Summary
Setting
Programmatic implementation of decentralized rapid drug susceptibility testing (DST) in Lima, Peru.
Objective
Pre-post analysis compared time to diagnosis, treatment outcome and survival among patients tested with direct nitrate reductase assay (NRA) vs. indirect conventional methods.
Design
From 2005 to 2009, we prospectively followed all patients referred for DST before (control) and after (intervention) NRA implementation. Among those referred for DST, NRA was used for smear-positive samples of patients with no prior history of multidrug resistance or treatment for multidrug-resistant tuberculosis (TB). Data were abstracted from patient charts and laboratory registers. Endpoints were favorable outcomes, time to result and time to death.
Results
Of those patients who met the criteria for NRA, 740 underwent NRA and 621 underwent conventional DST. NRA yielded test results for 78.4% of cases vs. 68.8% for conventional DST (P < 0.0001); the median time to result was 44 vs. 133 days, respectively (adjusted HR 0.64, 95%CI 0.56–0.73). Among individuals without previous anti-tuberculosis treatment, NRA was associated with a favorable treatment outcome (adjusted OR 1.39, 95%CI 1.01–1.90) and prolonged survival (adjusted HR 0.53, 95%CI 0.31–0.90).
Conclusion
Direct NRA significantly shortened time to test result and improved treatment outcomes and survival in certain groups.
Keywords: nitrate reductase assay, multidrug resistance, implementation science, clinical outcomes, diagnosis
THE WORLD HEALTH ORGANIZATION (WHO) reports that only 23 165 (5%) of approximately 440 000 incident multidrug-resistant tuberculosis (MDR-TB) cases received treatment in 2009.1 The WHO's Global Plan to Stop TB currently calls for rapid drug susceptibility testing (DST) for more than 50% of new cases and more than 90% of previously treated cases by 2015;2 however, programmatic implementation and scale-up of rapid DST, including methods that have been validated for many years, have been slow in many settings.
The direct nitrate reductase assay (NRA) was developed by the Central Tuberculosis Research Institute in Moscow, Russia, to identify Mycobacterium tuberculosis strains, and later to identify MDR-TB.3 Independent evaluation in 2002 demonstrated that the NRA was an accurate, rapid and inexpensive method for first-line DST, easily adapted to any laboratory with capacity for culture using Löwenstein-Jensen (LJ) medium.4 Since then, repeated comparisons of direct and indirect NRA compared to classic and novel methods have consistently shown NRA to be accurate, relatively rapid, inexpensive and adaptable to any laboratory that can perform M. tuberculosis cultures, all properties that are ideal for middle-and low-income countries.5–8 In a systematic review, NRA sensitivity and specificity were >94% for rifampin (RMP) and >92% for isoniazid (INH).9 NRA has compared favorably with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test, the resazurin microtiter method, the alamar blue method, the microscopic observation drug susceptibility (MODS) method, BACTEC™ MGIT™ (Mycobacteria Growth Indicator Tube) 960 (BD, Sparks, MD, USA), and Genotype® MTBDR and MTBDRplus (Hain Life Sciences, Nehren, Germany).10–13
In the present study, we describe the performance of direct NRA when deployed as part of a comprehensive strategy to strengthen laboratory infrastructure and accelerate the diagnosis of drug resistance to support early, aggressive treatment of MDR-TB. Implementation of rapid DST in resource-poor settings requires a comprehensive strategy, including adequate biosafe laboratory infrastructure, procedures for sustained quality of testing methods, efficient communication of DST results, and protocols for subsequent treatment optimization.14,15 Although several reports have described the implementation of rapid DST in resource-poor settings, to our knowledge there has been only one published report describing the impact of programmatic rapid DST on time to appropriate treatment, and none describing its impact on treatment outcome.16 We describe the impact of decentralized NRA in two district laboratories on time to DST result and clinical response.
