ABSTRACT
Apoptosis is a ubiquitous mode of programmed cell death that is found in healthy organs and can be stimulated by many toxic stresses. When it occurs in epithelia, apoptosis presents major challenges to tissue integrity. Apoptotic corpses can promote inflammatory and autoimmune responses if they are retained, and the cellular fragmentation that accompanies apoptosis can potentially compromise the epithelial barrier. Here we discuss 2 homeostatic mechanisms that allow epithelia to circumvent these potential risks: clearance of apoptotic corpses by professional and non-professional phagocytes and physical expulsion of apoptotic cells by apical extrusion. Extrusion and phagocytosis may represent complementary responses that preserve epithelial integrity despite the inevitable challenge of apoptosis.
KEYWORDS: apoptosis, epithelia, extrusion, phagocytosis, tissue mechanics
Introduction
Epithelia constitute fundamental tissue barriers of the body; they separate tissue compartments, enable exchange of essential nutrients and waste products, and protect the body from the external environment. Primary epithelial dysfunction can allow entry of toxins and microorganisms into the body, as well as the loss of fluid and solutes that are required for physiologic homeostasis. Moreover, professional pathogens can actively breach an intact epithelium to gain entry to underlying tissues, where they cause disease.1 Thus compromised barriers are commonly observed in both acute and chronic inflammatory disease, such as infective gastroenteritis and asthma.2,3
Effective epithelial barriers require healthy cells that are physically coupled to one another by specialized cell-cell junctions, notably tight junctions (TJ), which seal the paracellular pathway, and a variety of adhesion systems that maintain cell-cell cohesion. Thus epithelial cell injury and death present challenges to epithelial integrity. These challenges can arise from different modes of programmed cell death that are executed by dedicated signaling pathways and elicit distinct physiologic and pathophysiological consequences (reviewed in ref4). For example, pyroptosis is induced by caspases and executed by gasdermins, while necroptosis is signaled via RIPK3 and executed by MLKL. Like necrosis, a non-programmed form of cell death, pyroptosis and necroptosis are generally inflammatory, as they are associated with breakdown of plasma membrane integrity, leading to release of cellular contents that trigger an acute inflammatory response.4 In contrast, apoptosis, which is induced by caspases and executed by pro-apoptotic executioner proteins, is generally considered to be non-inflammatory in nature, as the plasma membrane remains intact.5,6
Apoptosis is a ubiquitous process during epithelial morphogenesis and homeostasis. Yet, at first glance, the cellular events of apoptosis might be expected to challenge epithelial integrity. During apoptosis, the moribund cell fragments are released as membrane-bound apoptotic bodies7 and cell-cell junctions are perturbed.8 Both these events constitute potentially fundamental challenges to barrier integrity. Nonetheless, epithelial barriers remain intact, even when apoptosis is stimulated.9 Thus, mechanisms must exist to preserve epithelial integrity in the face of the inevitable impact of apoptosis. In this review, we discuss 2 such mechanisms, clearance of apoptotic bodies by phagocytes (efferocytosis) and apical extrusion of apoptotic cells (Fig 1). We propose that these represent non-exclusive processes that serve to preserve barrier integrity and limit the inflammatory consequences of epithelial apoptosis. A better understanding of each of these pathways can also teach us how dysregulation in epithelial barrier function can contribute to its pathology.
Figure 1.
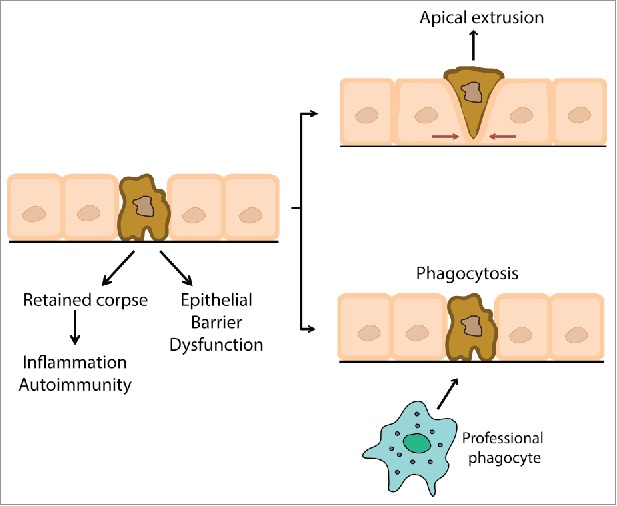
Epithelial responses to the challenge of apoptosis. Apoptosis presents 2 major challenges to epithelial integrity that can provoke inflammation and disrupt the epithelial barrier: cell fragmentation and junction disruption can perturb barrier integrity, while retained apoptotic corpses can provoke inflammation and autoimmune responses. These challenges are overcome by apical cell extrusion and phagocytsis of retained corpses.
The challenges of apoptosis for epithelial integrity
Apoptosis is a form of programmed cell death10 that was first identified by its distinctive morphological features, such as cell shrinkage, irreversible condensation of chromatin (pyknosis), and fragmentation of the nucleus (karyorrhexis). Apoptosis is ultimately mediated by the activation of a subset of caspases, cysteine proteases that cleave a diverse range of cellular substrates to initiate and execute the apoptotic program.
Three major pathways have been extensively characterized to mediate apoptosis (the mechanistic details of which have been the subject of many excellent reviews, to which the reader is directed).6,11 In brief, the intrinsic (or mitochondrial) pathway involves the release of pro-apoptotic proteins from the intermembrane space of mitochondria into the cytosol. Cellular stressors that can activate the intrinsic pathway include positive stimuli (injurious events that such as radiation, DNA damage and infection) or negative stimuli (i.e. the withdrawal of factors necessary for cell viability, such as growth factors). The extrinsic pathway is activated by death receptors of the Tumor Necrosis Factor (TNF) superfamily, such as TNFR1 and FasR and their ligands TNF-α and FasL, respectively. In general, TNF family receptor ligation leads to the cortical recruitment of cytoplasmic adaptor proteins, generating signaling complexes that can activate caspase cascades. Finally, the granzyme pathway is used by cytotoxic T lymphocytes and natural killer (NK) cells to induce apoptosis of their target cells via the action of secreted granzyme and performin.12 Perforin induces pores in the plasma membrane of the target cells, leading to calcium influx and triggering rapid dynamin-dependent endocytosis of granzymes.13,14 Granzymes are serine proteases which activate a variety of pathways to apoptosis.
