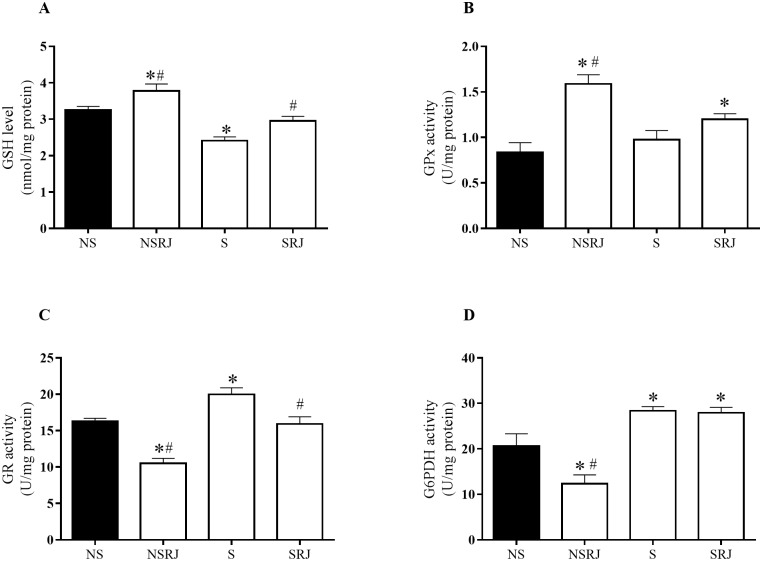
The glutathione defense system analysis in liver tissues is displayed in Fig 4. GSH content was lower in S compared to NS (F3, 51 = 17.65, p < 0.001), whereas GR and G6PDH levels were higher in S compared to NS (F3, 21 = 20.42, p < 0.05; F3, 20 = 17.24, p < 0.05, respectively). No significant difference was observed in GPx activity in S rats compared with NS rats. In addition, the glutathione defense system analysis of the RJ supplemented S rats showed an increase in GSH content (F3, 51 = 17.65, p < 0.05), a decrease in the GR level (F3, 21 = 20.42, p < 0.05), whereas no difference was observed in GPx and G6PDH activity compared to S rats.
Fig 4. Glutathione antioxidant defense system in liver tissue of rats stressed by immobilization and cold.
Glutathione level (GSH) (A). Glutathione peroxidase activity (GPx) (B). Glutathione reductase activity (GR) (C). Glucose-6-phosphate dehydrogenase activity (G6PDH) (D). No stress (NS), No Stress supplemented with Royal Jelly (NSRJ), Stress (S) and Stress supplemented with Royal Jelly (SRJ). Values are expressed as mean±SEM, * p < 0.05 vs. NS, # p < 0.05 vs. S (One-way ANOVA followed by Tukey teste). Outliers were detected by performing Grubb’s test using an online GraphPad outlier calculator (http://graphpad.com/quickcalcs/Grubbs1.cfm).
Discussion
Studies have been carried out to assess agents that could prevent the damage to liver tissues triggered by acute and chronic stress, which induce the formation of ROS followed by hepatic injury [26],[27]. In this study, the adaptogenic potential of RJ was assessed and associated with the antioxidant activity and anti-stress capacity, to improve the organism’s adaptation to stress induction and in absence of stress. Our results showed a decrease of corticosterone and blood glucose levels, weight loss as well as an improvement in the antioxidative parameters in the liver of stressed RJ-supplemented rats.
Stress promotes the activation of the HPA axis, thus stimulating the release of corticosterone by the adrenal gland [3]. This increase in corticosterone mobilizes energy substrates in liver tissues to maintain homeostasis in a stress situation [28]. In the present study, restraint and cold stress not only augmented corticosterone levels, blood glucose levels and biomarkers of oxidative stress, but also diminished the glutathione antioxidant defense system in the liver tissues. Our results corroborate other studies that reported an increase of corticosterone levels under several stress models such as restraint and cold, immobilization and cold, besides other models of stress induction [2, 4–6, 20, 29]. Besides that, we showed a decreased of the corticosterone level associated with RJ supplementation, even in absence of stress. Teixeira et al. (2017) also demonstrated the effect of RJ in reducing corticosterone levels in stressed rats [20]. Corticosterone synthesis is cholesterol-dependent, which indicates the possibility that RJ is able to inhibit the synthesis of cholesterol. Major royal jelly protein 1 was identified as a hypocholesterolemic protein [30], indicating a possible mechanism through which RJ could inhibit corticosterone synthesis. Furthermore, the decreased corticosterone levels improved glucose uptake [31], which could control glycemia even after stress induction.
