Abstract
Pain-depressed behavior can be defined as any behavior that decreases in rate, frequency, duration or intensity in response to a putative pain state. Common examples include pain-related decreases in feeding, locomotion and expression of positively reinforced operant behavior. In humans, depression of behavior is often accompanied by a co-morbid depression of mood. Measurements of pain-depressed behaviors are used to diagnose pain in both human and veterinary medicine, and restoration of pain-depressed behavior is often a priority of treatment. This article describes two strategies for integrating measures of pain-depressed behaviors into preclinical assays of pain and analgesia. Assays of pain-depressed behaviors may contribute both to improved translational efficiency in analgesic drug development and to new insights regarding the mechanisms and determinants of pain and analgesia.
Keywords: pain, behavioral assay, pain-depressed behavior, feeding, intracranial self-stimulation
1. Introduction
Preclinical behavioral assays of pain and analgesia necessarily include two elements: (1) a manipulation intended to produce a pain-like state (the independent variable), and (b) measurement of a response presumed to be indicative of that pain-like state (the dependent variable). In recent years, there have been significant advances in the methodology used to model acute, inflammatory, neuropathic and cancer pain states, and details of these methodologies are described elsewhere in this volume. However, the dependent measures in preclinical assays have been much slower to evolve (2, 14, 16, 24, 25). The most widely used measures fall into a category that we have described as “pain-stimulated behaviors,” which can be defined as behaviors that increase in rate, frequency, duration or intensity in response to a putative pain state (16, 23). Common examples include withdrawal responses from stimuli that can be escaped (e.g. paw withdrawal from a mechanical or thermal stimulus) or stretching/flinching responses from stimuli that cannot be escaped (e.g. stretching responses elicited by intraperitoneal injection of noxious chemical stimuli). Although a focus on pain-stimulated behaviors can be useful for many applications, an exclusive reliance on these behaviors as dependent measures can be problematic for a number of reasons. These problems have been discussed at length elsewhere, and they include a difficulty in dissociating analgesia from motor impairment (16), a dissociation between expression of pain-stimulated behaviors and verbal reports of pain in some types of chronic pain in humans (7, 22), and mediation by neural substrates that may exclude or under-represent key brain areas involved in affective components of pain (18, 19).
As an alternative to the use of pain-stimulated behaviors as dependent measures, we and others have begun to begun to explore strategies for incorporating PAIN-DEPRESSED BEHAVIOR into preclinical assays of pain and analgesia (12, 13, 15, 17, 21, 23). “Pain-depressed” behaviors can be defined as any behavior that decreases in rate, frequency, duration or intensity in response to a noxious stimulus, and common examples include pain-related decreases in feeding, locomotion and expression of positively reinforced operant behavior. The use of pain-depressed behaviors as dependent variables may be associated with several advantages relative to the use of pain-stimulated behaviors. First, drugs or other manipulations that produce analgesia would be expected to increase pain-depressed behaviors, and as a result, true analgesia would be readily dissociable from motor impairment. Second, pain states that requires clinical intervention are often associated with a depression of behavior rather than a stimulation of behavior, and in humans, pain-related depression of behavior is often accompanied by a co-morbid depression of mood (1, 8, 9, 11). Indeed, diagnostic tools that measure pain-related depression of behavior and mood are coming to play an increasingly prominent role in human medicine (4, 6, 10), and measures of functional impairment/depressed behavior are even more important for veterinary assessments of pain in animals (5). The utility of these measures of pain-depressed behavior in the clinical diagnosis of pain suggests that these measures may also be useful in preclinical research. Finally, a focus on pain-depressed behaviors could enable preclinical research on mechanisms and determinants of the affective components of pain (3, 17).