Apoptosis occurs in all tissues of the body. However, when it occurs within epithelia, apoptosis presents 2 homeostatic challenges that can lead to inflammation and barrier dysfunction (Fig 1). Firstly, apoptosis is associated with cellular fragmentation that can fundamentally compromise the epithelial barrier. Thus, the apoptotic pathways described above ultimately lead to fragmentation of the apoptotic cell, creating a variety of small membrane-bound vesicles (apoptotic bodies) that contain cytoplasmic contents and organelles.10,15-17 Apoptotic fragmentation often begins with the formation of plasma membrane blebs,7,18 which are local evaginations of the cell surface that occur at defects in the actomyosin cell cortex. Blebbing is driven by the executioner caspase-3, which cleaves and activates Rho kinase 1 (ROCK1)19,20 and LIM-kinase 1 (LIMK1).21 Activated ROCK1 stimulates non-muscle Myosin II (NMII) by promoting phosphorylation of its regulatory light chain,20 whereas LIMK1 inactivates the actin-severing protein, cofilin.22 Together, these lead to an increase in actomyosin contractility that creates local defects in the cell cortex. Taken with the increased hydrostatic pressure of the apoptotic cell, this causes the plasma membrane to bulge outwards, forming blebs.23 Of note for our later discussion, hypercontractility is therefore a distinctive feature of apoptosis. Plasma membrane integrity is preserved by rearrangement of the microtubule network.24 Membrane blebs can then dissociate from the dying cell to form a variety of smaller apoptotic bodies (reviewed in.7) Apoptotic fragmentation can also occur independently of blebbing, through the phenomenon of beaded apoptopodia, which are formed by segmentation of membrane protrusions, causing the rapid release of a large number of small apoptotic bodies.25
Even where apoptosis occurs as a sporadic event, individual cell defects by fragmentation and junctional dysfunction, can potentially compromise epithelial barrier function. For example, in addition to preventing entry of microbes and toxins, the permselective epithelial barrier plays a critical role in establishing the transepithelial ionic gradients that are necessary for fluid and solute transport by secretory and absorptive epithelia.26 These gradients can be rapidly short-circuited by the loss of even a few cells in monolayers or dysfunction of the specialized junctions that couple them to their neighbors.
Secondly, although the plasma membrane that encloses apoptotic bodies limits inflammation by preventing the release of cellular contents, this protection is time-limited. Apoptotic corpses can undergo secondary necrosis,27,28 an autolytic process that is characterized by mitochondrial dysfunction, lysosomal membrane permeabilization and depletion of the intracellular ATP pool.28 These changes cause rupture of cell membranes, releasing a variety of Damage-associated Molecular Patterns (DAMPs, also known as alarmins). DAMPs are potently inflammatory stimuli that act via diverse pathways. For example, double-stranded DNA and double-stranded RNA29-31 constitute a class of DAMPs that act via the nucleic acid sensing Toll-like Receptors (TLR), TLR929 and TLR7/8,30 to drive innate immune cell activation. They can also induce the production of autoantibodies by B cells, potentially leading to lupus-like syndromes through the deposition of autoantibody complexes.32
Despite these potential challenges, many epithelial cells undergo apoptosis every day, even in healthy organisms. Yet, barrier function is preserved and chronic inflammation is prevented. This implies that there must be physiologic mechanisms that circumvent the potential homeostatic challenges of epithelial apoptosis. Indeed, this is achieved through the phagocytosis of apoptotic bodies (efferocytosis) and apical extrusion of apoptotic cells, processes we will now consider.
Phagocytosis of apoptotic corpses
Phagocytosis of apoptotic fragments provides a ready mechanism to eliminate moribund corpses before they undergo secondary necrosis to release immune-stimulatory DAMPs. Corpse removal is primarily mediated by professional phagocytes, such as neutrophils, monocytes and macrophages, which support heterotypic engulfment.33,34 In addition, other cell types, including epithelial cells and fibroblasts, are able to take up a limited range of apoptotic particles.34 These, so called “non-professional” phagocytes, engulf cells of their own type (homotypic engulfment), typically when their neighboring cells undergo apoptosis. This phenomenon has been observed in a variety of epithelial tissues, including lung,35,36 thymus37 and breast, notably during the process of involution that follows lactation.38 However, non-professional phagocytes are likely to be less efficient in clearing apoptotic bodies, as they lack the extensive array of opsonic and non-opsonic phagocytic receptors characteristic of professional phagocytes;34,39 do not produce reactive oxygen species (ROS) in response to phagocytosis;34 and their lysosomes lack the substantial degradative capacity of macrophages, which are particularly adept at phagocytosis. Instead, these non-professional phagocytes may serve as an early response to apoptosis by cells that are present in the immediate vicinity, whereas professional phagocytes often must migrate to reach their targets. Professional phagocytosis may be more important in situations where there is a high apoptotic rate, such as during immune responses and organogenesis.
Professional phagocytes are guided to their targets by 2 sets of signals that derive from the apoptotic cells: “find me” signals that influence their migration, and subsequent “eat me” signals exposed on the apoptotic cells (Fig 2). “Find me” signals establish a chemotactic gradient, which stimulates and directs phagocyte migration toward the dying cell (Fig 2a). These signals are chemically diverse, but often signal to their target cells via G protein-coupled receptors (GPCRs). For example, lysophosphatidylcholine (LPC)40 is released from the apoptotic cell when caspase-3 activates phospholipase A2, and is recognized by G2A, a GPCR found on macrophages.41 S1P is synthesized by sphingosine kinase1 or 2 (SphK1, SphK2) and binds to its receptors, GPCRs which can be found on many cells, including macrophages and epithelial cells (a point that we discuss further below in the context of apoptotic extrusion).42,43 ATP and UTP are released from apoptotic cells via pannexin channels, which are opened in a caspase-dependent manner.44 As extracellular nucleotides are rapidly degraded by nucleotidases, ATP and UTP are thought to attract tissue-resident macrophages45 via binding to their P2Y2 receptors.46
Figure 2.