Fontella et al. (2005) demonstrated that the repeated exposure of adult rats to restraint stress causes a temporary suppression of food intake and reduction of body weight [32]. Herein, we observed a decrease of body weight in both groups of stressed rats and non-stressed rats supplemented with RJ, even without a diminution in food intake. These results corroborate a prior study [32] demonstrating that the diminution of body weight in restriction and cold stressed rats is attributable to an elevation of corticosterone levels. Furthermore, our data indicate that the RJ can also diminish body weight even in the absence of stress induction. Although we did not evaluate the mechanism by which supplementation with RJ reduces body weight, other studies have also showed that RJ supplementation can diminish body weight [33, 34]. New studies must be conducted to investigate the mechanism by which this effect occurs.
Stress alters the availability of metabolic substrates and can influence blood glucose levels, leading to an increase in oxidative stress [35]. Our results indicate that the glycemia of non-stressed rats supplemented with royal jelly did not differ from the non-stressed group. Although we did not measure the plasmatic insulin, a prior study demonstrated that RJ did not affect insulin levels [36]. Thus, the augmentation of corticosterone levels is probably responsible for increased the blood glucose level under this stress situation, due to its role in hepatic gluconeogenesis [37]. Furthermore, a strong positive correlation between glycemia and corticosterone levels was observed after the last stress induction session. These results indicate not only that glycemic control is corticosterone-dependent in the stress response, but also that after the last stress session (Sa and SRJa) the RJ supplementation diminished the increase of blood glucose levels compared to Sa rats. Thus, the capacity to inhibit the increase in blood glucose levels after stress induction indicates a potential anti-stress and adaptogenic effects of RJ.
Corticosterone acts on the liver, increasing glucose production especially through gluconeogenesis [37]. It can also increase oxidative stress and induce damage in the liver tissue [38]. Stressed rats presented increased plasma GGT compared with NS rats, indicating hepatic damage [39]. RJ supplementation reduced both GGT levels and AST compared with S rats, and also reduced AST and ALP compared with NS rats. Other studies using models of toxicity induced by lambda-cyhalothrin and azathioprine also found a decrease in these enzymes, supporting the protective effect of RJ towards liver tissue [40, 41]. Thus, data observed here suggests a hepatoprotective effect of RJ, not only by improving the liver enzymes, but also the antioxidative systems.
High levels of glucocorticoids and exposure to stress increase ROS [10, 11]. Herein, stressed rats had increased lipid peroxidation associated with the corticosterone level, corroborating other studies [38, 42]. However, RJ supplementation decreased the lipid peroxidation in the liver of stressed rats. RJ peptides can diminish the peroxidation of linoleic acid and eliminate hydroxyl radicals, inhibiting lipid peroxidation [43]. In addition, in the present study, stressed rats decreased total antioxidant capacity associated with the increase of corticosterone levels, whereas RJ supplementation restored FRAP to levels comparable to those of NS rats. RJ contains vitamins B and E, zinc, copper [44], phenolic compounds [45] and peptides with antioxidant actions [43]. These RJ compounds can act as antioxidant agents preventing the oxidative damage in the liver, suggesting that RJ supplementation may improve the antioxidant defense of stressed rats.
Furthermore, we analyzed the enzymatic and glutathione antioxidant defense system in the liver tissues of the stressed rats. SOD activity decreased in stressed rats compared with NS and increased in rats supplemented with RJ compared with S rats. Other studies have also shown a decrease in this enzyme activity in the liver of rats undergoing stress, thereby corroborating our results [46, 47]. Oishi and Mashida (2009) reported a decrease in hepatic SOD mRNA six hours after stress by immobilization and cold [48]. Furthermore, our findings of decreased GSH and increased GR and G6PDH activities in stressed rats, indicate a compensatory mechanism to maintain the redox cycle of GSH, as shown in other studies [11, 42].