We have proposed that development of preclinical assays of pain-depressed behavior might best proceed in three systematic steps: (a) identification of conditions under which a target behavior occurs at a high and stable rate to provide a stable and sensitive behavioral baseline, (b) identification of conditions under which a putative pain state reliably depresses the rate of that target behavior, and (c) assessment of effects of candidate analgesic drugs or other manipulations on the rate of pain-depressed behavior. In addition, it is useful to conduct control experiments to assess the degree to which the drug or other manipulation alters (a) control, non-depressed rates of the target behavior in the absence of a pain state, and (b) rates of the target behavior depressed by a putative non-painful manipulation. Under these circumstances, an optimal analgesic might be one that restores pain-depressed behavior without increasing baseline rates of that behavior in the absence of pain and without increasing rates of that behavior when it is depressed by non-painful manipulations. We have pursued this strategy in developing two different assays of pain-depressed behavior in rodents, and methods for both assays are provided. The first assay measures feeding behavior in mice, and it has the advantage of requiring a modest experimental infrastructure and limited training of experimental subjects (23). The second assay measures intracranial self-stimulation (ICSS) in rats. ICSS has been used extensively to evaluate effects of experimental manipulations on motivated behavior (3, 17, 20), and this technically more-demanding procedure may confer an advantage of heightened sensitivity in studies of pain-depressed behavior. In both assays, we have employed intraperitoneal injection of dilute acid to produce a pain state, although other approaches could also easily be used (e.g. manipulations to model inflammatory, neuropathic, cancer or other pain states). In addition, we have evaluated the sensitivity of these procedures using the opioid analgesic morphine. Again though, a wide variety of other pharmacologic or non-pharmacologic manipulations could be used. One final caveat is also warranted. The use of these assays is in its infancy, and these procedures will be a topic of research in their own right. The Materials and Methods provided below should be considered as starting point in the evolution of assays of pain-depressed behavior for preclinical research on pain and analgesia.
2. Materials
2.1. Acid-Depressed Feeding in Mice
Subjects: Adult male C57BL6/J Mice (e.g. Jackson Laboratories, Bar Harbor, ME) (see Note 1)
Scale with resolution to 0.1 gram suitable for weighing mice and feeding solutions
Observation cage (e.g. a clean, empty standard housing cage)
Vanilla-flavored Ensure™ protein drink
Wide flat dish capable of holding 8ml (8.6 grams) of Ensure (see Note 2)
Acetic acid solution
Injection supplies (syringes, saline for dilution of acid and drug solutions)
2.2. Acid-Depressed Intracranial Self-Stimulation (ICSS) in Rats
This procedure uses the same materials and methods for ICSS that have been described in detail previously (3). The reader is encouraged to refer to this excellent resource for a detailed description of the general methodology.
Subjects: Adult rats weighing approximately 300–350 grams at the time of surgery (Note 4)
-
Equipment for Operant Conditioning and ICSS Delivery
Rat operant conditioning stations housed in sound-attenuating chambers and equipped with at least one response lever, stimulus and house lights, ICSS stimulator, interface, and computer control equipment with software to operate ICSS experiments (e.g. Med Associates, St. Albans, VT) (Note 5)
ICSS electrodes (e.g. MS303/1-AIU/SPC bipolar electrodes with one 0.25 mm insulated wire cut to 10 mm and one 0.125 mm uninsulated wire cut to 15 mm; Plastics One, Roanoke, VA)
Two-channel commutator to allow rat to rotate (e.g. SL2C, Plastics One; one per operant station)
Bipolar cables [one to connect ICSS stimulator to commutator (e.g. 305–491, Plastics One) and one to connect commutator to electrode (e.g. 305–305, Plastics One)] (Note 6)
Oscilloscope to monitor ICSS (e.g. 2120B Dual Trace 30MHZ Oscilloscope, BK Precision, Yorba Linda, CA 92887)
Ring stand, clamp holder and 3 prong clamp to hold commutator above operant chamber (e.g. catalog numbers 11-474-207 Cast-iron L-shaped base support, 12621-250 Talon clamp holder, and 21570-126 three-prong extension clamp, VWR International, Marietta, GA)
-
Equipment and Supplies for Surgical Implantation of Electrode
Rat stereotax (e.g. Model 900; Kopf Instruments, Tujunga, CA)
Electrode holder for stereotax (e.g. MH-300, Plastics One)
Skull screws (e.g. 0–80 × 1/8, Plastics One)
Drill holder and drill bits to drill holes in skull for electrode and skull screws (e.g. DH-1 and D#56, Plastics One)
Surgical supplies (e.g. anesthetic, atropine sulfate, antibiotic ointment, instruments (scalpel, hemostats), suture material; other supplies as required by institutional regulations for rodent surgery)
Acrylic cement (e.g. catalog number 651006 Orthodontic resin powder and 651002 orthodonitic resin pink liquid, Dentsply Caulk, Densply International, Milford, DE)
Supplies for Experimental Manipulations (Note 7)
3. Methods
3.1. Acid-Depressed Feeding in Mice
3.1.1. Subjects
Mice are group housed under standard laboratory conditions. Access to food and water is controlled to optimize the likelihood of high, stable rates of feeding during daily feeding sessions. Specifically, a water bottle and the daily ration of mouse chow (8 grams) are provided immediately after each daily session, and the water bottle and any uneaten food are removed 4 hr before the next session. We tested mice during the light phase of the light-dark cycle.