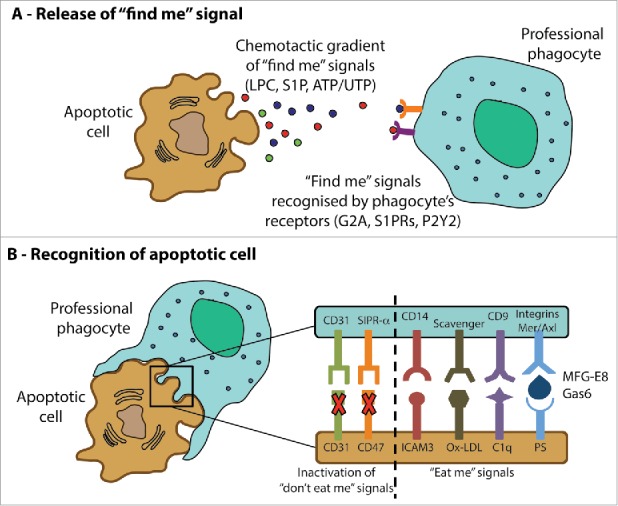
Guidance of professional phagocytes to apoptotic cells. Professional phagocytes migrate to the site of apoptotic cells in response to a variety chemotactic signals (“find me” signals, A). Subsequently, recognition of the target cell is mediated by the activation signals that promote adhesion and phagocytosis of apoptotic corpses (“eat me signals”) as well as the inactivation of inhibitory “don't eat me” signals (B). See text for further details.
“Eat me” signals allow professional phagocytes to discriminate apoptotic cells from their healthy neighbors (Fig 2b). Many such “eat me” signals have been reported45,47 but much interest has focused on phosphatidylserine (PS) as potentially the major and universally detected “eat me” signal.48 PS is found in the inner leaflet of the plasma membrane in healthy cells, but in apoptotic cells PS becomes exposed on the outer leaflet, where it can be recognized by phagocytes.49-51 The precise recognition mechanism remains unclear. The phosphatidylserine receptor (PSR) was once thought to directly mediate engulfment of PS-marked cells, but it is now believed not to function as a surface receptor.52 Alternatively, PS may be recognized by bridging molecules, such as milk fat globule-EGF factor 8 (MFG-E8) and growth arrest-specific gene product 6 (Gas6) which mediate its association with receptors on the phagocytes (integrins and the membrane receptor for kinases Mer and Axl, respectively).53-55 Apart from the “eat me” signal, apoptotic cells also lose so-called “don't eat me” signals from their surface, such as CD3156 and CD47,57 which inhibit engulfment of viable cells. Suppression or loss of these inhibitory molecules during apoptosis also promotes interactions with phagocytes.
After the apoptotic cell is recognized, the phagocyte undergoes cytoskeletal rearrangements necessary for internalization and engulfment of the corpse.58 Following internalization, phagosomes fuse with lysosomes containing digestive enzymes required for the final degradation of the apoptotic cells.59 Efficient processing and degradation of the apoptotic cell also triggers the secretion of the regulatory cytokines IL-10 and TNF-β, which play important roles in resolving and limiting inflammation.60,61
Apoptotic cell extrusion
As noted above, apoptosis poses the dual challenges to epithelial integrity of clearing apoptotic corpses and preserving the epithelial barrier. Interestingly, even when the rate of apoptosis was increased by UV irradiation, cultured epithelial cell monolayers were able to maintain effective barriers, as measured by resistance to ionic flux.9 This implied that epithelia possess an intrinsic mechanism to preserve their permeability barriers in the face of apoptosis. However, while efferocytosis presents a powerful mechanism to eliminate apoptotic corpses, it does not readily explain how the epithelial barrier can be maintained. This is because even if non-professional phagocytes were invoked to mediate apoptotic clearance, it is not clear how they would prevent the at least-transient barrier dysfunction that is predicted to occur when apoptotic cells fragment.
Cell extrusion presents an alternative response to apoptosis that can potentially serve to eliminate moribund cells while simultaneously preserving epithelial integrity. First described by Rosenblatt et al9 in the context of apoptosis, extrusion involves the physical expulsion of apoptotic cells from the epithelium. Since then, the extrusion process has been implicated in allowing epithelia to eliminate oncogene-expressing cells62,63 and even removing otherwise healthy live cells to relieve overcrowding.64,65 Apoptotic extrusion has several features that make it an efficient epithelial response to apoptosis. Firstly, the apoptotic cell is typically extruded in an apical direction.9,66-68 For most simple epithelia, this would lead to their expulsion into the external environment and hence their elimination from the body. Secondly, although extrusion is initiated by injury, characteristic features of irreversible apoptosis, such as the expression of PS on the extrafacial surface of the plasma membrane, only occur after the extrusion process begins.9 Thus, extrusion is a relatively early response to apoptotic injury. Thirdly, extrusion of apoptotic cells is accompanied by the simultaneous rearrangement of the neighboring cells, which form rosettes that ensure that overt gaps in the epithelium do not form at the site of the apoptotic cell.9,69 Finally, extrusion effectively occurs in cultured epithelial monolayers, indicating that it is an epithelium-intrinsic process that does not require the involvement of other cells (e.g. stromal or immune cells).