Methods
TB treatment strategy
In 1996, the Peruvian Strategy for Tuberculosis Control incorporated treatment of MDR-TB into its national program, in collaboration with Partners In Health, Harvard University, the Massachusetts State Laboratory Institute, Socios En Salud and the Peruvian National Institute of Health.17 As part of this effort, the Laboratory Improvement Project was established in 2000, with specialists from the above organizations plus the US Centers for Diseases Control and Prevention (CDC), to strengthen and expand Peru's TB laboratory network.18 Decentralized NRA was an integral component of this strategic plan to reduce the bottleneck of DST at the National Reference Laboratory (NRL) and reduce delays in obtaining DST results.14,19 Lima Ciudad and Lima Este, adjacent health districts in Lima, were selected for decentralized DST and comprise the setting for this study. The district laboratories of Lima Ciudad and Lima Este collectively cover a catchment area of 5.7 million inhabitants and perform approximately 14 000 mycobacterial cultures annually.
Study population
We performed an observational prospective cohort study to evaluate the impact of NRA implementation in Lima Ciudad and Lima Este. Individuals meeting the criteria for DST testing (Table 1) were enrolled in the study. Criteria for NRA testing included no prior MDR-TB diagnosis or MDR-TB treatment and a smear-positive sample. Our cohort therefore comprised patients referred for NRA (intervention group) compared with patients referred for conventional DST who met the criteria for NRA testing but were referred prior to NRA implementation (historical control group).
Table 1. National TB Program criteria for DST referral.
| A Smear- or culture-positive patients at risk for MDR-TB without prior treatment history |
Subjects may be referred for DST if they 1) are diagnosed with smear-positive pulmonary TB, 2) have no prior history of anti-tuberculosis treatment, and 3) have at least one of the following risk factors:
|
| B Patients who have received at least one previous course of treatment |
Subjects may be referred for DST if they have any of the prior TB treatment histories:
|
| C Confirmed or suspected smear-negative TB among high-risk groups (tested by BACTEC™) |
Subjects may be referred for DST if they 1) are suspected or confirmed to have active pulmonary TB, 2) are smear-negative, and 3) have at least one of the following risk factors:
|
TB = tuberculosis; DST = drug susceptibility testing; MDR-TB = multidrug-resistant TB; HIV = human immunodeficiency virus; ELISA = enzyme-linked immunosorbent assay.
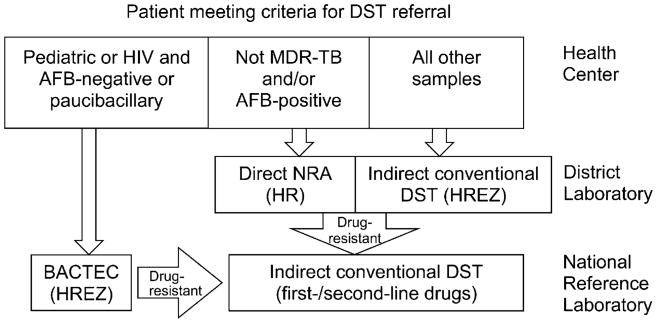
Peruvian norms for MDR-TB treatment, as per WHO guidelines, have been described elsewhere.20,21 Patients who met the criteria for DST were identified by providers at local health centers. Prior to the implementation of NRA, samples were processed at the district laboratory for DST against first-line drugs using the conventional indirect proportion method. Since the implementation of NRA at district laboratories (Figure 1), smear-positive sputum specimens from individuals with no prior diagnosis of MDR-TB or MDR-TB treatment have been processed using NRA, while samples that are smear-negative and/or from individuals with MDR-TB are processed using indirect conventional DST or BACTEC 460; the latter method is reserved for smear-negative and paucibacillary sputum samples from human immunodeficiency virus (HIV) positive patients and children.22 Isolates identified as resistant to first-line drugs (FLDs) are sent to the NRL for conventional DST against first- and second-line drugs (SLDs). Results from district and national laboratories are entered into the information system and transmitted electronically to the referring health establishment.
Figure 1.
NRA implementation at district laboratories. DST = drug susceptibility testing; HIV = human immunodeficiency virus; AFB = acid-fast bacilli; MDR-TB = multidrug-resistant tuberculosis; NRA = nitrate reductase assay; H = isoniazid; R = rifampicin; E = ethambutol; Z = pyrazinamide.