3.1.2. Behavioral Procedure (see Note 3)
Feeding sessions are conducted Monday–Friday beginning at 12:00 noon. For each session, mice are removed from their home cages, weighed and placed individually in observation cages equipped with a dish containing 8 ml (8.6 g) of liquid food (vanilla-flavored Ensure™ protein drink). After a specified period of access (usually 30 min), mice are returned to their home cages, and the amount of Ensure consumed during the access period is determined by subtracting the weight of the dish at the end of the session from its weight at the beginning of the session. To control for any weight changes during the experiment, consumption is expressed as grams of liquid food consumed ÷ weight of the mouse in grams.
In our initial studies, mice were initially trained under these conditions until feeding stabilized (3 consecutive days with ≥ 20% variability in consumption). Testing was then initiated in three phases to (1) identify conditions that would generate high and stable rates of feeding, (2) identify conditions under which acetic acid would depress feeding, and (3) characterize the effects of morphine on acid-suppressed feeding and other control behaviors. The effects of the dopamine D2 receptor antagonist haloperidol were also examined as a representative non-analgesic drug that impairs motor function. During Phase 1, the concentration of Ensure (0 – 100% in water) and the duration of the access period (7.5 – 120 min) were manipulated. On the basis of our results, an Ensure concentration of 32% and access duration of 30 min were used for subsequent studies. During Phase 2, feeding behavior was examined after intraperitoneal administration of acetic acid in the same group of mice. A range of acetic acid concentrations (0 – 1% in saline) and pretreatment times (0 – 240 min before the feeding session) were examined, and acetic acid was administered no more than once per week. On remaining days of each week, mice had access to 32% Ensure for 30 min. On the basis of these results, a concentration of 0.56% acetic acid given immediately before the feeding session (0 min pretreatment time) was used for subsequent studies. During Phase 3, the effects of morphine and haloperidol were examined on acetic acid-suppressed feeding and a variety of other behaviors including (1) control rates of feeding determined without acid pretreatment, (2) rates of feeding suppressed by non-pain manipulations (substitution of water for Ensure, prefeeding subjects immediately prior to test session), and (3) expression of acetic acid-induced writhing, a pain-stimulated behavior.
3.1.3. Illustrative Results
Figure 1 shows selected results for effects of acetic acid alone or after pretreatment with morphine and haloperidol on a pain-stimulated behavior (writhing, top panels) or a pain-suppressed behavior (feeding, bottom panels). Under baseline conditions in the absence of acetic acid treatment, animals displayed no writhing and relatively high and stable levels of feeding (~0.14 grams liquid food/gram of body weight) (see points above “BL” in all panels). Acetic acid produced a concentration-dependent increase in writhing and a decrease in feeding (left panels, bars over “Acid Alone” in right panels). Morphine attenuated both acid-stimulated writhing and acid-suppressed feeding, whereas the non-analgesic haloperidol decreased acid-stimulated writhing but failed to block acid-suppressed feeding (right panels). The effects of morphine were time dependent and naltrexone reversible, and a dose of morphine that blocked acid-suppressed feeding had little or no effect on baseline feeding or on feeding suppressed by other manipulations that did not involve noxious stimulation and pain (23). Overall, morphine produced antinociceptive effects in assays of both pain-stimulated and pain-depressed behavior, whereas the non-analgesic drug haloperidol produced an antinociceptive effect only in the assay of pain-stimulated behavior. These results illustrate the utility of using complementary assays of pain-stimulated and pain-depressed behavior to evaluate candidate analgesics.
Figure 1. Comparison of drug effects on pain-stimulated and pain-depressed behaviors in mice.