Therefore, apical extrusion presents a potential first line of response for epithelia to deal with the challenge of apoptosis for barrier integrity. If accomplished effectively, extrusion can occur anterior to efferocytosis and limit the need for phagocytes to be recruited and engaged. The capacity for extrusion to preserve epithelial integrity is likely to be limited by the extent of cell injury and may be most effective for low levels of apoptosis. In contrast to the major efforts devoted to characterizing the cell biology of efferocytosis, however, less is known about the mechanisms that underlie apoptotic extrusion. Two features are key to understanding this process. Firstly, apoptotic extrusion is a fundamentally biomechanical process that involves active regulation of mechanical forces. Secondly, extrusion is a cell non-autonomous phenomenon, which entails communication between injured cells and their surrounding epithelial neighbors.
The biomechanics of epithelial apoptotic extrusion
Early description of apoptotic extrusion revealed that it was characterized by the assembly of a contractile actomyosin ring in the neighboring cells immediately adjacent to the injured cells (Fig 3). Time-lapse imaging revealed that this neighbor-cell contractile ring progressively constricted during the process of extrusion,9 suggesting that it provided the mechanical force responsible for expelling the injured cells. Indeed, this contractile ring moved downwards, in a basal direction, as extrusion occurred, as might be expected if it were driving expulsion of the injured cell. More detailed imaging further revealed that actomyosin accumulates in a broad zone of the junctions between apoptotic cells and their immediate neighbors.66 This suggested that the downward movement of the contractile ring might represent a cortical flow of contractility. Finally, the basal-directed actomyosin merged with lamellipodial protrusions in the nearest neighbors,70 which may mediate cell crawling, to bring neighbors together and prevent gaps forming in the epithelium. Together, these suggested that extrusion is driven by a contractile response of neighboring cells to the local presence of fatally injured cells.
Figure 3.
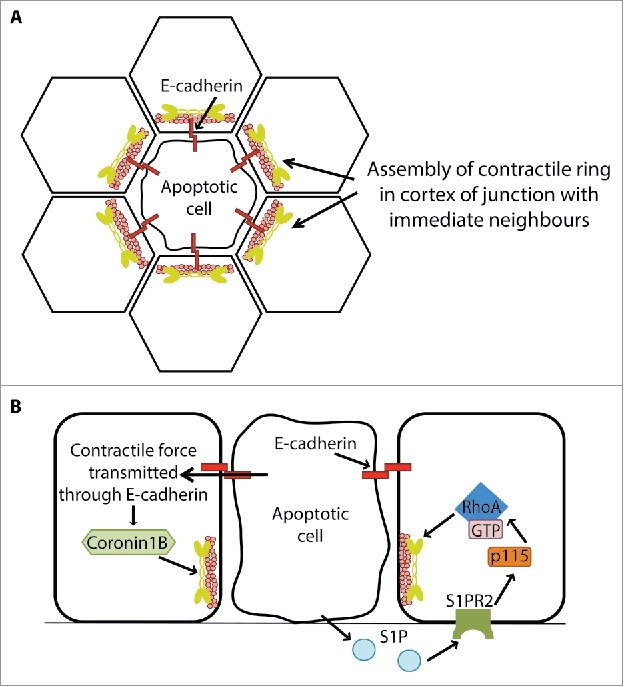
Apical cell extrusion is a biomechanical process that involves cell-cell communication between apoptotic cells and their neighbors. Apoptotic extrusion is distinguished by the assembly of an actomyosin contractile ring in the nearest neighbors adjacent to the apoptotic cell (A). Biogenesis of this contractile ring involves both soluble chemical signals generated by apoptotic cells (such as sphingosine 1-phosphate, S1P, see text for discussion of possible S1P generating mechanisms; B, right-hand cell) and transmission of contractile forces from the apoptotic cell to its neighbors via E-cadherin (left-hand cell). See text for details.
Contractile events also occur in the apoptotic epithelial cell that precede the formation of a nearest-neighbor contractile ring.68 These include the accumulation of apical F-actin in the apoptotic cell, which coincides with an inward pulling of junctions. Indeed, as noted above, earlier studies with other cell types demonstrated that apoptosis is associated with a pronounced hypercontractile phenotype.19,20 In isolated cells, such as fibroblasts, this manifests in the appearance of prominent membrane blebs. It has been attributed to the caspase 3-dependent cleavage of Rho kinase (ROCK), which eliminates the N-terminus that is responsible for autoinhibition, thereby generating an active enzyme that is no longer subject to physiologic regulation.19 Indeed, mosaic expression in epithelia of an equivalent truncated molecule that was constitutively-active was sufficient to promote the accumulation of actomyosin in neighbor cells that abutted the transfectants; this led to extrusion of the transfected cells, but without evidence of apoptosis.66,69
Thus extrusion appears to entail contractile events in both the apoptotic cell and its immediate neighbors. Furthermore, the E-cadherin system that mediates cell-cell adhesion in epithelia can make important contributions to these modes of contractility.66,69 Firstly, cell-cell adhesion mechanically couples together cortical contractility in the apoptotic cell with that in its nearest neighbors. This is because cadherins physically interact with cortical actomyosin; thus, when they engage in homophilic adhesion, cadherins physically link the contractile cortices of cells together.71 Secondly, E-cadherin adhesion can also promote contractility by facilitating RhoA signaling that activates Myosin II72,73 as well as recruiting actin regulators that facilitate actin assembly and organization.74,75 Consistent with this, E-cadherin is necessary for extrusion to occur.66,69 Of course, cadherin adhesions must ultimately be disassembled to allow extruded cells to be released from the epithelium,8 but this is likely to be a relatively late event. Overall, then, E-cadherin may contribute to the coordinated patterns of contractility between apoptotic cells and their neighbors responsible for extrusion.
