ABSTRACT
The claudin family of tetraspan transmembrane proteins is essential for tight junction formation and regulation of paracellular transport between epithelial cells. Claudins also play a role in apical-basal cell polarity, cell adhesion and link the tight junction to the actin cytoskeleton to exert effects on cell shape. The function of claudins in paracellular transport has been extensively studied through loss-of-function and gain-of-function studies in cell lines and in animal models, however, their role in morphogenesis has been less appreciated. In this review, we will highlight the importance of claudins during morphogenesis by specifically focusing on their critical functions in generating epithelial tubes, lumens, and tubular networks during organ formation.
KEYWORDS: animal model, claudin, epithelial tubulogenesis, lumen, morphogenesis, neural tube, tight junction
Introduction
Claudins are well-known for their roles in regulating the tight junction barrier function in mammalian organs to create physiologically distinct compartments. Claudins are also expressed at critical time points during embryogenesis and have unique roles in orchestrating events that underlie formation of these organs during development.1-8 Here, we review the evidence that claudins regulate morphogenesis in tissues and organs that form from epithelial tubes – polarized epithelial layers in which the apical cell surface faces a central lumen. Claudins are essential for maintaining the integrity of both the tubule lumen and the surrounding epithelial cell layer, and are critical for the morphogenetic movements that give shape to the organs derived from tubes. We begin by describing changes in cell and tissue behaviors that are essential for epithelial tube formation. We then describe the data demonstrating that claudins regulate cellular events intrinsic to tubulogenesis. Finally, we consider the potential molecular mechanisms used by claudins to direct epithelial cell behaviors that contribute to morphogenesis.
Creating organs from epithelial tubes
Polarized epithelial cells are the building blocks that give rise to tubes of all organs. Some organs, including the central nervous system, heart, and digestive tract, begin as a linear tube that then undergoes bending and/or looping, followed by regional specialization to generate discrete functional domains that remain connected. In other organs, such as the lung and kidney, the epithelial tube undergoes branching morphogenesis to generate a complex network of connected tubules that become segmentally differentiated to fulfill distinct physiologic functions. The process of creating an epithelial tube can be subdivided into 4 phases: (i) formation of the primitive tube, (ii) tube elongation, (iii) lumen expansion, and, finally, (iv) tube maturation during which the tube will bend, loop and/or branch. Throughout these steps, claudin-based tight junctions have a critical role in maintaining both the integrity of the epithelial layer and the barrier between the lumen and the rest of the tissue. Below we outline the cellular events that regulate these steps in tube formation.
Forming a tube
Epithelial tubes form by diverse mechanisms including wrapping, budding, cord or cell hollowing, and cavitation (Fig. 1). While tubular epithelia are generated in many ways, in all cases the simplest structural unit of the tube is an epithelial cell. Neighboring epithelial cells are linked through apical junctional complexes consisting of tight and adherens junctions to form a polarized cell sheet. In the early embryo, the ectoderm and much of the endoderm is already epithelial and, therefore, tubes derived from these cells remodel from a flat epithelial sheet into a tube through wrapping or budding (Fig. 1A, B).9 Wrapping and budding refer to the invagination or evagination of polarized sheets of epithelial cells to form a tube, respectively. In contrast, tubes derived from cord hollowing, cell hollowing and cavitation arise from loosely adherent mesenchymal cells that undergo polarization and thus, acquire epithelial identity.9 Both mechanisms require actomyosin-driven apical constriction of a subset of cells within the epithelial sheet to initiate the process.10
Figure 1.
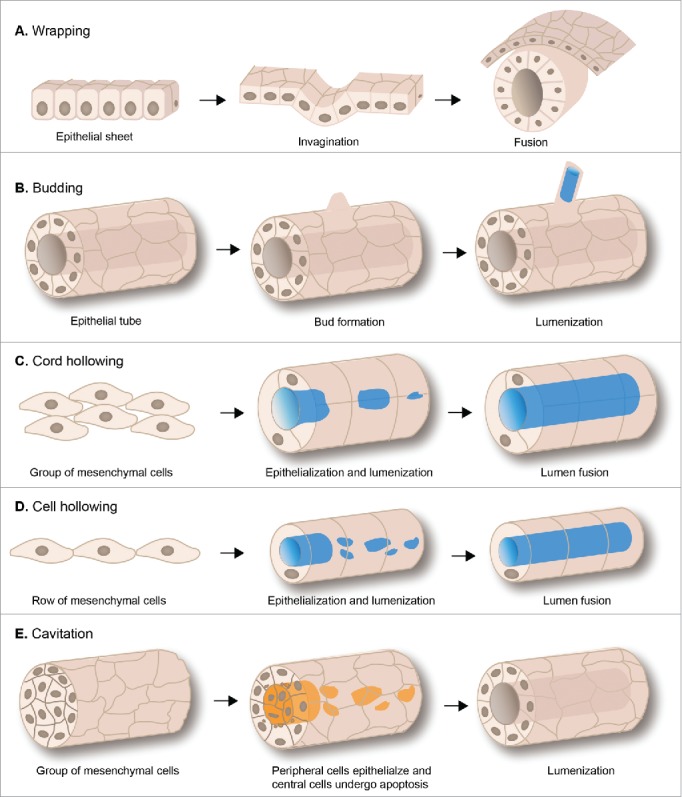
Mechanisms of epithelial tube formation. The schematics illustrate the 5 mechanisms that can give rise to epithelial tubes. (A) Wrapping. Tubes derived from epithelial wrapping are formed when cells within a flat sheet of epithelial cells (left) invaginate (middle), and then more lateral cells fuse to form a closed tube (right). (B) Budding begins with an existing epithelial tube (left). A group of cells migrates out to form a bud (middle), which then extends and forms a lumen (blue) generating an epithelial tube that is a direct extension of the original tube (right). (C) Cord hollowing. A group of mesenchymal cells condenses to form a cylindrical cord (left). The cord undergoes epithelialization and small lumens (blue) are created between cells (middle). The individual lumens then fuse to generate an epithelial tube with a single central lumen (right). (D) Cell hollowing. A group of mesenchymal cells condense to form a cord of single cells (left). The cells epithelialize and small lumens form within individual cells (blue) (middle) that then fuse to form a tube with a common central lumen (right). (E) Cavitation. A group of mesenchymal cells condense forming a solid cylindrical mass of cells (left). Cells in the center of the cylindrical mass undergo apoptosis (orange), while those on the periphery epithelialize (middle) resulting in formation of an epithelial tube (right). Nuclei (brown) are shown only in cells at the transverse view of the tube.
An example of an epithelial tube that forms by wrapping is the primary neural tube of mammalian and avian embryos.11 Cells at the midline of the neural epithelium undergo apical constriction causing the neural epithelium to bend. Subsequent elevation of the lateral neural ectoderm allows the edges to meet and fuse to form a continuous tube which is separated from surrounding tissue. Epithelial wrapping generates tubes that run parallel to the plane of embryonic growth. In contrast, with epithelial budding the final tubes are perpendicular to the plane of the epithelium of origin.9 Cell shape changes regulate the position of the bud and direct its outgrowth and the elongated bud often remains contiguous with the epithelium of origin. The Drosophila trachea and salivary glands and the primary bronchi of the vertebrate lung are examples of epithelial tubes that form by budding.12 With both wrapping and budding, the integrity and polarity of the epithelium is maintained throughout the process of tube formation by apical junctional complexes and the lumens of tubes are formed passively as a consequence of the inward folding of the epithelium to form a tube.