Abscissae (left panels): % Acetic acid solution injected IP in mice. Points above “BL” show baseline in the absence of an IP injection. Abscissae (right panels): Treatment condition—baseline, 0.56% acetic acid alone (Acid Alone), 0.56% acetic acid + 1.0 mg/kg morphine (+ morphine), 0.56% acetic acid + 1.0 mg/kg haloperidol (+ haloperidol). Ordinates (top panels). # Writhes counted during observation period. Ordinates (bottom panels): Consumption of liquid food solution (g consumed per g body weight). All points/bars show mean ± SEM of 6–12 mice. * Indicates significantly different from “BL,” p<0.05. † Indicates significantly different from “Acid Alone,” p<0.05. Reprinted from (16) with permission from the Journal of Pharmacology and Experimental Therapeutics.
3.2. Acid-Depressed Intracranial Self-Stimulation (ICSS) in Rats
3.2.1. Subjects
Rats are typically ordered at a weight of approximately 250–275 grams to allow some room for growth during an initial 1 week acclimation period before surgery. Rats are singly housed to prevent inter-animal tampering with head-mounted ICSS electrodes, and they are given free access to food and water except during experimental sessions. We tested rats during the light phase of the light-dark cycle.
3.2.2. Surgical Procedure
Electrodes are surgically implanted to target the medial forebrain bundle at the level of the lateral hypothalamus (Note 8). In rats weighing 300–350 gm, this can be achieved by securing the rat in the stereotax with level skull (i.e. bregma and lamda at identical dorso-ventral coordinates), and targeting the electrode at 2.8 mm posterior to bregma, 1.7 mm lateral to the midsaggital suture, and 7.8 mm below dura.
Details of the anesthesia, surgical procedure and post-operative care should comply with institutional regulations. Briefly, the rat is anesthetized and secured in the stereotax, an incision is made in the scalp to expose the skull and the landmarks bregma and lamda, and holes are drilled in the skull to accommodate 3 or 4 skull screws and the electrode. Skull screws are placed before insertion of the electrode. When using the electrodes described above (MS303/1-AIU/SPC, Plastics One, Roanoke, VA), the thicker, insulated wire serves as the stimulating electrode, and it is inserted into the brain at the specified coordinates. The thinner, uninsulated wire serves as a ground, and this wire is wrapped around one or two of the skull screws. Once the electrode is in place, it is secured with acrylic cement poured around the pedestal of the electrode and the skull screws. After the acrylic cement dries, the incision is sutured, and the rat can be removed from the stereotax.
3.2.3. Behavioral Procedure
In our studies, training and testing for each rat have proceeded in three phases. The first phase (Lever Press Training) begins seven days after surgery, and daily sessions last 30 to 60 min (duration is not critical). Each lever press results in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration, 141 Hz), and stimulation is accompanied by the illumination of a 2-W “house” light located on the ceiling of the sound-attenuating chamber. Responses during the 0.5-s stimulation period do not earn additional stimulation. The stimulation intensity for each rat is set initially at 150μA, and it is adjusted gradually to the lowest value that sustains a reliable rate of responding (≥20 responses/min) (Notes 9 and 10). In the second phase (Multiple-Frequency Training), daily sessions consist of multiple 15-min components. During each component, a descending series of 15 current frequencies (141-28 Hz in 0.05 log increments) is presented, with a 60-s trial at each frequency. Each frequency trial begins with a 5-sec timeout period followed by 5 sec of period of non-contingent stimulation (five 0.5 sec pulses separated by 0.5 sec intervals), which in turn is followed by a 50-s “response” phase during which stimulus lights are illuminated and each response produces electrical stimulation. Under these conditions, response rates are typically high during the initial high-frequency trials of each component, and response decline to low rates as frequency declines (see Figure 2). The intensity may again be adjusted during this phase so that the subject responds at high rates for the first 4–6 frequency trials and at lower rates for the remaining trials. The third phase (Testing) begins once responding stabilizes under Multiple-Frequency Training. Test sessions consist of three consecutive “baseline” components followed by up to six consecutive “test” components, and experimental manipulations are introduced during an interval between the baseline and test components. Test sessions are conducted one or two times per week, with Multiple Frequency Training sessions between test sessions.