Cell-cell communication and apoptotic extrusion
The assembly of the contractile ring in the nearest-neighbor cells is believed to be driven by activation of the RhoA GTPase,9 a canonical regulator of actomyosin that exerts its impact through downstream effectors, such as ROCK and formins.76,77 The activity of RhoA is determined by the balance between its activators (guanine nucleotide exchange factors, GEFs) and inactivators (GTPase activating proteins, GAPs).78 Thus, activation of RhoA in the nearest-neighbor cells might be predicted to involve a RhoA GEF. Indeed, p115 RhoGEF has been implicated in the formation of contractile rings in the nearest-neighbor cells.79
More generally, the active response of nearest-neighbor cells implies that some form(s) of cell-cell communication must occur between these cells and the apoptotic cells. Minimally, neighbor cells might detect some form of signal that alerts them to the nearby presence of an injured cell (Fig 3). One possibility is that chemical signals released from the injured cell are detected by its neighbors to stimulate contractility, akin to the signals that guide phagocytes during efferocytosis. Interestingly, one such candidate signal is S1P, which we have already discussed as implicated as a “find-me” signal. Both of the sphingosine kinases, SphK1 and SphK2, are reported to mediate the production of S1P by apoptotic cells,42,80 with the caveat that their involvement has principally been studied in non-epithelial cells. Activated SphK1 may translocate to the plasma membrane of apoptotic cells, generating S1P that is then released from cells through a variety of membrane transporters.81 SphK1 may also itself be exported from cells, generating S1P in the cell exterior.82 SphK2 was reported to be cleaved by caspase-1, leading to its transport from the cell and extracellular production of S1P.80 Of the 5 S1PRs that have been identified (termed S1PR1-S1PR5), S1PR1–3 are ubiquitously expressed, whereas S1PR4 and S1PR5 are mainly expressed by lymphoid and neuronal cells, respectively.83
The first evidence that implicated S1P signaling in epithelial apoptotic extrusion came from the observation that SphK inhibitors (SKI II, SKI V and tDHS) could inhibit formation of the nearest-neighbor contractile ring and led to gaps appearing between apoptotic cells and their neighbors. Furthermore, the S1PR2 antagonist, JTE-013, as well as genetic inhibition of S1PR2 itself, also significantly lowered the extrusion rate, suggesting that S1PR2 mediates S1P signaling during apoptotic extrusion.84 It was proposed that S1P secreted from apoptotic cells activated S1PR2 on its neighbors, leading to the G12/13-coupled activation of p115 RhoGEF.79,84 Consistent with this, immunostaining revealed that S1P could be detected first in apoptotic cells, and later in its nearest neighbors.84 Interestingly, microtubules appeared to play a role in controlling the precise cortical localization of p115 RhoGEF, focusing it at the basal regions of the nearest-neighbor cortex to promote contractility there, thus facilitating extrusion of the apoptotic cell in the apical direction.67,79
A complementary mechanism for apoptotic cells to communicate with their neighbors lies in the transmission of contractile force from the apoptotic cell that is sensed by neighbor cells through mechanotransduction (Fig 3). This was first suggested by the observation that mosaic expression of a constitutively-active ROCK transgene, based on the caspase-induced cleavage that causes hypercontractility in apotosis, led to the extrusion of those cells without concomitant apoptosis.19,66,69 This was accompanied by morphological changes in the neighboring cells, consistent with their being pulled and stretched by the hypercontractile ROCK-expressing cells. Interestingly, however, the morphological responses of its nearest neighbors or extrusion of the ROCK-expressing cells did not occur if E-cadherin was depleted by RNAi.66,69 Similar morphological changes are seen in response to apoptotic cells and both these and the extrusion event itself require cadherin. This suggested that cadherin might transmit forces from contractile cells to their neighbors that in some way contributed to the extrusion process.
Insight into how that force transmission might affect extrusion came from the observation that contractility in the nearest-neighbor actomyosin ring was compromised in E-cadherin-depleted cells. Although a contractile ring formed, it did not constrict effectively.66,69 This was attributable to a defect in regulation of actin assembly and organization in the nearest-neighbor cortex. Specifically, the actin regulator, Coronin 1B, is recruited by E-cadherin adhesion during biogenesis of the actomyosin cortex at cell-cell junctions, where it promotes the formation of bundled actin networks for optimal contractile force generation.66 Coronin 1B is also recruited during assembly of the nearest-neighbor contractile ring in response to apoptosis. However, this recruitment is compromised in E-cadherin-depleted cells, leading to suboptimal organization of F-actin in the contractile ring.66 Therefore, E-cadherin was necessary for optimal regulation of the actin cytoskeleton to generate an effective contractile ring in the neighbors of apoptotic cells.
How might this occur? E-cadherin participates in many forms of cell signaling, but an exciting possibility is that E-cadherin may transmit forces from apoptotic cells that regulate the nearest-neighbor response. This is supported by the observation that mosaic expression of constitutively-active ROCK also promoted Coronin 1B recruitment to the cortex in nearest-neighbor cells in an E-cadherin-dependent fashion.66 This suggests that contractility alone, independent of other chemical signals from apoptotic cells, can signal to regulate the nearest-neighbor cytoskeleton via E-cadherin. If so, then, force-sensing and mechanotransduction may also contribute to the cytoskeletal response of nearest-neighbor cells in response to the hypercontractility of an apoptotic cell. Indeed, it is increasingly apparent that cadherin-based cell-cell junctions are sites of mechanotransduction, which can involve many potential molecules. In particular, α-catenin is a force-sensitive molecule that is under tensile stress at cadherin adhesions.76,85 In vitro studies have shown that this can alter the conformation of α-catenin86 and allow it to promote binding of the cadherin-catenin complex to F-actin.87 α-catenin can also influence cortical signaling, including RhoA,72 although whether this is involved in apoptotic extrusion remains to be tested. In addition, many components of the junctional cortex are themselves mechanosensitive, including Myosin II88 and formins.89 Individually or in combination these may contribute to sensing forces from apoptotic cells.
It should be emphasized that these 2 models for cell-cell communication, via chemical and mechanical signals, are not mutually exclusive. Instead, they may have complementary properties. Mechanical signals can be rapidly propagated within tissues, with a speed and extent that is determined by the viscoelastic properties of the tissue.85 Mechanical changes can also occur in response to many different biochemical changes in the cell. In contrast, chemical signals provide a greater degree of specificity to cell-cell communication than is provided by mechanical signals. Further, it has recently been reported that tissue mechanics can define sites that are susceptible to apoptosis and subsequent extrusion.90 Thus, it is possible that secreted chemical and mechanical modes of communication may collaborate to drive apoptotic cell extrusion.