RJ supplementation also increased the GSH level, corroborating Karadeniz et al. (2011), who showed that RJ-supplemented rats present increased GSH in the liver and kidney in an oxidative stress model induced by cisplatin [49]. Furthermore, RJ supplementation decreased GR activity compared to S rats, and increased GPx and G6PDH activities similar to NS rats. Studies employing models of toxicity for cisplatin [49] and paracetamol [50] also showed increased GPx activity in rat livers. The unchanged GPx activity between stressed rats shown in the present study was not found in previous studies. Our finding may be attributable to the stress model used in this study. To the best of our knowledge, this is the first study that has observed the effect of RJ supplementation on the activity of GR and G6PDH in the liver during a stress situation. The RJ antioxidant property may be derived from the short-chain peptides [43], phenolic compounds (flavonoids and cinnamic acid derivatives) [45], some antioxidant type vitamins (A and E) and fatty acids (trans-10-Hydroxy-2-decenoic) [19]. Therefore, these results support the antioxidant activity of RJ and indicate a prevention of hepatic oxidative damage caused by stress.
Conclusion
RJ decreases corticosterone and improves glycemia control after stress induction. Moreover, RJ showed a hepatoprotective effect against oxidative damage, reducing lipoperoxidation and increasing the total antioxidant capacity in liver tissues of restraint and cold stressed rats. Taken together, these results highlight an adaptogenic role of RJ in situations of stress and oxidative damage.
Acknowledgments
The authors gratefully acknowledge the Apiário Girassol Ltda. (Uberlândia, Brazil) for donating the royal jelly for the development of this study; Center of Animal Experimentation (CBEA-UFU) of the Federal University of Uberlândia for supplying the animals and infrastructural support; Foundation of Support Research of the State of Minas Gerais (FAPEMIG) for financial support; Laboratory of Clinical Analyses, School of Veterinary Medicine, Federal University of Uberlândia for help with the plasma biochemical analysis, Laboratory of Bromatology and Animal Nutrition of the Federal University of Uberlândia for centesimal analysis of the royal jelly and the support from the National Institute of Science and Technology in Theranostics and Nanobiotechnology (INCT). DCC, HLM, AVS and DDV received graduate fellowships from the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). LGP received postdoctoral fellowships from the National Postdoctoral Program PNPD/CAPES and FSE is recipient of grant from CNPq (308965/2015-9).
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received funding support for this work by Foundation of Support Research of the State of Minas Gerais (FAPEMIG), National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Budni J, Zomkowski AD, Engel D, Santos DB, dos Santos AA, Moretti M, et al. Folic acid prevents depressive-like behavior and hippocampal antioxidant imbalance induced by restraint stress in mice. Experimental neurology. 2013;240:112–21. Epub 2012/11/13. doi: 10.1016/j.expneurol.2012.10.024 . [DOI] [PubMed] [Google Scholar]
- 2.Klenerová V, Jurcovicová J, Kaminský O, Sída P, Krejcí I, Hlinák Z, et al. Combined restraint and cold stress in rats: effects on memory processing in passive avoidance task and on plasma levels of ACTH and corticosterone. Behav Brain Res. 2003;142(1–2):143–9. . [DOI] [PubMed] [Google Scholar]
- 3.Djordjevic J, Cvijic G, Davidovic V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol Res. 2003;52(1):67–72. Epub 2003/03/11. . [PubMed] [Google Scholar]