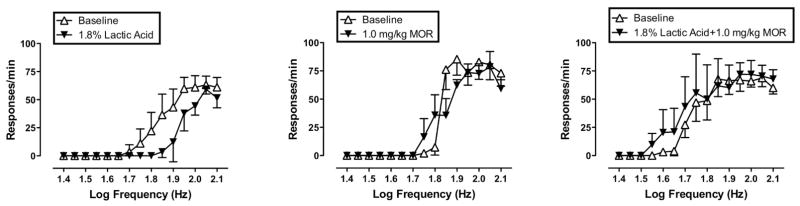
Figure 2. Morphine effects on pain-depressed intracranial self-stimulation (ICSS) in rats.
Abscissae: Frequency of electrical stimulation (log Hz). Ordinates: Response rate in responses/min. “Baseline” curves show data collected from the second and third baseline components conducted during each test session (the first component served as an acclimation period, and data were discarded). Test curves show data from the component of peak effect after delivery of each treatment. Left panel shows effects of 1.8% lactic acid delivered IP in a volume of 1 ml/kg. The center panel shows effects of 1.0 mg/kg morphine IP. The right panel shows effects of 1.0 mg/kg morphine administered as a pretreatment to lactic acid. All points show mean ±SEM from 5 rats.
3.2.4. Illustrative Results
Figure 2 shows illustrative results using this procedure to evaluate pain-depressed behavior. Each panel shows response rates as a function of log frequency for one test session in a group of five rats. During the baseline components at the beginning of each test session, there was a frequency-dependent increase in response rates. IP administration of 1.8% lactic acid (1ml/kg injection volume) depressed ICSS as indicated by a rightward shift in the frequency-rate curve (Fig. 2, left panel). Notably, response rates were depressed at intermediate stimulus frequencies (i.e. 1.75 to 2.0 log Hz) but not at high frequencies (2.05–2.1 log Hz). These high response rates at high stimulus frequencies demonstrate that rats were still motorically capable of responding at high rates. Overall, these results suggest that a noxious stimulus (IP injections of 1.8% lactic acid) depressed responding by decreasing the reinforcing efficacy of brain stimulation rather than by decreasing the motoric ability of rats to respond.
The center panel of Figure 2 shows that a relatively low dose of morphine (1.0 mg/kg IP) alone had little effect on ICSS. However, the right panel of Figure 2 shows that pretreatment with this morphine dose completely blocked the effect of 1.8% lactic acid. Thus, pretreatment with a morphine dose that had no effect alone completely prevented lactic acid-induced depression of ICSS.
Footnotes
Mice, rats or subjects of a variety of other species could conceivably be used for this type of procedure. Mice were selected for these initial studies for two reasons: (1) they have been used extensively in assays of pain-stimulated behavior, including an assay of acetic acid induced stretching on which this assay is based, and (2) they are a useful species of experimental subject for genetic manipulations. In addition, we used young adult mice for our initial studies, but mice of different ages could conceivably be used for developmental studies.
We used recycled caps from Starbucks™ bottled Frappuccino™ beverages. These had the advantage of being relatively wide and shallow with vertical side walls. These features were useful for minimizing spillage by promoting stability of the dish and facilitating access. To further promote stability, the dishes were secured to the floor of the observation cage with a putty adhesive (e.g. 3M Scotch Removable Adhesive Putty).
Our initial studies were conducted using a repeated-measures, within-subject design in which each mouse received all manipulations. This approach was taken to minimize animal usage, and animals displayed stable responses to repeated manipulations and little or no evidence of toxicity/pathology associated with repeated testing. However, studies proceeded at a relatively slow rate, because acid injections were delivered no more often than once per week. It is likely that rate of throughput could be increased by using a between-subjects design in which each mouse received one acclimation session followed by one test session.
Rats or mice are the species most often used for ICSS studies, although other species could also be used. Rats were selected for these studies to build on the relatively large literature of ICSS studies with this species. In addition, we used young adult rats for our initial studies, but rats of different ages could conceivably be used for developmental studies, although it would be necessary to adjust coordinates for electrode implantation to accommodate animals of various sizes.
Multiple vendors offer operant conditioning equipment. Med Associates offers operant conditioning stations for ICSS in both rats and mice, and these stations can be customized to include different types or numbers of response manipulanda, different stimulus light configurations, and devices for delivering different types of consequent stimuli (e.g. food pellets, liquid reinforcers). Med Associates also uses a proprietary programming language that is relatively accessible to non-specialists, and Med Associates also has programmers who can write custom software.