Concluding remarks
Apoptosis is a fundamental mode of cell death in health and disease; consistent with its ubiquity, much is known about the cell-autonomous pathways that trigger apoptosis. In this review, we have sought to highlight the modes of tissue response that ensure that epithelial integrity is preserved, despite the inherent challenges that apoptosis can present to these tissue barriers. Both the processes of phagocytosis and extrusion that we have discussed represent cell non-autonomous responses that ensure that apoptosis occurs with as little impact on epithelial integrity as possible. It seems likely that these 2 modes of response can complement one another. Extrusion may work as a first response, squeezing the dying cell out of the monolayer and sealing the gap to maintain integrity of the epithelial sheet. Efferocytosis, by professional or non-professional phagocytes, may then provide a second line of response if apoptotic cells persist in the epithelium. How these may be coordinated will be interesting questions for future research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
KD is the recipient of an International Postgraduate Research Scholarship and a UQ Centennial Scholarship; GAG and KS are Future Fellows of the Australian Research Council (FT160100366 and FT130100361, respectively); and MJS and ASY are Research Fellows of the NHMRC Australia (1107914, 1044041 respectively).
References
- [1].Shawki A, McCole DF. Mechanisms of intestinal epithelial barrier dysfunction by Adherent-Invasive Escherichia coli. Cell Mol Gastroenterol Hepatol 2017; 3(1):41-50; PMID:28174756; https://doi.org/ 10.1016/j.jcmgh.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Georas SN, Rezaee F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol 2014; 134(3):509-20; PMID:25085341; https://doi.org/ 10.1016/j.jaci.2014.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Catalioto RM, Maggi CA, Giuliani S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem 2011; 18(3):398-426; PMID:21143118 [DOI] [PubMed] [Google Scholar]
- [4].Blander JM. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nat Rev Immunol 2014; 14(9):601-18; PMID:25145756; https://doi.org/ 10.1038/nri3720 [DOI] [PubMed] [Google Scholar]
- [5].Yang Y, Jiang G, Zhang P, Fan J. Programmed cell death and its role in inflammation. Mil Med Res 2015; 2:12; PMID:26045969; https://doi.org/ 10.1186/s40779-015-0039-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol 2007; 35(4):495-516; PMID:17562483; https://doi.org/ 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Atkin-Smith GK, Poon IK. Disassembly of the dying: Mechanisms and functions. Trends Cell Biol 2017; 27(2):151-62; PMID:27647018; https://doi.org/ 10.1016/j.tcb.2016.08.011 [DOI] [PubMed] [Google Scholar]
- [8].Grieve AG, Rabouille C. Extracellular cleavage of E-cadherin promotes epithelial cell extrusion. J Cell Sci 2014; 127(Pt 15):3331-46; PMID:24895403; https://doi.org/ 10.1242/jcs.147926 [DOI] [PubMed] [Google Scholar]
- [9].Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 2001; 11(23):1847-57; PMID:11728307 [DOI] [PubMed] [Google Scholar]
- [10].Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26(4):239-57; PMID:4561027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006; 25(34):4798-811; PMID:16892092; https://doi.org/ 10.1038/sj.onc.1209608 [DOI] [PubMed] [Google Scholar]
- [12].Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol 2002; 20:323-70; PMID:11861606; https://doi.org/ 10.1146/annurev.immunol.20.100201.131730 [DOI] [PubMed] [Google Scholar]
- [13].Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 2005; 23(3):249-62; PMID:16169498; https://doi.org/ 10.1016/j.immuni.2005.08.001 [DOI] [PubMed] [Google Scholar]
- [14].Veugelers K, Motyka B, Frantz C, Shostak I, Sawchuk T, Bleackley RC. The granzyme B-serglycin complex from cytotoxic granules requires dynamin for endocytosis. Blood 2004; 103(10):3845-53; PMID:14739229; https://doi.org/ 10.1182/blood-2003-06-2156 [DOI] [PubMed] [Google Scholar]
- [15].Hacker DL, Fowler BC. Complementation of the host range restriction of southern cowpea mosaic virus in bean by southern bean mosaic virus. Virology 2000; 266(1):140-9; PMID:10612668; https://doi.org/ 10.1006/viro.1999.0072 [DOI] [PubMed] [Google Scholar]
- [16].Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, Lowe M. Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol 2002; 156(3):495-509; PMID:11815631; https://doi.org/ 10.1083/jcb.200110007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 2001; 1(4):515-25; PMID:11703942 [DOI] [PubMed] [Google Scholar]
- [18].Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 1998; 140(3):627-36; PMID:9456322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 2001; 3(4):339-45; PMID:11283606; https://doi.org/ 10.1038/35070009 [DOI] [PubMed] [Google Scholar]
- [20].Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 2001; 3(4):346-52; PMID:11283607; https://doi.org/ 10.1038/35070019 [DOI] [PubMed] [Google Scholar]
- [21].Tomiyoshi G, Horita Y, Nishita M, Ohashi K, Mizuno K. Caspase-mediated cleavage and activation of LIM-kinase 1 and its role in apoptotic membrane blebbing. Genes Cells 2004; 9(6):591-600; PMID:15189451; https://doi.org/ 10.1111/j.1356-9597.2004.00745.x [DOI] [PubMed] [Google Scholar]
- [22].Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393(6687):805-9; PMID:9655397; https://doi.org/ 10.1038/31729 [DOI] [PubMed] [Google Scholar]
- [23].Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 2005; 435(7040):365-9; PMID:15902261; https://doi.org/ 10.1038/nature03550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moss DK, Betin VM, Malesinski SD, Lane JD. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci 2006; 119(Pt 11):2362-74; PMID:16723742; https://doi.org/ 10.1242/jcs.02959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IK. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 2015; 6:7439; PMID:26074490; https://doi.org/ 10.1038/ncomms8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1(2):a002584. https://doi.org/ 10.1101/cshperspect.a002584. PMID: 20066090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Silva MT. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett 2010; 584(22):4491-9; PMID:20974143; https://doi.org/ 10.1016/j.febslet.2010.10.046 [DOI] [PubMed] [Google Scholar]