- 4.Solin AV, Lyashev YD. Stress-induced changes in the liver of rats with different resistance to stress. Bull Exp Biol Med. 2014;157(5):571–3. doi: 10.1007/s10517-014-2617-7 . [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A, Jaggi AS, Singh N. Pharmacological investigations on adaptation in rats subjected to cold water immersion stress. Physiol Behav. 2011;103(3–4):321–9. doi: 10.1016/j.physbeh.2011.02.014 . [DOI] [PubMed] [Google Scholar]
- 6.Bali A, Singh N, Jaggi AS. Investigations into mild electric foot shock stress-induced cognitive enhancement: possible role of angiotensin neuropeptides. Journal of the renin-angiotensin-aldosterone system: JRAAS. 2013;14(3):197–203. Epub 2012/09/01. doi: 10.1177/1470320312456579 . [DOI] [PubMed] [Google Scholar]
- 7.Sierra M. Depersonalization disorder: pharmacological approaches. Expert review of neurotherapeutics. 2008;8(1):19–26. Epub 2007/12/20. doi: 10.1586/14737175.8.1.19 . [DOI] [PubMed] [Google Scholar]
- 8.Mlekusch W, Paletta B, Truppe W, Paschke E, Grimus R. Plasma concentrations of glucose, corticosterone, glucagon and insulin and liver content of metabolic substrates and enzymes during starvation and additional hypoxia in the rat. Horm Metab Res. 1981;13(11):612–4. Epub 1981/11/01. doi: 10.1055/s-2007-1019352 . [DOI] [PubMed] [Google Scholar]
- 9.Spiers JG, Chen HJ, Cuffe JS, Sernia C, Lavidis NA. Acute restraint stress induces rapid changes in central redox status and protective antioxidant genes in rats. Psychoneuroendocrinology. 2016;67:104–12. doi: 10.1016/j.psyneuen.2016.02.005 . [DOI] [PubMed] [Google Scholar]
- 10.Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005;30(1):105–11. . [DOI] [PubMed] [Google Scholar]
- 11.Sahin E, Gümüşlü S. Cold-stress-induced modulation of antioxidant defence: role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int J Biometeorol. 2004;48(4):165–71. doi: 10.1007/s00484-004-0205-7 . [DOI] [PubMed] [Google Scholar]
- 12.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. Epub 2012/01/13. doi: 10.1097/WOX.0b013e3182439613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419–30. Epub 1969/01/01. doi: 10.1146/annurev.pa.09.040169.002223 . [DOI] [PubMed] [Google Scholar]
- 14.Fujii A, Kobayashi S, Kuboyama N, Furukawa Y, Kaneko Y, Ishihama S, et al. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Japanese journal of pharmacology. 1990;53(3):331–7. Epub 1990/07/01. . [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga KH, Yoshida C, Suzuki KM, Maruyama H, Futamura Y, Araki Y, et al. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biological & pharmaceutical bulletin. 2004;27(2):189–92. Epub 2004/02/06. . [DOI] [PubMed] [Google Scholar]
- 16.Chan QW, Melathopoulos AP, Pernal SF, Foster LJ. The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC genomics. 2009;10:387 Epub 2009/08/22. doi: 10.1186/1471-2164-10-387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto I, Taniguchi Y, Kunikata T, Kohno K, Iwaki K, Ikeda M, et al. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life sciences. 2003;73(16):2029–45. Epub 2003/08/06. . [DOI] [PubMed] [Google Scholar]
- 18.Kamakura M, Moriyama T, Sakaki T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. The Journal of pharmacy and pharmacology. 2006;58(12):1683–9. Epub 2007/03/03. doi: 10.1211/jpp.58.12.0017 . [DOI] [PubMed] [Google Scholar]
- 19.Nagai T, Inoue R, Suzuki N, Nagashima T. Antioxidant properties of enzymatic hydrolysates from royal jelly. Journal of medicinal food. 2006;9(3):363–7. Epub 2006/09/29. doi: 10.1089/jmf.2006.9.363 . [DOI] [PubMed] [Google Scholar]
- 20.Teixeira RR, de Souza AV, Peixoto LG, Machado HL, Caixeta DC, Vilela DD, et al. Royal jelly decreases corticosterone levels and improves the brain antioxidant system in restraint and cold stressed rats. Neuroscience letters. 2017;655:179–85. Epub 2017/07/16. doi: 10.1016/j.neulet.2017.07.010 . [DOI] [PubMed] [Google Scholar]