Both individualized and empiric regimens for drug-resistant TB are used. For patients previously treated for TB, empiric MDR-TB regimens are initiated while awaiting DST results. All drug resistance data, including those emitted from district laboratories, are used to make regimen changes pending final DST data from the NRL, which are considered definitive and result in a finalized individualized regimen.
Laboratory methods
Prior to NRA implementation, biosafe laboratory facilities were constructed at the NRL and in both districts; first- and second-line DST and BACTEC 460 (BD) were validated and implemented at the NRL, and conventional DST was decentralized to the district laboratories. We also implemented methods to ensure rapid transport of sputum specimens to the laboratories, trained providers on criteria for DST testing, created and deployed an electronic information system for prompt communication of DST results,15,23,24 and validated direct NRA at district laboratories.18 Lima Ciudad implemented NRA in January 2006; Lima Este followed in March 2007.
NRA has been described in detail elsewhere.6 Tubes are incubated at 37°C and read at 14, 21 and 28 days by introducing 0.5 ml of freshly-made NRA reagent containing one part 50% concentrated hydrochloric acid mixed with two parts 0.2% sulfanilamide and two parts 0.1% n-1-naphthylethylenediamine dihydrochloride. If the control tube turns purple, the same amount of reagent is introduced into the drug-containing tubes, and the color intensity is compared to the control tube. For drug-resistant isolates, the remaining drug-free control tubes are sent to the NRL for first- and second-line DST.
Conventional DST against FLDs is performed in district laboratories for non-NRA samples. Samples are decontaminated with 4% sodium hydroxide for 15 min and inoculated without centrifugation onto Ogawa medium. For positive cultures, the district laboratory performs DST for the FLDs INH, RMP, streptomycin and ethambutol on LJ medium using the indirect proportion method.
The NRL performs confirmation of M. tuberculosis using Capilia TB (BD), as well as first- and second-line DST using the agar plate proportion method. District and national laboratories have standard operating procedures and internal quality control protocols for all methods. The NRL performs external quality assurance for district laboratories. External quality assurance of the NRL is performed by the Massachusetts State Laboratory Institute, Jamaica Plain, MA, USA.
Enrollment and data collection
Individuals were consecutively identified at the time of referral for DST and followed prospectively. We collected socio-demographic, clinical and laboratory data, as well as all changes in TB treatment. A study team abstracted data from patient charts, laboratory registries and information systems. Individuals were followed until they completed treatment. If they were still in treatment at the time of study completion, subjects were censored after a minimum of 6 months' follow-up.
Ethical considerations
The study was approved by the institutional review boards at Brigham and Women's Hospital and the Peruvian National Institute of Health. This activity was approved by the CDC as program evaluation and not human subjects research.
Analysis
We compared the proportion of DSTs yielding results for both INH and RMP on NRA vs. conventional DST. We also compared the time to DST result, i.e., the number of days from DST request to DST result. We assessed the clinical impact of NRA on TB treatment outcomes.25 Among individuals with a final outcome, cure and treatment completion were considered favorable TB treatment responses; failure, death from any cause, and default were unfavorable. Among those who transferred or were censored, those who achieved culture conversion (two consecutive negative cultures at least 30 days apart, with no subsequent positive cultures) were considered to have a favorable response. To identify those subgroups that benefited most from NRA, we stratified the analysis by drug resistance patterns and by previous treatment history. We hypothesized that individuals with drug resistance (i.e., those requiring a regimen change to receive appropriate treatment) and those without prior treatment history (i.e., those less likely to receive empiric treatment while awaiting a DST result) would benefit most from NRA.
Binary outcomes were compared using χ2 analysis or Fisher's exact test, when appropriate. We compared time to event endpoints using Cox proportional hazards models. Multivariable analysis used logistic regression analyses and Cox proportional hazards models for binary outcomes and time to event, respectively. In these models, we controlled for significant baseline differences between groups.