In contrast, when epithelial tubes arise from unpolarized mesenchymal precursors or from the coalescence of epithelial cells, a central lumen is actively created.13 Three general mechanisms have been described for the formation of epithelial tubes from unpolarized precursors: cord hollowing, cell hollowing and cavitation (Fig. 1C, D, E). In all cases, signaling cues trigger mesenchymal cells to form condensations that have adhesive properties and transition through a cord-like structure. The cells in the cord then undergo mesenchymal-to-epithelial transition to generate polarized epithelial cells that are connected to each other by apical junctional complexes. Cord hollowing (Fig. 1C) involves the formation of multiple small polarized lumens between cells that coalesce to form a single common large lumen (e.g. the zebrafish gut and the nephric duct that will form the mammalian kidney).13 The related process of cell hollowing (Fig. 1D) involves creating a lumen within a cell, as occurs in the C. elegans excretory cell and in zebrafish blood vessel formation.13 Lumen formation by cavitation (Fig. 1E) begins with polarization of cells on the periphery of the condensation, followed by apoptosis of cells in the center.13 An example of tube formation by cavitation is the primary neural tube of fish which, in contrast to birds and mammals, occurs via coalescence of neural epithelial cells to form a rod called the neural keel, which then undergoes cavitation to generate a central lumen called the neurocoel.14
Finally, some tubes are dependent both on epithelial wrapping and condensation of non-polarized mesenchymal cells. One example is the mammalian neural tube, which is the embryonic precursor of the brain and the spinal cord.11 At the anterior end of the embryo, a flat sheet of epithelial cells rolls up to form a closed elongated tube in a process known as primary neurulation. The posterior end of the neural tube is formed during secondary neurulation, which involves condensation of mesenchymal cells in the tail bud of the embryo to form an epithelial rod that undergoes canalization to form a lumen that is continuous with that of the primary neural tube.11,14 Both primary and secondary neurulation are required to generate the complete neural tube in mammals, birds and frogs, while in fish, neural tube formation is exclusively by secondary neurulation.14
Tube elongation
Elongation of the epithelial tube ensures that it grows in parallel with the developing embryo. During elongation, epithelial cells undergo cell division, cell shape changes, and cell rearrangements that are coordinated in time and space.9 Throughout these processes, cells are connected via tight junctions, which maintain their epithelial integrity. Cell proliferation can contribute to tube elongation when the cell divisions are oriented with respect to the direction of the tube.9 During neural tube closure in avian and mouse embryos, cell divisions are preferentially oriented along the anterior-posterior axis compared with the medial-lateral axis contributing to the lengthening of the neural plate.15 Similarly, in the developing mouse lung, oriented cell divisions allow the tubules to grow in length without affecting the overall diameter of the tube.16 In tubes that elongate with few cell divisions, change in cell shape is the predominant mechanism driving tube growth. For example, elongation of individual cells causes epithelial tube lengthening during Drosophila salivary gland and trachea development.12
Another important contributor to tube elongation is cell rearrangement. Elongation of the neural tube in chick and mouse embryos is dependent on convergent extension cell rearrangements.17 Neural ectoderm cells intercalate contributing to the overall narrowing (convergence) and anterior-posterior lengthening (extension) of the neural plate.18 Convergent extension is regulated by the Wnt/planar cell polarity pathway (PCP), which controls cell polarity in the plane of the epithelium that is perpendicular to apical-basal polarity.19-21 This allows cells to orient themselves relative to their neighbors and move in a coordinated manner thereby altering the shape of the tissue. PCP signaling has also been implicated in tube elongation in the mouse kidney by controlling tubule diameter through oriented cell division.22,23
Lumen expansion
Expansion and maintenance of the lumen are also critical for tube development. Lumens that are formed passively as a result of epithelial folding primarily rely on fluid accumulation inside the lumen to generate sufficient hydrostatic pressure to maintain the lumen with the correct morphology.13 This is regulated by tight junctions in vertebrates and their functional equivalent, septate junctions, in invertebrates. These junctions create paracellular barriers or pores, depending on the composition of claudin proteins, to regulate the movement of ions and small molecules between cells in epithelial layers, thereby regulating the amount of fluid that accumulates within the lumen.24 In contrast, lumens that are formed de novo may depend on the deposition of negatively-charged anti-adhesive matrix factors that force the membranes of the lumen apart due to repulsion and provide structural support.13 The fibrous polysaccharide chitin serves this purpose in Drosophila tracheal cells.25,26 A third model suggests that lumen expansion depends on changes in cell shape driven by expansion of the apical membrane through fusion of intracellular membrane vesicles.27 Relaxation of the apical actin-myosin contractile network will also contribute to expansion of the apical surface and lumen.
Tube maturation: Bending, looping and branching morphogenesis
The neural tube is an example of a simple epithelial tube that undergoes segment-specific constriction events that demarcate the future lobes of the fore-, mid- and hindbrain.28,29 The gut and heart undergo more complex tube maturation and exhibit bending and looping to generate the final 3-dimensional shape of the organ.30,31 These stereotypic, and often evolutionarily-conserved, steps in tubulogenesis are critical for the organs to acquire their final shape that allows them to fit into compact body cavities. In organs that require a large tubular surface area, individual tubes undergo branching events that rapidly expand their surface area. The vertebrate lung, kidney, and liver require a large surface area for gas, waste and nutrient exchange to occur efficiently and thus are composed of an elaborate network of tubes.32 Positioning of the buds and bifurcations that lead to branch points depend on local signaling cues that direct cell shape changes, including apical constriction.33 This highly reiterative process leads to a stereotypic branched network in the mature organ.
The events that contribute to reshaping of the tubes during organogenesis are dependent on temporally- and spatially-regulated changes in the shape of individual cells, which are largely driven by local dynamic regulation of the actin-cytoskeleton. Several reviews have described the cellular and tissue movements that control tube formation during organogenesis.28-30,34-36 The purse-string hypothesis has been proposed to be a mechanism to change epithelial cells from columnar to wedge-shaped via contraction of the apical actin cytoskeleton and may explain how buds or branch points arise within an epithelial layer.37 To exert individual cell shape change, junctional complexes within the epithelial layer may undergo remodelling to effect changes in the actin-cytoskeleton and/or their interactions with neighboring cells within the epithelial cell layer. The remainder of this review will focus on the roles played by claudins, a family of small integral tight junction proteins, in epithelial tube formation and morphogenesis.
The claudin family of tight junction proteins and epithelial tube morphogenesis
Claudins
In vertebrates, tight junctions are essential to maintain the integrity of the epithelial cell layer that surrounds the tubular structures and the tensile forces that keep the lumen expanded.24,38,39 Tight junction formation depends upon the presence of claudins,40,41 a family of ∼24 tetraspan transmembrane proteins that range in size from 20–27kDa.42 Claudins have 4 transmembrane domains, 2 extracellular loops, a short intracellular loop and cytoplasmic N- and C-termini. The second extracellular loop (∼16–33 amino acids) participates in trans-interactions (head-to-head) of claudin molecules between apposing cells,41,43 and in cis-interactions (side-to-side) with other claudins in the same cell.41,44,45 Cis-interactions of the claudin transmembrane domains with those of other claudins in the cell membrane also promote oligomerization.46 Together these cis- and trans-interactions form the backbone of tight junction strands and segregate the apical and basolateral membrane components (Fig. 2).
Figure 2.
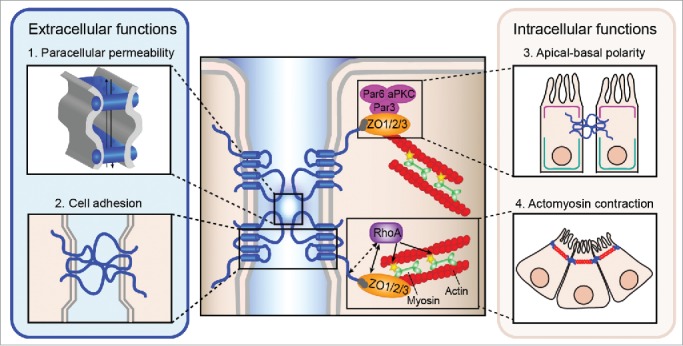
Interactions of claudin extracellular and intracellular domains. The extracellular (left) and intracellular (right) functions of claudins involved in epithelial tube formation are depicted. (1) Paracellular permeability: interactions between extracellular domains of claudins form charge and size-selective pores/barriers to regulate the paracellular movements of ions and small molecules. (2) Cell adhesion: claudins act as adhesion molecules through trans-interactions between adjacent cells. (3) Apical-basal polarity: claudins maintain apical-basal polarity by preventing the lateral diffusion of proteins and through direct and indirect interactions with the Par polarity complex (pink) through interactions at their C-terminal cytoplasmic tail. (4) Actomyosin contraction: claudins anchor RhoA (purple) to the apical cell membrane, where active RhoA-ROCK signaling leads to phosphorylation (yellow stars) and activation of myosin light chain (green) and actin-myosin contraction (actin fibers in red). This causes cells to constrict apically resulting in bending or invagination of epithelial cells. The interaction between claudins and RhoA may be direct or indirect via the interaction of their PDZ-binding domain (gray circles) with tight junction scaffolding proteins like the zonula occludens family (orange).