- [28].Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem 2011; 22(6):527-34; PMID:20801629; https://doi.org/ 10.1016/j.jnutbio.2010.04.007 [DOI] [PubMed] [Google Scholar]
- [29].Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev 2008; 60(7):795-804; PMID:18262306; https://doi.org/ 10.1016/j.addr.2007.12.004 [DOI] [PubMed] [Google Scholar]
- [30].Hornung V, Barchet W, Schlee M, Hartmann G. RNA recognition via TLR7 and TLR8. Handb Exp Pharmacol 2008; 183:71-86; PMID:18071655; https://doi.org/ 10.1007/978-3-540-72167-3_4 [DOI] [PubMed] [Google Scholar]
- [31].Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med 2012; 18(8):1286-90; PMID:22772463; https://doi.org/ 10.1038/nm.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006; 6(11):823-35; PMID:17063184; https://doi.org/ 10.1038/nri1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Silva MT, Correia-Neves M. Neutrophils and macrophages: The main partners of phagocyte cell systems. Front Immunol 2012; 3:174; PMID:22783254; https://doi.org/ 10.3389/fimmu.2012.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rabinovitch M. Professional and non-professional phagocytes: An introduction. Trends Cell Biol 1995; 5(3):85-7; PMID:14732160 [DOI] [PubMed] [Google Scholar]
- [35].Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA. Epithelial cells as phagocytes: Apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ 2005; 12(2):107-14; PMID:15647754; https://doi.org/ 10.1038/sj.cdd.4401517 [DOI] [PubMed] [Google Scholar]
- [36].Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, Ravichandran KS. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 2013; 493(7433):547-51; PMID:23235830; https://doi.org/ 10.1038/nature11714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cao WM, Murao K, Imachi H, Hiramine C, Abe H, Yu X, Dobashi H, Wong NC, Takahara J, Ishida T. Phosphatidylserine receptor cooperates with high-density lipoprotein receptor in recognition of apoptotic cells by thymic nurse cells. J Mol Endocrinol 2004; 32(2):497-505; PMID:15072554 [DOI] [PubMed] [Google Scholar]
- [38].Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod 2008; 78(4):586-94; PMID:18057312; https://doi.org/ 10.1095/biolreprod.107.065045 [DOI] [PubMed] [Google Scholar]
- [39].Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect Immun 2000; 68(12):6939-45; PMID:11083817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, et al.. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003; 113(6):717-30; PMID:12809603 [DOI] [PubMed] [Google Scholar]
- [41].Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem 2008; 283(9):5296-305; PMID:18089568; https://doi.org/ 10.1074/jbc.M706586200 [DOI] [PubMed] [Google Scholar]
- [42].Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J 2008; 22(8):2629-38; PMID:18362204; https://doi.org/ 10.1096/fj.08-107169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat Rev Immunol 2005; 5(7):560-70; PMID:15999095; https://doi.org/ 10.1038/nri1650 [DOI] [PubMed] [Google Scholar]
- [44].Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al.. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010; 467(7317):863-7; PMID:20944749; https://doi.org/ 10.1038/nature09413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J Exp Med 2010; 207(9):1807-17; PMID:20805564; https://doi.org/ 10.1084/jem.20101157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al.. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009; 461(7261):282-6; PMID:19741708; https://doi.org/ 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li W. Eat-me signals: Keys to molecular phagocyte biology and “appetite” control. J Cell Physiol 2012; 227(4):1291-7; PMID:21520079; https://doi.org/ 10.1002/jcp.22815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992; 148(7):2207-16; PMID:1545126 [PubMed] [Google Scholar]
- [49].Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995; 182(5):1545-56; PMID:7595224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol 2003; 65:701-34; PMID:12471163.; https://doi.org/ 10.1146/annurev.physiol.65.092101.142459 [DOI] [PubMed] [Google Scholar]
- [51].Borisenko GG, Matsura T, Liu SX, Tyurin VA, Jianfei J, Serinkan FB, Kagan VE. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells–existence of a threshold. Arch Biochem Biophys 2003; 413(1):41-52; PMID:12706340 [DOI] [PubMed] [Google Scholar]
- [52].Bratton DL, Henson PM. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Curr Biol 2008; 18(2):R76-9; PMID:18211846; https://doi.org/ 10.1016/j.cub.2007.11.024 [DOI] [PubMed] [Google Scholar]
- [53].Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002; 417(6885):182-7; PMID:12000961; https://doi.org/ 10.1038/417182a [DOI] [PubMed] [Google Scholar]
- [54].Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell 2010; 140(5):619-30; PMID:20211132; https://doi.org/ 10.1016/j.cell.2010.02.014 [DOI] [PubMed] [Google Scholar]
- [55].Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem 1997; 272(47):29411-4; PMID:9367994 [DOI] [PubMed] [Google Scholar]
- [56].Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 2002; 418(6894):200-3; PMID:12110892; https://doi.org/ 10.1038/nature00811 [DOI] [PubMed] [Google Scholar]
- [57].Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005; 123(2):321-34; PMID:16239148; https://doi.org/ 10.1016/j.cell.2005.08.032 [DOI] [PubMed] [Google Scholar]
- [58].Kinchen JM. A model to die for: Signaling to apoptotic cell removal in worm, fly and mouse. Apoptosis 2010; 15(9):998-1006; PMID:20461556; https://doi.org/ 10.1007/s10495-010-0509-5 [DOI] [PubMed] [Google Scholar]
- [59].Kinchen JM, Ravichandran KS. Phagosome maturation: Going through the acid test. Nat Rev Mol Cell Biol 2008; 9(10):781-95; PMID:18813294; https://doi.org/ 10.1038/nrm2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elliott MR, Ravichandran KS. Clearance of apoptotic cells: Implications in health and disease. J Cell Biol 2010; 189(7):1059-70; PMID:20584912; https://doi.org/ 10.1083/jcb.201004096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Henson PM. Dampening inflammation. Nat Immunol 2005; 6(12):1179-81; PMID:16369556; https://doi.org/ 10.1038/ni1205-1179 [DOI] [PubMed] [Google Scholar]