- 21.Paula-Freire LI, Mendes FR, Molska GR, Duarte-Almeida JM, Carlini EA. Comparison of the chemical composition and biological effects of the roots, branches and leaves of Heteropterys tomentosa A. Juss. Journal of ethnopharmacology. 2013;145(2):647–52. Epub 2012/12/15. doi: 10.1016/j.jep.2012.12.004 . [DOI] [PubMed] [Google Scholar]
- 22.Arnold M, Langhans W. Effects of anesthesia and blood sampling techniques on plasma metabolites and corticosterone in the rat. Physiol Behav. 2010;99(5):592–8. Epub 2010/02/10. doi: 10.1016/j.physbeh.2010.01.021 . [DOI] [PubMed] [Google Scholar]
- 23.Reis FM, Albrechet-Souza L, Franci CR, Brandao ML. Risk assessment behaviors associated with corticosterone trigger the defense reaction to social isolation in rats: role of the anterior cingulate cortex. Stress. 2012;15(3):318–28. Epub 2011/10/14. doi: 10.3109/10253890.2011.623740 . [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–54. Epub 1976/05/07. . [DOI] [PubMed] [Google Scholar]
- 25.Diniz Vilela D, Gomes Peixoto L, Teixeira RR, Belele Baptista N, Carvalho Caixeta D, Vieira de Souza A, et al. The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxid Med Cell Longev. 2016;2016:6978625 doi: 10.1155/2016/6978625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafari M, Salehi M, Zardooz H, Rostamkhani F. Response of liver antioxidant defense system to acute and chronic physical and psychological stresses in male rats. EXCLI journal. 2014;13:161–71. Epub 2014/01/01. . [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, et al. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16(11):26087–124. Epub 2015/11/02. doi: 10.3390/ijms161125942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12(2):167–77. doi: 10.1080/10253890802234168 . [DOI] [PubMed] [Google Scholar]
- 29.Celikbilek A, Gocmen AY, Tanik N, Yaras N, Yargicoglu P, Gumuslu S. Serum lipid peroxidation markers are correlated with those in brain samples in different stress models. Acta Neuropsychiatr. 2014;26(1):51–7. doi: 10.1017/neu.2013.32 . [DOI] [PubMed] [Google Scholar]
- 30.Kashima Y, Kanematsu S, Asai S, Kusada M, Watanabe S, Kawashima T, et al. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS One. 2014;9(8):e105073 doi: 10.1371/journal.pone.0105073 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piato AL, Detanico BC, Linck VM, Herrmann AP, Nunes DS, Elisabetsky E. Anti-stress effects of the "tonic"Ptychopetalum olacoides (Marapuama) in mice. Phytomedicine. 2010;17(3–4):248–53. Epub 2009/08/18. doi: 10.1016/j.phymed.2009.07.001 . [DOI] [PubMed] [Google Scholar]
- 32.Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275(6 Pt 2):R1928–38. . [DOI] [PubMed] [Google Scholar]
- 33.Guo H, Saiga A, Sato M, Miyazawa I, Shibata M, Takahata Y, et al. Royal jelly supplementation improves lipoprotein metabolism in humans. J Nutr Sci Vitaminol (Tokyo). 2007;53(4):345–8. . [DOI] [PubMed] [Google Scholar]
- 34.Pourmoradian S, Mahdavi R, Mobasseri M, Faramarzi E. Effects of royal jelly supplementation on body weight and dietary intake in type 2 diabetic females. Health Promot Perspect. 2012;2(2):231–5. doi: 10.5681/hpp.2012.028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CM, Hsieh CJ, Huang JC, Huang IC. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49 Suppl 1:S171–7. doi: 10.1007/s00592-012-0398-x . [DOI] [PubMed] [Google Scholar]
- 36.Ghanbari E, Nejati V, Khazaei M. Improvement in Serum Biochemical Alterations and Oxidative Stress of Liver and Pancreas following Use of Royal Jelly in Streptozotocin-Induced Diabetic Rats. Cell journal. 2016;18(3):362–70. Epub 2016/09/08. doi: 10.22074/cellj.2016.4564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nirupama R, Devaki M, Yajurvedi HN. Chronic stress and carbohydrate metabolism: persistent changes and slow return to normalcy in male albino rats. Stress. 2012;15(3):262–71. doi: 10.3109/10253890.2011.619604 . [DOI] [PubMed] [Google Scholar]