Within the paracellular space, the larger first extracellular loop (∼50 amino acids) determines the size and charge of the ions and small molecules that can move between the cells.47,48 It has a conserved W-GLW-C-C motif where the 2 cysteine residues are predicted to form a disulfide bond that stabilizes the conformation of the loop and several charged residues that determine the permeability properties of the junction.49 The conformation of the loop and the charged amino acid residues within the first loop determine the charge and size selectivity of the paracellular pore (Fig. 2).50-55
The C-terminal domain shows the most sequence and size heterogeneity between claudin family members.42 Compared to the other domains, the C-terminus is enriched in serine, threonine and tyrosine residues that can undergo post-translational modifications that affect stability of the protein, its localization to the tight junction complex,56,57,58 and the paracellular permeability properties of the tight junction.59-61 All claudins, except for Claudin-12, have a conserved PDZ binding motif at their C-termini. This motif interacts with the PDZ domain of tight junction cytoplasmic proteins, including the zonula occludens family (ZO-1, -2, -3) and MUPP1,62-64 which link claudins to the actin cytoskeleton. Thus the C-terminal domain is essential to bridge the apical tight junction complex to intracellular signaling events, thereby linking cell polarity to cell proliferation and differentiation, and morphogenesis (Fig. 2). Through these activities, claudins have the potential to coordinate changes in cell shape and tissue movements that regulate morphogenesis.
Most cells express multiple claudin family members; the combination and proportion of individual claudins expressed within an epithelial or endothelial layer creates functional diversity in the size and ion selectivity of the tight junction paracellular barrier.65-67 In addition to their roles in tight junction formation, the extracellular loops act as receptors for viruses and enterotoxins. For example, the first extracellular loop of Claudin-1 serves as a receptor for the Hepatitis C virus (HCV),68 while the second extracellular loops of Claudin-3, -4, -6, -7, -8 and -14 act as a receptor for Clostridium perfringens enterotoxin (CPE).69-73
The exact origin and evolution of claudins remains unknown. Claudins arose before the development of chordates, suggesting that the claudin gene family predates that of structural tight junctions.74 Consistent with this hypothesis, claudin-like molecules have been identified in sponges (e.g., Amphimedon queenslandica) and lower chordates (e.g., the ascidian Halocynthia roretzi).42,74 C. elegans has 5 claudins (clc-1 to -5) and 5 claudin-like molecules including nsy-1 to -4 and vab-9. These proteins are functionally similar to vertebrate claudins regulating cell adhesion and paracellular permeability, however, they localize to adherens junctions.75-77 In invertebrates, claudin-like proteins are also present in septate junctions, the functional equivalent of the vertebrate tight junction. In contrast to the vertebrate tight junction, septate junctions lie immediately basal to adherens junctions. The Drosophila septate junction contains 3 claudin-like molecules, Sinuous, Kune-kune, and Megatrachea, which regulate the barrier function of tight junctions, but not apical-basal polarity.4-6 Thus, claudins and claudin-like proteins have an evolutionarily-conserved role in mediating epithelial permeability and cell adhesion. However, their relative position within the membrane and role in maintaining apical-basal polarity is not.
The appearance of tight junctions in vertebrates was accompanied by the expansion of the claudin gene family. There are 20 claudins in Xenopus tropicalis,78 17 in the chick,65 and ∼24 family members in mouse and human.42 Teleosts have a much larger number of claudins with 56 family members identified in the pufferfish Takifugu79 and 54 in zebrafish.78 Several pairs of highly homologous CLDN genes (CLDN3 and 4, CLDN6 and 9, CLDN8, 14 and 17, and CLDN22 and 24) are located in close proximity in the mammalian genome. This genomic organization suggests that gene duplication events contributed to the expansion and evolution of claudins from an ancient common ancestor.
Claudins are expressed in tubular epithelia that arise from each of the germ layers: the ectodermally-derived neural tube, mesodermally-derived tubes of the kidney, and endodermally-derived tubes that give rise to the lung, liver, gut, and pancreas.65 Claudins have been shown to play a critical role in regulating ion permeability and in maintaining apical-basal polarity in the mature epithelia of most of these organs.80-92 But what about in development? There is also a growing body of evidence demonstrating that claudins influence the formation of these tissues by affecting the behavior of the epithelial cell layers during organogenesis in the developing embryo.1-7,93 Below we describe examples of claudin function in regulating cell polarity, adhesion and shape changes, as well as in maintaining the epithelial barrier and promoting lumen formation during tubular morphogenesis.
Claudins in cell polarity
Cells in epithelial layers exhibit both apical-basal polarity as well as planar polarity with respect to their relative orientation to other cells within the epithelium. Several examples exist that demonstrate the importance of claudins in maintaining apical-basal polarity in epithelial tubes (Fig. 3A), including in the zebrafish intrahepatic biliary system, a tubular network that drains bile away from hepatocytes into the biliary tree. In the liver, hepatocytes are arranged in rows with their basal side facing the sinusoidal endothelial cells that carry blood and their apical domains forming canaliculi that empty into the bile duct that is lined by biliary epithelial cells. The canaliculi form a dilated intercellular space between adjacent hepatocytes through which bile flows. During zebrafish development, claudin 15-like b (cldn15lb) is expressed in tight junctions of the biliary epithelial cells and hepatocytes.93 Mutant livers from zebrafish that have a nonsense point mutation, C290T, in cldn15lb have fewer polarized hepatocytes and their clustered non-polarized hepatocytes are unable to form a continuous epithelial layer. Consequently, in cldn15lb mutants the vascular and biliary networks are intermixed and the canaliculi are shorter and wider. The biliary epithelial cells are closer to each other and their cytoplasmic extensions that normally form the tubular network of the biliary system appear dilated. These data reveal an essential role for cldn15lb in establishing hepatocyte apical-basal polarity and the intrahepatic vascular and biliary tubular networks.
Figure 3.
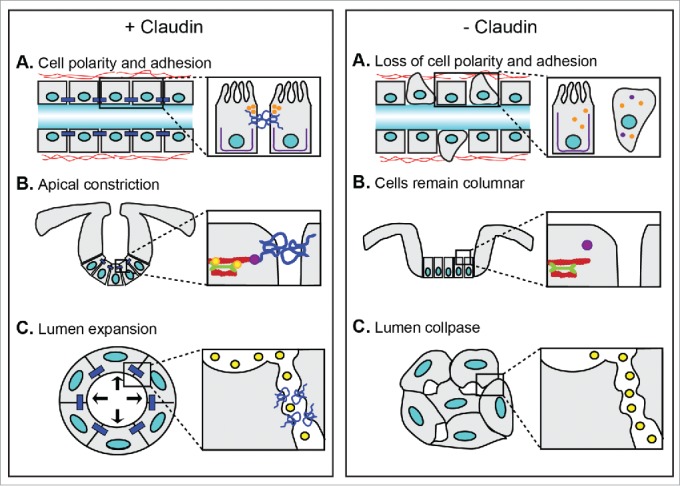
Events in tubulogenesis that are regulated by claudins. (A) Left, claudins maintain apical-basal polarity by preventing the mixing of apical (orange) and basolateral (purple) proteins. This is required for cells to maintain their epithelial identity. They also function as adhesion molecules. Right, in the absence of claudins, apical and basolateral proteins mix and some cells lose their epithelial identity and become mesenchymal. Cells also detach from one another. As a result, the epithelial tube becomes disorganized. (B) Left, claudins anchor RhoA (purple) to the apical cell membrane. This interaction is important for actomyosin contraction and invagination of epithelial cells by ensuring that RhoA/ROCK signaling phosphorylates (yellow circles) and activates myosin light chain (green). Right, in the absence of claudins, RhoA is distributed throughout the cytoplasm where it does not activate ROCK and phosphorylation and activation of myosin light chain fails to occur. Consequently, cells do not undergo apical constriction and invagination does not occur. (C) Left, claudins form paracellular pores to restrict the paracellular movement of water molecules (yellow circles) and maintain hydrostatic pressure that is required for lumen expansion. Right, in the absence of claudins, water molecules can freely pass between cells leading to loss of hydrostatic pressure and collapse of the lumen.