The bipolar cable from commutator to electrode should be custom ordered at a length that will permit movement of rat throughout the operant chamber but without excessive slack. An appropriate length is the distance from the commutator (mounted in a fixed location above the chamber) to a corner of the chamber at floor level. In addition, it is necessary to attach this cable to the electrode in such a way that it delivers cathodal pulses to the target. To determine the cathodal wire in the bipolar cable, an ICSS program can be activated to deliver pulses while current amplitude is monitored by touching the red and black leads of an ammeter to the two pins at the end of the cable. If the sign of the current reading from each pulse is positive, the wire touching the black lead on the ammeter is the cathode, and the cable above this pin can be marked with an indelible marker for future reference. (If the sign of the current reading is negative, the leads of the ammeter can be switched to the opposite pins on the cable to generate a positive reading). The cathode should then be connected to the electrode port for the thick, insulated electrode.
In our initial studies, we used dilute lactic acid as the noxious stimulus, and morphine was evaluated for its ability to produce antinociceptive effects. These manipulations required lactic acid, sterile saline (for dilution of lactic acid and as vehicle for drug solutions), and syringes for delivery of solutions. Manipulations designed to model inflammatory, neuropathic or other pain states would obviously require different supplies.
In our initial studies, we targeted the medial forebrain bundle of the lateral hypothalamus, because this region maintains high rates of ICSS and innervates cell bodies of the mesolimbic dopamine system, a neural system considered to be important in mediating behavior reinforced by various stimuli. However, other electrode targets may also be of interest, such as targets in pathways thought to be important in modulating pain (e.g. the periaqueductal gray).
During initial training, it is helpful to “shape” lever-press responding by delivering a stimulus pulse for successive approximations of the target behavior. Thus, for initial training sessions, the rat is connected to the bipolar cable and allowed to explore the chamber while an observer operates a remote switch to deliver pulses. (For example, for standard responses levers from Med Associates, the microswitch in the response lever can be operated from outside the chamber by inserting a paperclip through an access hole on the underside of the lever housing.) Initially, pulses can be delivered if the rat faces the lever. Subsequently, pulses can be delivered if the rat approaches or touches the lever. Ultimately, the rat will begin to press the lever on its own, and observer-delivered pulses are no longer necessary.
The effectiveness of intracranial stimulation as a reinforcer depends on the placement of the electrode. Although there is some room for error in electrode placement, larger displacements may place the electrode in contact with areas mediating motor effects, and stimulation may produce stereotyped motor responses, such as a turning of the head or lifting of the paw. In other cases, stimulation may promote seizures. In these cases, undesired effects of stimulation can sometimes be reduced or eliminated by reducing current intensity.
References
- 1.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn-Munro G. Pain-like behaviours in animals - how human are they? Trends Pharmacol Sci. 2004;25:299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 4.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 5.Council NR. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- 6.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 8.Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Lepine JP, Angermeyer MC, Levinson D, de Girolamo G, Iwata N, Karam A, Guimaraes Borges GL, de Graaf R, Browne MO, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27:1571–87. doi: 10.1592/phco.27.11.1571. [DOI] [PubMed] [Google Scholar]
- 10.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23:345–56. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 11.Lepine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(Suppl 1):S3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 12.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 14.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–5. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Morgan D, Carter CS, Dupree JP, Yezierski RP, Vierck CJ. Evaluation of prescription opioids using operant-based pain measures in rats. Exp Clin Psychopharmacol. 2008;16:367–75. doi: 10.1037/a0013520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 17.Pereira Do Carmo G, Schrode KA, Carlezon WA, Jr, Negus SS. College on Problems of Drug Dependence. San Juan, PR: 2008. Effects of pain and analgesia on intracranial self-stimulation (ICSS) in rats. [Google Scholar]
- 18.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 19.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv. 2002;2:392–402. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 20.Reid LD. Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs. In: Bozarth MJ, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer; Berlin: 1987. pp. 391–420. [Google Scholar]
- 21.Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008 doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans -- review article. Pain. 2002;96:3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- 24.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Whiteside GT, Adedoyin A, Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 2008;54:767–75. doi: 10.1016/j.neuropharm.2008.01.001. [DOI] [PubMed] [Google Scholar]