- 38.Sahin E, Gümüşlü S. Stress-dependent induction of protein oxidation, lipid peroxidation and anti-oxidants in peripheral tissues of rats: comparison of three stress models (immobilization, cold and immobilization-cold). Clin Exp Pharmacol Physiol. 2007;34(5–6):425–31. doi: 10.1111/j.1440-1681.2007.04584.x . [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Wang H, Guo Y, Lei W, Wang J, Hu X, et al. Metal Ion Imbalance-Related Oxidative Stress Is Involved in the Mechanisms of Liver Injury in a Rat Model of Chronic Aluminum Exposure. Biol Trace Elem Res. 2016. doi: 10.1007/s12011-016-0627-1 . [DOI] [PubMed] [Google Scholar]
- 40.Cavusoglu K, Yapar K, Oruc E, Yalcin E. The protective effect of royal jelly on chronic lambda-cyhalothrin toxicity: serum biochemical parameters, lipid peroxidation, and genotoxic and histopathological alterations in swiss albino mice. Journal of medicinal food. 2011;14(10):1229–37. Epub 2011/06/15. doi: 10.1089/jmf.2010.0219 . [DOI] [PubMed] [Google Scholar]
- 41.Cemek M, Aymelek F, Buyukokuroglu ME, Karaca T, Buyukben A, Yilmaz F. Protective potential of Royal Jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem Toxicol. 2010;48(10):2827–32. Epub 2010/07/20. doi: 10.1016/j.fct.2010.07.013 . [DOI] [PubMed] [Google Scholar]
- 42.Hidalgo J, Gasull T, Garcia A, Blanquez A, Armario A. Role of glucocorticoids and catecholamines on hepatic thiobarbituric acid reactants in basal and stress conditions in the rat. Horm Metab Res. 1991;23(3):104–9. doi: 10.1055/s-2007-1003626 . [DOI] [PubMed] [Google Scholar]
- 43.Guo H, Ekusa A, Iwai K, Yonekura M, Takahata Y, Morimatsu F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J Nutr Sci Vitaminol (Tokyo). 2008;54(3):191–5. . [DOI] [PubMed] [Google Scholar]
- 44.Karaali A, Meydanoǧlu F, Eke D. Studies on Composition, Freeze-Drying and Storage of Turkish Royal Jelly. 2015. [Google Scholar]
- 45.Kolayli S, Sahin H, Can Z, Yildiz O, Malkoc M, Asadov A. A Member of Complementary Medicinal Food: Anatolian Royal Jellies, Their Chemical Compositions, and Antioxidant Properties. J Evid Based Complementary Altern Med. 2015. doi: 10.1177/2156587215618832 . [DOI] [PubMed] [Google Scholar]
- 46.Su D, Zhang R, Zhang C, Huang F, Xiao J, Deng Y, et al. Phenolic-rich lychee (Litchi chinensis Sonn.) pulp extracts offer hepatoprotection against restraint stress-induced liver injury in mice by modulating mitochondrial dysfunction. Food Funct. 2016;7(1):508–15. doi: 10.1039/c5fo00975h . [DOI] [PubMed] [Google Scholar]
- 47.Samarghandian S, Farkhondeh T, Samini F, Borji A. Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat’s Brain, Liver, and Kidney. Biochem Res Int. 2016;2016:2645237. doi: 10.1155/2016/2645237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oishi K, Machida K. Different effects of immobilization stress on the mRNA expression of antioxidant enzymes in rat peripheral organs. http://dx.doi.org/10.1080/003655102753611735. 2009. [DOI] [PubMed] [Google Scholar]
- 49.Karadeniz A, Simsek N, Karakus E, Yildirim S, Kara A, Can I, et al. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid Med Cell Longev. 2011;2011:981793 Epub 2011/09/10. doi: 10.1155/2011/981793 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanbur M, Eraslan G, Beyaz L, Silici S, Liman BC, Altinordulu S, et al. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp Toxicol Pathol. 2009;61(2):123–32. doi: 10.1016/j.etp.2008.06.003 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.