Another example of the importance of claudins in establishing apical-basal polarity comes from studies of the Xenopus pronephros, a transient kidney structure formed during vertebrate development. Pronephros morphogenesis involves a series of events including induction, mesenchymal-to-epithelial transition, patterning and differentiation, lumenization and elongation.94 In Xenopus, XCldn6 is expressed in the pronephric anlage and in the pronephric tubule and duct but not in the glomus.7 Morpholino knockdown of XCldn6 caused polarization defects during pronephric tubule morphogenesis, such that they were unable to complete epithelialization and failed to differentiate.7 Phosphorylated ezrin was no longer restricted to the apical surface and expression of the basolateral marker Na+/K+-ATPase was reduced, as were the differentiated tubular cell marker PDZK1 and the duct marker CLC-K. In our studies of the kidney development in chick and mouse embryos, we observed that several claudins are highly expressed in the pronephros, nephric duct and branched collecting duct network of the developing and mature kidney.1,65,67 However, embryonic kidney phenotypes in the single claudin knockout mouse models have been underwhelming.80,87,95-101 For example, we did not observe any kidney defects in either the Claudin-7 knockout or in the Claudin-16 knockdown mutant mouse models despite their strong expression during kidney organogenesis.67 Similar observations have been made in the analyses of many claudin mutant models in a range of organisms, and we expect that this is most likely due to functional redundancy among claudin members. Studies using reagents that target multiple claudin family members, such as the C-terminal domain of Clostridium perfringens enterotoxin (C-CPE), will clarify the role of claudins in regulating the morphogenesis of the developing kidney and other organs.
Recently, we discovered that claudins also regulate planar cell polarity (PCP), which allows cells to maintain their correct orientation relative to their neighbors in epithelial layers. PCP is regulated by the evolutionarily-conserved non-canonical Wnt/PCP pathway102 and is essential for convergent extension movements and oriented cell division during neural tube formation.19,20,103-105 These processes contribute to the lengthening of the epithelium and are necessary to bring the 2 edges of the neural folds sufficiently close for the epithelial remodelling events that result in neural tube closure. Using C-CPE72,106 to remove Claudin-3, Claudin-4 and Claudin-8 from tight junctions during neural tube closure caused open neural tube defects in all treated embryos.8 Both convergent extension and oriented cell division in the neural ectoderm were affected in C-CPE-treated embryos, and molecular analysis revealed a significant reduction in the level of the core PCP protein Vangl2 following depletion of Claudin-4 and Claudin-8 from tight junctions in the neural ectoderm.8 Our data suggest that in addition to maintaining apical-basal polarity, claudins can regulate the localization of planar polarity proteins at the apical cell surface. These data also suggest that individual claudins may specifically contribute to protein localization and complex formation at the tight junction cytoplasmic face to regulate the intracellular actin-cytoskeleton during cell shape changes.
Claudins regulate changes in cell shape
Cell shape changes are an integral part of tubule formation. It is well known that claudins are upregulated when cells undergo cell shape changes associated with mesenchymal-to-epithelial transition and that their expression is downregulated when cells undergo epithelial-to-mesenchymal transition.107-111 However, there are also several examples of claudin-dependent cell shape changes in developing embryos. In Drosophila, 3 claudin-like molecules – Sinuous, Kune-kune and Megatrachea - regulate tracheal tube morphogenesis.4-6 The Drosophila tracheal system is a branched network of epithelial tubes that functions as a combined pulmonary and vascular system to exchange gas and deliver oxygen to tissues. The trachea forms from a cluster of ∼80 epithelial cells that undergo a series of coordinated morphogenetic movements to produce a complex network of polarized tubes with a characteristic diameter and length. The apical sides of tracheal cells face the lumen while their basal surface makes contact with the interstitial space. Mutations in the Drosophila claudin-like molecules lead to abnormally shaped tracheal cells that have an elongated morphology rather than their characteristic cuboidal shape. This change in cell shape results in tracheal tubules that are elongated and tortuous. Apical-basal polarity is maintained in these mutants and polarity markers such as Crumbs, E-cadherin, and Dlg are appropriately localized,4-6 indicating that the shape change is not due to a disruption of cell polarity. As with most vertebrate claudins, the C-terminal domain of Megatrachea, Sinuous, and Kune-kune has a PDZ binding domain that interacts with PDZ domain adaptor proteins that bridge septate junctions to the actin cytoskeleton. Thus, these data suggest that the organization of the septate junctions and their interactions with the actin-cytoskeleton is necessary for regulating the shape of tracheal cells, which is important for ultimately regulating tracheal morphogenesis.
Claudin-dependent cell shape changes are also critical during neural tube closure.8 In chick and mouse embryos, cells at the midline of the neural plate transition from columnar to wedge-shaped to form the median hinge point.112-115 Wedging of the median hinge point cells generates intrinsic forces that deform the midline of the neural plate causing the neural folds to elevate.112-115 During neural tube closure, apical localization of RhoA-ROCK signaling components at the neural plate midline is required for phosphorylation of myosin light chain (pMLC), which then moves along actin filaments to generate the contractile force required for apical constriction.116-118 We recently showed that claudins act upstream of the actomyosin-driven apical constriction and microtubule-mediated basal nuclear migration required for the cell shape changes at the median hinge point.8 In C-CPE-treated embryos, where Claudin-4 and Claudin-8 are selectively removed from the neural ectoderm, RhoA and pMLC showed reduced localization to the membrane suggesting that Claudin-4 and/or Claudin-8 participate in the recruitment of RhoA-ROCK signaling components to the cytoplasmic plaque.8 In addition, the apical-basal microtubule network was discontinuous in the Claudin-4 and Claudin-8-depleted embryos. Together these data demonstrate that C-CPE-sensitive claudins regulate cell shape changes that are dependent on the actin-myosin and microtubule cytoskeletons (Fig. 3B) and further support the importance of claudin interactions with cytoplasmic proteins to regulate morphogenesis.
Claudins as adhesion molecules
The above studies have demonstrated intracellular roles for claudins during tube morphogenesis, but what about the extracellular loops, which have well established roles in paracellular transport and “trans” interactions between cells? First we will consider the second extracellular loop and the potential for its trans- interactions to function in cell adhesion. Cell adhesion is particularly important for condensing mesenchymal cells that form a cord which transitions into an epithelial tube. Prior to acquiring apical-basal polarity, they must first be able to adhere to one another.119 Direct evidence for claudins being able to function as adhesion molecules (Fig. 3A) comes from studies in gastrula-stage zebrafish and Xenopus embryos.120,121 The first coordinated cell movement during zebrafish development is epiboly.122 During epiboly, a multilayered cell sheet consisting of cells that give rise to embryonic tissues (the deep cells), an extraembryonic epithelial monolayer (the enveloping layer), and a membrane-enclosed group of nuclei that lies at the interface between the yolk cell and enveloping layer (the yolk syncytial layer) spread and thin, ultimately engulfing the yolk cell. Tight junctions not only connect the cells of the enveloping layer to each other, but they also attach the enveloping layer to the yolk syncytial layer. This attachment allows the yolk syncytial layer to pull the overlying enveloping layer along with it as epiboly progresses. Morpholino knockdown of cldne delays initiation and progression of epiboly.120 While cells in the enveloping layer of morphant embryos differentiate normally, they have a rounded shape and have reduced cell-cell contacts with the cells of the yolk syncytial layers. These data suggest that Claudin E is able to act as an adhesion molecule to maintain tight junction contacts between these 2 cell layers,120 thereby maintaining the tension between the cells of the enveloping layer and those of the yolk syncytial layer during epiboly. Cldne has no mammalian ortholog, although it is most closely related to mammalian Claudin-3 and -4.120
Further evidence that claudins affect cell adhesion in vivo comes from studies in Xenopus. At the blastula stage, the Xenopus embryo is divided into 3 regions: the animal cap, which forms ectodermal derivatives, the marginal zone that forms mesoderm tissues, and the vegetal pole, which develops into endodermal tissue. As gastrulation occurs, the cells or blastomeres of the animal cap undergo epiboly. In contrast to control blastomeres, overexpression of the Xenopus claudin, Xcla, caused these cells to remain in tight clusters and fail to undergo epiboly.121 In addition, overexpression of Xcla cells derived from animal cap explants increased adherence and made them difficult to dissociate. These data demonstrate a role for Xcla in cell adhesion through its interaction with claudins in neighboring cells. In addition to being expressed in blastomeres at the animal pole, Xcla is expressed in epithelial tissues including the neural ectoderm, the pronephros, and the nephric duct. Xcla, subsequently named Xcla 4a, is the homolog of human CLDN4.