- [62].Hogan C, Dupre-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-Lopez LA, Vincent JP, Itoh Y, et al.. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol 2009; 11(4):460-7; PMID:19287376; https://doi.org/ 10.1038/ncb1853 [DOI] [PubMed] [Google Scholar]
- [63].Wu SK, Lagendijk AK, Hogan BM, Gomez GA, Yap AS. Active contractility at E-cadherin junctions and its implications for cell extrusion in cancer. Cell Cycle 2015; 14(3):315-22; PMID:25590779; https://doi.org/ 10.4161/15384101.2014.989127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012; 484(7395):546-9; PMID:22504183; https://doi.org/ 10.1038/nature10999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 2012; 484(7395):542-5; PMID:22504180; https://doi.org/ 10.1038/nature10984 [DOI] [PubMed] [Google Scholar]
- [66].Michael M, Meiring JC, Acharya BR, Matthews DR, Verma S, Han SP, Hill MM, Parton RG, Gomez GA, Yap AS. Coronin 1B reorganizes the architecture of F-actin networks for contractility at steady-state and apoptotic adherens junctions. Dev Cell 2016; 37(1):58-71; PMID:27046832; https://doi.org/ 10.1016/j.devcel.2016.03.008 [DOI] [PubMed] [Google Scholar]
- [67].Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell 2011; 22(21):3962-70; PMID:21900494; https://doi.org/ 10.1091/mbc.E11-05-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kuipers D, Mehonic A, Kajita M, Peter L, Fujita Y, Duke T, Charras G, Gale JE. Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J Cell Sci 2014; 127(Pt 6):1229-41; PMID:24463819; https://doi.org/ 10.1242/jcs.138289 [DOI] [PubMed] [Google Scholar]
- [69].Lubkov V, Bar-Sagi D. E-cadherin-mediated cell coupling is required for apoptotic cell extrusion. Curr Biol 2014; 24(8):868-74; PMID:24704076; https://doi.org/ 10.1016/j.cub.2014.02.057 [DOI] [PubMed] [Google Scholar]
- [70].Kocgozlu L, Saw TB, Le AP, Yow I, Shagirov M, Wong E, Mege RM, Lim CT, Toyama Y, Ladoux B. Epithelial cell packing induces distinct modes of cell extrusions. Curr Biol 2016; 26(21):2942-50; PMID:27746027; https://doi.org/ 10.1016/j.cub.2016.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol 2015; 17(5):533-9; PMID:25925582; https://doi.org/ 10.1038/ncb3136 [DOI] [PubMed] [Google Scholar]
- [72].Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 2012; 14(8):818-28; PMID:22750944; https://doi.org/ 10.1038/ncb2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 2010; 12(7):696-702; PMID:20543839; https://doi.org/ 10.1038/ncb2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ratheesh A, Yap AS. A bigger picture: Classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol 2012; 13(10):673-9; PMID:22931853; https://doi.org/ 10.1038/nrm3431 [DOI] [PubMed] [Google Scholar]
- [75].Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol 2014; 24(15):1689-99; PMID:25065757; https://doi.org/ 10.1016/j.cub.2014.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Acharya BR, Wu SK, Lieu ZZ, Parton RG, Grill SW, Bershadsky AD, Gomez GA, Yap AS. Mammalian diaphanous 1 mediates a pathway for E-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep 2017; 18(12):2854-67. https://doi.org/ 10.1016/j.celrep.2017.02.078. PMID: 28329679 [DOI] [PubMed] [Google Scholar]
- [77].Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010; 67(9):545-54; PMID:20803696; https://doi.org/ 10.1002/cm.20472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420(6916):629-35; PMID:12478284; https://doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- [79].Slattum G, McGee KM, Rosenblatt J. P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J Cell Biol 2009; 186(5):693-702; PMID:19720875; https://doi.org/ 10.1083/jcb.200903079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Weigert A, Cremer S, Schmidt MV, von Knethen A, Angioni C, Geisslinger G, Brune B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood 2010; 115(17):3531-40; PMID:20197547; https://doi.org/ 10.1182/blood-2009-10-243444 [DOI] [PubMed] [Google Scholar]
- [81].Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol 2010; 688:141-55; PMID:20919652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem 2002; 277(8):6667-75; PMID:11741921; https://doi.org/ 10.1074/jbc.M102841200 [DOI] [PubMed] [Google Scholar]
- [83].Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol 2014; 171(15):3575-94; PMID:24602016; https://doi.org/ 10.1111/bph.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol 2011; 193(4):667-76; PMID:21555463; https://doi.org/ 10.1083/jcb.201010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. Alpha-catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12(6):533-42; PMID:20453849; https://doi.org/ 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- [86].Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mege RM, et al.. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun 2014; 5:4525; PMID:25077739; https://doi.org/ 10.1038/ncomms5525 [DOI] [PubMed] [Google Scholar]
- [87].Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 2014; 346(6209):1254211; PMID:25359979; https://doi.org/ 10.1126/science.1254211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr Opin Cell Biol 2004; 16(1):61-7; PMID:15037306; https://doi.org/ 10.1016/j.ceb.2003.11.011 [DOI] [PubMed] [Google Scholar]
- [89].Shao X, Li Q, Mogilner A, Bershadsky AD, Shivashankar GV. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc Natl Acad Sci U S A 2015; 112(20):E2595-601; PMID:25941386; https://doi.org/ 10.1073/pnas.1504837112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Saw TB, Doostmohammadi A, Nier V, Kocgozlu L, Thampi S, Toyama Y, Marcq P, Lim CT, Yeomans JM, Ladoux B. Topological defects in epithelia govern cell death and extrusion. Nature 2017; 544(7649):212-6; PMID:28406198; https://doi.org/ 10.1038/nature21718 [DOI] [PMC free article] [PubMed] [Google Scholar]