CLDN3 and CLDN4 are thought to have arisen through a gene duplication event42; they are similar in sequence and located within 50 kb of each other on human chromosome 7, mouse chromosome 5, and chicken chromosome 19.42,123 While the individual knockout mice for CLDN-3124 and -4100 do not exhibit defects in cell adhesion, it is tempting to speculate that these 2 claudins may act in a functionally redundant manner. Expression of Cldn-3 and -4, in addition to Cldn-6, and -7, is regulated by the grainyhead-like-2 (Grhl2) transcription factor, which is expressed in the non-neural ectoderm.125 Up- or downregulation of Grhl2 expression causes neural tube defects due to a failure in the fusion event of the neural folds.125-127 Further experiments are needed to confirm that knockdown of claudins is responsible for the neural tube defects observed in these mice and whether it is a cell adhesion defect. Similarly in the mouse kidney, inactivation of Grhl2 results in impaired lumen expansion in the nephric duct and in impaired lumen formation in mIMCD-3 cells grown in Matrigel. Interestingly, the lumen phenotype in Grlh2-/- collecting duct cells was rescued when Claudin-4 was overexpressed.128 New technologies such as the CRISPR/Cas9 system will enable the creation of a double knockout for Cldn-3 and -4 and allow us to answer the question of whether theses claudins act in a functionally redundant manner to regulate cell adhesion during morphogenesis.
Roles for claudins in maintaining lumen shape
The integrity of liquid-filled lumens is maintained by hydrostatic pressure, which requires maintenance of the appropriate ionic balance. The claudin first extracellular loop is the major contributor to the permeability/ barrier properties of the tight junction, with respect to ion charge and size of molecules that can pass through the junction. Thus it is not surprising that claudins contribute to shaping lumens (Fig. 3C). In early mouse development, the trophectoderm functions as a barrier that covers the surface of the blastocyst to protect the inner cell mass from the external environment. Transport of ions, water and small molecules across the trophectoderm generates the blastocoel cavity.129 Once the blastocoel cavity has formed, tight junctions function as a barrier that prevents the paracellular movement of materials across the trophectoderm and maintains the hydrostatic pressure that is required for expansion of the blastocoel cavity. In the mouse, Claudin-4 and -6 are required in the blastocyst to maintain the hydrostatic pressure that gives the blastocoel its shape.24 When in vitro cultured mouse blastocysts were treated with C-CPE, which removes Claudin-4 and -6, the trophectoderm paracellular barrier was compromised and exhibited increased permeability, consequently the blastocoel cavity failed to expand. However, apical-basal polarity was maintained as localization of occludin and Claudin-7 to tight junctions was not affected and expression of the basolateral marker Na+/K+-ATPase was normal. These data suggest that Claudin-4 and Claudin-6 are required in the trophectoderm tight junctions to appropriately regulate the paracellular barrier and maintain the appropriate pressure within the blastocyst (Fig. 3C).
Claudins also play a role in maintaining hydrostatic pressure that drives lumen formation during zebrafish brain and gut development. Hydrostatic pressure is required for expansion of the brain ventricular lumen during brain morphogenesis.130 In zebrafish, Claudin-5a forms a barrier in the neuroepithelium lining the brain ventricles to maintain the hydrostatic pressure required for ventricular lumen expansion.38 Morpholino knockdown of claudin-5a increased leakiness through the neuroepithelial cells and ventricle size was decreased. Again apical-basal polarity was unaffected. These data suggest that claudin-5a is required to establish the neuroepithelial-ventricular barrier, which maintains ventricular fluid pressure necessary for brain ventricle morphogenesis. Similarly to what occurs in the brain, hydrostatic pressure is required to generate a lumen in the gut tube. In zebrafish, fusion of multiple small lumens to form a single lumen is driven by fluid accumulation, which provides an intraluminal force that causes expansion and coalescence of these smaller lumens. Claudin-15 creates a paracellular pore in the gut that allows the passage of ions into the lumen to create hydrostatic pressure necessary for lumen formation.131 Morpholino knockdown of claudin-15 causes a multiple lumen phenotype in the gut due to defective transport of ions. Together these studies demonstrate that the claudin extracellular domains also play critical roles in tubular morphogenesis.
Persepctives
Functional analyses of claudin variants and the effects of claudin depletion during embryonic development clearly demonstrate that claudins are required throughout all phases of tubular morphogenesis. These studies also indicate that some claudins are likely to be functionally redundant during early stages of embryonic development. Tools that target multiple claudins, such as the C-terminal domain of Clostridium perfringens enterotoxin (C-CPE) or using the CRISPR/Cas9 approach to delete claudins that are linked on the same chromosome, will identify morphogenetic events that are regulated by functionally redundant claudins but masked when claudins are studied individually.
There remain several gaps in our understanding of claudin biology to precisely delineate the unique and overlapping roles of individual family members. For example, data describing the temporal and spatial dynamics of claudin expression during embryogenesis is limited. Detailed analysis of claudin mRNA and protein expression patterns during morphogenesis is essential to identify the tissues and developing tubules where each family member can contribute to the formation of epithelial tubes. Moreover, we are also limited in our knowledge about the complexity of the heteromeric interactions between claudin family members and how they are positioned within the strands of the tight junction complex. Approaches that interrogate interactions of the claudin cytoplasmic C-terminal tail with signaling and polarity complexes at the tight junction cytoplasmic plaque will provide further insight into how claudins affect cellular behaviors and tissue morphogenesis. Increasing our knowledge of claudin-claudin intercellular interactions and the ability of different combinations of claudins to uniquely regulate paracellular barriers is critical to our understanding of events in tubular morphogenesis that are regulated by claudins. Undoubtedly these studies will contribute to the identification of claudin-specific functions during tissue morphogenesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank S. LaCharité-Harbec and M.P. Laverde for critical reading of the manuscript. AIB is the recipient of a doctoral studentship from Fonds de recherche du Québec– Santé (FRQS).
Funding
This work was supported by the Kidney Foundation of Canada (AKR and IRG), the Natural Sciences and Engineering Research Council of Canada (AKR), and Canadian Institutes of Health Research (IRG). AKR and IRG are members of the RI-MUHC, which is supported in part by the FRQS.
References
- [1].Haddad N, El Andalousi J, Khairallah H, Yu M, Ryan AK, Gupta IR. The tight junction protein claudin-3 shows conserved expression in the nephric duct and ureteric bud and promotes tubulogenesis in vitro. Am J Physiol Renal Physiol. 2011;301:F1057-65. doi: 10.1152/ajprenal.00497.2010. PMID:21775479 [DOI] [PubMed] [Google Scholar]
- [2].Simard A, Di Pietro E, Young CR, Plaza S, Ryan AK. Alterations in heart looping induced by overexpression of the tight junction protein Claudin-1 are dependent on its C-terminal cytoplasmic tail. Mech Dev. 2006;123:210-27. doi: 10.1016/j.mod.2005.12.004. PMID:16500087 [DOI] [PubMed] [Google Scholar]
- [3].Collins MM, Baumholtz AI, Simard A, Gregory M, Cyr DG, Ryan AK. Claudin-10 is required for relay of left-right patterning cues from Hensen's node to the lateral plate mesoderm. Dev Biol. 2015;401:236-48. doi: 10.1016/j.ydbio.2015.02.019. PMID:25744724 [DOI] [PubMed] [Google Scholar]
- [4].Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313-23. doi: 10.1083/jcb.200309134. PMID:14734539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611-20. doi: 10.1016/S1534-5807(03)00275-2. PMID:14536062 [DOI] [PubMed] [Google Scholar]
- [6].Nelson KS, Furuse M, Beitel GJ. The Drosophila Claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010;185:831-9. doi: 10.1534/genetics.110.114959. PMID:20407131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun J, Wang X, Li C, Mao B. Xenopus Claudin-6 is required for embryonic pronephros morphogenesis and terminal differentiation. Biochem Biophys Res Commun. 2015;462:178-83. doi: 10.1016/j.bbrc.2015.04.065. PMID:25979361 [DOI] [PubMed] [Google Scholar]
- [8].Baumholtz AI, Simard A, Nikolopoulou E, Oosenbrug M, Collins MM, Piontek A, Krause G, Piontek J, Greene NDE, Ryan AK. Claudins are essential for cell shape changes and convergent extension movements during neural tube closure. Dev Biol. 2017;428(1):25-38. doi: 10.1016/j.ydbio.2017.05.013. PMID:28545845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341:34-55. doi: 10.1016/j.ydbio.2009.09.024. PMID:19778532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341:5-19. doi: 10.1016/j.ydbio.2009.09.009. PMID:19751720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nikolopoulou E, Galea GL, Rolo A, Greene ND, Copp AJ. Neural tube closure: cellular, molecular and biomechanical mechanisms. Development. 2017;144:552-66. doi: 10.1242/dev.145904. PMID:28196803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maruyama R, Andrew DJ. Drosophila as a model for epithelial tube formation. Dev Dyn. 2012;241:119-35. doi: 10.1002/dvdy.22775. PMID:22083894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sigurbjornsdottir S, Mathew R, Leptin M. Molecular mechanisms of de novo lumen formation. Nat Rev Mol Cell Biol. 2014;15:665-76. doi: 10.1038/nrm3871. PMID:25186133 [DOI] [PubMed] [Google Scholar]
- [14].Lowery LA, Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 2004;121:1189-97. doi: 10.1016/j.mod.2004.04.022. PMID:15327780 [DOI] [PubMed] [Google Scholar]
- [15].Sausedo RA, Smith JL, Schoenwolf GC. Role of nonrandomly oriented cell division in shaping and bending of the neural plate. J Comp Neurol. 1997;381:473-88. doi: 10.1002/(SICI)1096-9861(19970519)381:4%3c473::AID-CNE7%3e3.0.CO;2-. PMID:9136804 [DOI] [PubMed] [Google Scholar]
- [16].Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science. 2011;333:342-5. doi: 10.1126/science.1204831. PMID:21764747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schoenwolf GC, Alvarez IS. Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development. 1989;106:427-39. PMID:2598817 [DOI] [PubMed] [Google Scholar]
- [18].Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547-56. PMID:10498689 [DOI] [PubMed] [Google Scholar]
- [19].Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165-81. doi: 10.1006/dbio.2002.0673. PMID:12074560 [DOI] [PubMed] [Google Scholar]
- [20].Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815-25. doi: 10.1242/dev.00123. PMID:12421719 [DOI] [PubMed] [Google Scholar]
- [21].Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59-72. doi: 10.1016/S0925-4773(98)00029-X. PMID:9545534 [DOI] [PubMed] [Google Scholar]
- [22].Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010-5. doi: 10.1038/ng.179. PMID:18604206 [DOI] [PubMed] [Google Scholar]
- [23].Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, Dean CH. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010;19:4663-76. doi: 10.1093/hmg/ddq397. PMID:20843830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moriwaki K, Tsukita S, Furuse M. Tight junctions containing claudin 4 and 6 are essential for blastocyst formation in preimplantation mouse embryos. Dev Biol. 2007;312:509-22. doi: 10.1016/j.ydbio.2007.09.049. PMID:17980358 [DOI] [PubMed] [Google Scholar]
- [25].Luschnig S, Batz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16:186-94. doi: 10.1016/j.cub.2005.11.072. PMID:16431371 [DOI] [PubMed] [Google Scholar]
- [26].Bagnat M, Navis A, Herbstreith S, Brand-Arzamendi K, Curado S, Gabriel S, Mostov K, Huisken J, Stainier DY. Cse1l is a negative regulator of CFTR-dependent fluid secretion. Curr Biol. 2010;20:1840-5. doi: 10.1016/j.cub.2010.09.012. PMID:20933420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21:R126-36. doi: 10.1016/j.cub.2010.12.003. PMID:21300279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Filas BA, Oltean A, Beebe DC, Okamoto RJ, Bayly PV, Taber LA. A potential role for differential contractility in early brain development and evolution. Biomech Model Mechanobiol. 2012;11:1251-62. doi: 10.1007/s10237-012-0389-4. PMID:22466353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Filas BA, Oltean A, Majidi S, Bayly PV, Beebe DC, Taber LA. Regional differences in actomyosin contraction shape the primary vesicles in the embryonic chicken brain. Phys Biol. 2012;9:066007. doi: 10.1088/1478-3975/9/6/066007. PMID:23160445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, Tabin CJ. On the growth and form of the gut. Nature. 2011;476:57-62. doi: 10.1038/nature10277. PMID:21814276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi Y, Yao J, Xu G, Taber LA. Bending of the looping heart: differential growth revisited. J Biomech Eng. 2014;136; pp.081002.1-081002.15; doi: 10.1115/1.4026645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ochoa-Espinosa A, Affolter M. Branching morphogenesis: from cells to organs and back. Cold Spring Harb Perspect Biol. 2012;4: 1-14. doi: 10.1101/cshperspect.a008243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol. 2009;10:831-42. doi: 10.1038/nrm2797. PMID:19888266 [DOI] [PubMed] [Google Scholar]
- [34].Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987-98. doi: 10.1242/dev.102228. PMID:24803648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taber LA. Morphomechanics: transforming tubes into organs. Curr Opin Genet Dev. 2014;27:7-13. doi: 10.1016/j.gde.2014.03.004. PMID:24791687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Linask KK, Vanauker M. A role for the cytoskeleton in heart looping. Sci World J. 2007;7:280-98. doi: 10.1100/tsw.2007.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Martin AC. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol. 2010;341:114-25. doi: 10.1016/j.ydbio.2009.10.031. PMID:19874815 [DOI] [PubMed] [Google Scholar]
- [38].Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci U S A. 2010;107:1425-30. doi: 10.1073/pnas.0911996107. PMID:20080584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213-28. doi: 10.1152/ajpcell.00558.2003. PMID:15151915 [DOI] [PubMed] [Google Scholar]
- [40].Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or −2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391-401. doi: 10.1083/jcb.143.2.391. PMID:9786950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Piontek A, Rossa J, Protze J, Wolburg H, Hempel C, Gunzel D, Krause G, Piontek J. Polar and charged extracellular residues conserved among barrier-forming claudins contribute to tight junction strand formation. Ann N Y Acad Sci. 2017;1397:143-56. doi: 10.1111/nyas.13341. PMID:28415153 [DOI] [PubMed] [Google Scholar]
- [42].Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. PMID:19706201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 2008;22:146-58. doi: 10.1096/fj.07-8319com. PMID:17761522 [DOI] [PubMed] [Google Scholar]
- [44].Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O, Fujiyoshi Y. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science. 2014;344:304-7. doi: 10.1126/science.1248571. PMID:24744376 [DOI] [PubMed] [Google Scholar]
- [45].Milatz S, Piontek J, Schulzke JD, Blasig IE, Fromm M, Gunzel D. Probing the cis-arrangement of prototype tight junction proteins claudin-1 and claudin-3. Biochem J. 2015;468:449-58. doi: 10.1042/BJ20150148. PMID:25849148 [DOI] [PubMed] [Google Scholar]
- [46].Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Gunzel D, Wolburg H, Krause G, Piontek J. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem. 2014;289:7641-53. doi: 10.1074/jbc.M113.531012. PMID:24478310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408-17. doi: 10.1128/MCB.24.19.8408-8417.2004. PMID:15367662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346-54. doi: 10.1152/ajpcell.00547.2002. PMID:12700140 [DOI] [PubMed] [Google Scholar]
- [49].Li J, Angelow S, Linge A, Zhuo M, Yu AS. Claudin-2 pore function requires an intramolecular disulfide bond between two conserved extracellular cysteines. Am J Physiol Cell Physiol. 2013;305:C190-6. doi: 10.1152/ajpcell.00074.2013. PMID:23677799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li J, Zhuo M, Pei L, Yu AS. Conserved aromatic residue confers cation selectivity in claudin-2 and claudin-10b. J Biol Chem. 2013;288:22790-7. doi: 10.1074/jbc.M113.484238. PMID:23760508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291:F1288-99. doi: 10.1152/ajprenal.00138.2006. PMID:16804102 [DOI] [PubMed] [Google Scholar]
- [52].Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109-18. doi: 10.1242/jcs.02631. PMID:16234325 [DOI] [PubMed] [Google Scholar]
- [53].Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142-7. doi: 10.1152/ajpcell.00038.2002. PMID:12055082 [DOI] [PubMed] [Google Scholar]
- [54].Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878-91. doi: 10.1172/JCI26323. PMID:16528408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Krug SM, Gunzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci. 2012;69:2765-78. doi: 10.1007/s00018-012-0949-x. PMID:22402829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729-56. doi: 10.1016/j.bbamem.2007.08.018. PMID:17950242 [DOI] [PubMed] [Google Scholar]
- [57].Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118:1427-36. doi: 10.1242/jcs.01735. PMID:15769849 [DOI] [PubMed] [Google Scholar]
- [58].Van Itallie CM, Tietgens AJ, LoGrande K, Aponte A, Gucek M, Anderson JM. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J Cell Sci. 2012;125:4902-12. doi: 10.1242/jcs.111237. PMID:22825868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol. 2004;199:29-38. doi: 10.1007/s00232-004-0673-z. PMID:15366421 [DOI] [PubMed] [Google Scholar]
- [60].Fujibe M, Chiba H, Kojima T, Soma T, Wada T, Yamashita T, Sawada N. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res. 2004;295:36-47. doi: 10.1016/j.yexcr.2003.12.014. PMID:15051488 [DOI] [PubMed] [Google Scholar]
- [61].Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, Sawada N. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res. 2004;300:202-12. doi: 10.1016/j.yexcr.2004.07.012. PMID:15383327 [DOI] [PubMed] [Google Scholar]
- [62].Fredriksson K, Van Itallie CM, Aponte A, Gucek M, Tietgens AJ, Anderson JM. Proteomic analysis of proteins surrounding occludin and claudin-4 reveals their proximity to signaling and trafficking networks. PLoS One. 2015;10:e0117074. doi: 10.1371/journal.pone.0117074. PMID:25789658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351-63. doi: 10.1083/jcb.147.6.1351. PMID:10601346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455-61. doi: 10.1074/jbc.M109005200. PMID:11689568 [DOI] [PubMed] [Google Scholar]
- [65].Collins MM, Baumholtz AI, Ryan AK. Claudin family members exhibit unique temporal and spatial expression boundaries in the chick embryo. Tissue Barriers. 2013;1:e24517. doi: 10.4161/tisb.24517. PMID:24665397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291:F1132-41. doi: 10.1152/ajprenal.00063.2006. PMID:16774906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Khairallah H, El Andalousi J, Simard A, Haddad N, Chen YH, Hou J, Ryan AK, Gupta IR. Claudin-7, −16, and −19 during mouse kidney development. Tissue Barriers. 2014;2:e964547. doi: 10.4161/21688362.2014.964547. PMID:25610756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801-5. doi: 10.1038/nature05654. PMID:17325668 [DOI] [PubMed] [Google Scholar]
- [69].Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1997;136:1239-47. doi: 10.1083/jcb.136.6.1239. PMID:9087440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652-8. doi: 10.1074/jbc.272.42.26652. PMID:9334247 [DOI] [PubMed] [Google Scholar]
- [71].Shrestha A, McClane BA. Human claudin-8 and −14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. MBio. 2013;4:1-11. doi: 10.1128/mBio.00594-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Winkler L, Gehring C, Wenzel A, Muller SL, Piehl C, Krause G, Blasig IE, Piontek J. Molecular determinants of the interaction between Clostridium perfringens enterotoxin fragments and claudin-3. J Biol Chem. 2009;284:18863-72. doi: 10.1074/jbc.M109.008623. PMID:19429681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mitchell LA, Koval M. Specificity of interaction between clostridium perfringens enterotoxin and claudin-family tight junction proteins. Toxins (Basel). 2010;2:1595-611. doi: 10.3390/toxins2071595. PMID:22069652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mukendi C, Dean N, Lala R, Smith J, Bronner ME, Nikitina NV. Evolution of the vertebrate claudin gene family: insights from a basal vertebrate, the sea lamprey. Int J Dev Biol. 2016;60:39-51. doi: 10.1387/ijdb.150364nn. PMID:27002805 [DOI] [PubMed] [Google Scholar]
- [75].Simske JS. Claudins reign: The claudin/EMP/PMP22/gamma channel protein family in C. elegans. Tissue Barriers. 2013;1:e25502. doi: 10.4161/tisb.25502. PMID:24665403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Simske JS, Hardin J. Claudin family proteins in Caenorhabditis elegans. Methods Mol Biol. 2011;762:147-69. doi: 10.1007/978-1-61779-185-7_11. PMID:21717355 [DOI] [PubMed] [Google Scholar]
- [77].Simske JS, Koppen M, Sims P, Hodgkin J, Yonkof A, Hardin J. The cell junction protein VAB-9 regulates adhesion and epidermal morphology in C. elegans. Nat Cell Biol. 2003;5:619-25. doi: 10.1038/ncb1002. PMID:12819787 [DOI] [PubMed] [Google Scholar]
- [78].Baltzegar DA, Reading BJ, Brune ES, Borski RJ. Phylogenetic revision of the claudin gene family. Mar Genomics. 2013;11:17-26. doi: 10.1016/j.margen.2013.05.001. PMID:23726886 [DOI] [PubMed] [Google Scholar]
- [79].Loh YH, Christoffels A, Brenner S, Hunziker W, Venkatesh B. Extensive expansion of the claudin gene family in the teleost fish, Fugu rubripes. Genome Res. 2004;14:1248-57. doi: 10.1101/gr.2400004. PMID:15197168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li G, Flodby P, Luo J, Kage H, Sipos A, Gao D, Ji Y, Beard LL, Marconett CN, DeMaio L, et al.. Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis. Am J Respir Cell Mol Biol. 2014;51:210-22. PMID:24588076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Matsumoto K, Imasato M, Yamazaki Y, Tanaka H, Watanabe M, Eguchi H, Nagano H, Hikita H, Tatsumi T, Takehara T, et al.. Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterology. 2014;147:1134-45 e10. doi: 10.1053/j.gastro.2014.07.033. PMID:25068494 [DOI] [PubMed] [Google Scholar]
- [82].Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology. 2013;144:369-80. doi: 10.1053/j.gastro.2012.10.035. PMID:23089202 [DOI] [PubMed] [Google Scholar]
- [83].Hayashi D, Tamura A, Tanaka H, Yamazaki Y, Watanabe S, Suzuki K, Suzuki K, Sentani K, Yasui W, Rakugi H, et al.. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1beta, and atrophic gastritis in mice. Gastroenterology. 2012;142:292-304. doi: 10.1053/j.gastro.2011.10.040. PMID:22079592 [DOI] [PubMed] [Google Scholar]
- [84].Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, Cao J, Chen YH. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142:305-15. doi: 10.1053/j.gastro.2011.10.025. PMID:22044670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tanaka H, Takechi M, Kiyonari H, Shioi G, Tamura A, Tsukita S. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut. 2015;64:1529-38. doi: 10.1136/gutjnl-2014-308419. PMID:25691495 [DOI] [PubMed] [Google Scholar]
- [86].Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, et al.. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A. 2010;107:8011-6. doi: 10.1073/pnas.0912901107. PMID:20385797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PloS One. 2012;7:e52272. doi: 10.1371/journal.pone.0052272. PMID:23284964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, Jeansonne BG, Ding L, Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol. 2010;298:F24-34. doi: 10.1152/ajprenal.00450.2009. PMID:19759267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A. 2015;112:4340-5. doi: 10.1073/pnas.1421441112. PMID:25831548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Müller D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A. 2012;109:14241-6. doi: 10.1073/pnas.1203834109. PMID:22891322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350-5. doi: 10.1073/pnas.0907724106. PMID:19706394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114-22. doi: 10.1074/jbc.M700632200. PMID:17442678 [DOI] [PubMed] [Google Scholar]
- [93].Cheung ID, Bagnat M, Ma TP, Datta A, Evason K, Moore JC, Lawson ND, Mostov KE, Moens CB, Stainier DY. Regulation of intrahepatic biliary duct morphogenesis by Claudin 15-like b. Dev Biol. 2012;361:68-78. doi: 10.1016/j.ydbio.2011.10.004. PMID:22020048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wessely O, Tran U. Xenopus pronephros development–past, present, and future. Pediatr Nephrol. 2011;26:1545-51. doi: 10.1007/s00467-011-1881-2. PMID:21499947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099-111. doi: 10.1083/jcb.200110122. PMID:11889141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649-59. doi: 10.1016/S0092-8674(00)81553-6. PMID:10612400 [DOI] [PubMed] [Google Scholar]
- [97].Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, et al.. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049-61. doi: 10.1093/hmg/ddg210. PMID:12913076 [DOI] [PubMed] [Google Scholar]
- [98].Tamura A, Kitano Y, Hata M, Katsuno T, Moriwaki K, Sasaki H, Hayashi H, Suzuki Y, Noda T, Furuse M, et al.. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523-34. doi: 10.1053/j.gastro.2007.11.040. PMID:18242218 [DOI] [PubMed] [Google Scholar]
- [99].Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, Okamura Y, Sasaki H, Miyachi Y, et al.. Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J Cell Biol. 2005;169:527-38. doi: 10.1083/jcb.200501154. PMID:15883201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kage H, Flodby P, Gao D, Kim YH, Marconett CN, DeMaio L, Kim KJ, Crandall ED, Borok Z. Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L524-36. doi: 10.1152/ajplung.00077.2014. PMID:25106430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Anderson WJ, Zhou Q, Alcalde V, Kaneko OF, Blank LJ, Sherwood RI, Guseh JS, Rajagopal J, Melton DA. Genetic targeting of the endoderm with claudin-6CreER. Dev Dyn. 2008;237:504-12. doi: 10.1002/dvdy.21437. PMID:18213590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120-33. doi: 10.1016/j.devcel.2011.06.011. PMID:21763613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767-78. doi: 10.1242/dev.02347. PMID:16571627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220-4. doi: 10.1038/nature04375. PMID:16407953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789-99. doi: 10.1242/dev.000380. PMID:17229766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195-204. doi: 10.1083/jcb.147.1.195. PMID:10508866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Che J, Yang Y, Xiao J, Zhao P, Yan B, Dong S, Cao B. Decreased expression of claudin-3 is associated with a poor prognosis and EMT in completely resected squamous cell lung carcinoma. Tumour Biol. 2015;36:6559-68. doi: 10.1007/s13277-015-3350-1. PMID:25820701 [DOI] [PubMed] [Google Scholar]
- [108].Bhat AA, Pope JL, Smith JJ, Ahmad R, Chen X, Washington MK, Beauchamp RD, Singh AB, Dhawan P. Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis. Oncogene. 2015;34:4570-80. doi: 10.1038/onc.2014.385. PMID:25500541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449-54. doi: 10.1073/pnas.1004900107. PMID:20713713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Godde NJ, Galea RC, Elsum IA, Humbert PO. Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. J Mammary Gland Biol Neoplasia. 2010;15:149-68. doi: 10.1007/s10911-010-9180-2. PMID:20461450 [DOI] [PubMed] [Google Scholar]
- [111].Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959-67. doi: 10.1242/jcs.00389. PMID:12668723 [DOI] [PubMed] [Google Scholar]
- [112].Smith JL, Schoenwolf GC. Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec. 1987;218:196-206. doi: 10.1002/ar.1092180215. PMID:3619087 [DOI] [PubMed] [Google Scholar]
- [113].McShane SG, Mole MA, Savery D, Greene ND, Tam PP, Copp AJ. Cellular basis of neuroepithelial bending during mouse spinal neural tube closure. Dev Biol. 2015;404:113-24. doi: 10.1016/j.ydbio.2015.06.003. PMID:26079577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Smith JL, Schoenwolf GC, Quan J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J Comp Neurol. 1994;342:144-51. doi: 10.1002/cne.903420113. PMID:8207124 [DOI] [PubMed] [Google Scholar]
- [115].Schoenwolf GC, Franks MV. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol. 1984;105:257-72. doi: 10.1016/0012-1606(84)90284-7. PMID:6479439 [DOI] [PubMed] [Google Scholar]
- [116].Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical accumulation of Rho in the neural plate is important for neural plate cell shape change and neural tube formation. Mol Biol Cell. 2008;19:2289-99. doi: 10.1091/mbc.E07-12-1286. PMID:18337466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Escuin S, Vernay B, Savery D, Gurniak CB, Witke W, Greene ND, Copp AJ. Rho-kinase-dependent actin turnover and actomyosin disassembly are necessary for mouse spinal neural tube closure. J Cell Sci. 2015;128:2468-81. doi: 10.1242/jcs.164574. PMID:26040287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Itoh K, Ossipova O, Sokol SY. GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J Cell Sci. 2014;127:2542-53. doi: 10.1242/jcs.146811. PMID:24681784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chung S, Andrew DJ. The formation of epithelial tubes. J Cell Sci. 2008;121:3501-4. doi: 10.1242/jcs.037887. PMID:18946020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Siddiqui M, Sheikh H, Tran C, Bruce AE. The tight junction component Claudin E is required for zebrafish epiboly. Dev Dyn. 2010;239:715-22. doi: 10.1002/dvdy.22172. PMID:20014098 [DOI] [PubMed] [Google Scholar]
- [121].Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Dev Biol. 2001;230:217-29. doi: 10.1006/dbio.2000.0116. PMID:11161574 [DOI] [PubMed] [Google Scholar]
- [122].Warga RM, Kimmel CB. Cell movements during epiboly and gastrulation in zebrafish. Development. 1990;108:569-80. PMID:2387236 [DOI] [PubMed] [Google Scholar]
- [123].Consortium Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695-716. doi: 10.1038/nature03154. PMID:15592404 [DOI] [PubMed] [Google Scholar]
- [124].Chakraborty P, William Buaas F, Sharma M, Smith BE, Greenlee AR, Eacker SM, Braun RE. Androgen-dependent sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol Endocrinol. 2014;28:1055-72. doi: 10.1210/me.2013-1134. PMID:24825397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev Biol. 2011;353:38-49. doi: 10.1016/j.ydbio.2011.02.027. PMID:21377456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rifat Y, Parekh V, Wilanowski T, Hislop NR, Auden A, Ting SB, Cunningham JM, Jane SM, et al.. Regional neural tube closure defined by the Grainy head-like transcription factors. Dev Biol. 2010;345:237-45. doi: 10.1016/j.ydbio.2010.07.017. PMID:20654612 [DOI] [PubMed] [Google Scholar]
- [127].Brouns MR, De Castro SC, Terwindt-Rouwenhorst EA, Massa V, Hekking JW, Hirst CS, Savery D, Munts C, Partridge D, Lamers W, et al.. Over-expression of Grhl2 causes spina bifida in the Axial defects mutant mouse. Hum Mol Genet. 2011;20:1536-46. doi: 10.1093/hmg/ddr031. PMID:21262862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Aue A, Hinze C, Walentin K, Ruffert J, Yurtdas Y, Werth M, Chen W, Rabien A, Kilic E, Schulzke JD, et al.. A Grainyhead-Like 2/Ovo-Like 2 Pathway Regulates Renal Epithelial Barrier Function and Lumen Expansion. J Am Soc Nephrol. 2015;26:2704-15. doi: 10.1681/ASN.2014080759. PMID:25788534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kidder GM. Trophectoderm development and function: the roles of Na+/K(+)-ATPase subunit isoforms. Can J Physiol Pharmacol. 2002;80:110-5. doi: 10.1139/y02-017. PMID:11934253 [DOI] [PubMed] [Google Scholar]
- [130].Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132:2057-67. doi: 10.1242/dev.01791. PMID:15788456 [DOI] [PubMed] [Google Scholar]
- [131].Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954-60. doi: 10.1038/ncb1621. PMID:17632505 [DOI] [PubMed] [Google Scholar]