Results
Of 1846 individuals referred for DST in the study period, 468 (25.4%) were excluded from analysis as they were referred for BACTEC (n = 307) or did not meet the criteria for NRA testing (n = 161). Of the remaining 1378 cases, 752 underwent NRA and 626 underwent conventional DST. Individuals in the intervention and control groups were similar (Table 2), except for age (respective mean ages 35.6 years vs. 33.3 years, P = 0.004) and fewer referrals for suspected failure of first-line therapy (16.1% vs. 20.5%, P = 0.04).
Table 2. Baseline characteristics (n = 1378).
| NRA DST (n = 752) n (%) or mean ± SD | Conventional DST (n = 626) n (%) or mean ± SD | P value | |
|---|---|---|---|
| Socio-demographic characteristics | |||
| Female sex | 256 (34.0) | 223 (35.6) | 0.54 |
| Age, years | 35.6 ± 15.8 | 33.3 ± 14.2 | 0.004 |
| Married/living together (n = 1376) | 289 (38.4) | 246 (39.3) | 0.74 |
| Employed | 246 (32.8) | 237 (37.9) | 0.05 |
| Did not start secondary education (n = 1374) | 147 (19.7) | 113 (18.1) | 0.45 |
| Clinical characteristics | |||
| Tobacco use (n = 1377) | 293 (25.5) | 163 (26.1) | 0.82 |
| Alcohol use (n = 1377) | 289 (38.4) | 231 (37.0) | 0.58 |
| Drug use (n = 1376) | 146 (19.4) | 106 (17.0) | 0.24 |
| Heart rate (n = 1360) | 78.5 ± 11.7 | 78.1 ± 12.2 | 0.48 |
| Respiratory rate (n = 1361) | 21.9 ± 10.1 | 22.4 ± 6.6 | 0.28 |
| Weight, kg (n = 1377) | 55.6 ± 10.6 | 55.1 ± 11.1 | 0.46 |
| Baseline culture-positive | 594 (79.0) | 507 (81.0) | 0.36 |
| Cavitary disease | 144 (19.2) | 96 (15.4) | 0.07 |
| Risk factors for DST referral (see Table 1) | |||
| Group A: patients at risk for MDR-TB without prior treatment history | 340 (45.2) | 305 (48.7) | 0.19 |
| Household contact of documented or suspected MDR-TB case | 184 (24.5) | 148 (23.6) | 0.72 |
| HIV-positive | 20 (2.7) | 24 (3.8) | 0.22 |
| Diabetes mellitus | 99 (13.2) | 65 (10.4) | 0.13 |
| Health care worker or student* | 44 (5.9) | 31 (2.3) | 0.48 |
| Incarcerated or worked in penitentiary system* | 37 (4.9) | 23 (3.7) | 0.29 |
| Suspected treatment failure with Category I or II regimen | 121 (16.1) | 128 (20.5) | 0.04 |
| Other risk factor | 21 (2.8) | 20 (3.2) | 0.75 |
| Group B: patients with prior treatment history | 412 (54.8) | 321 (51.3) | 0.19 |
| Prior default | 102 (13.6) | 89 (14.2) | 0.73 |
| Prior relapse | 50 (6.7) | 37 (5.9) | 0.66 |
| Prior treatment failure | 69 (9.2) | 60 (9.6) | 0.85 |
| Multiple courses of anti-tuberculosis treatment | 138 (18.4) | 142 (22.7) | 0.05 |
| Prior private or self-administered treatment | 63 (8.4) | 52 (8.3) | 1.00 |
In the past 2 years.
NRA = nitrate reductase assay; DST = drug susceptibility testing; SD = standard deviation; MDR-TB = multidrug-resistant TB; HIV = human immunodeficiency virus.
DST results were obtained more frequently among those tested using NRA vs. conventional DST (78.4% vs. 68.8%, P < 0.0001). Of the 1020 individuals for whom DST results were obtained, 349 (34.2%) had MDR-TB. MDR-TB rates did not differ significantly among NRA vs. conventional DST groups (32.2% vs. 37.0%, P = 0.14, Table 3).
Table 3. Baseline resistance patterns among individuals with DST results (n = 1020).
| Characteristic | NRA DST (n = 590) n (%) | Conventional DST (n = 430) n (%) | P value |
|---|---|---|---|
| INH- and RMP-susceptible | 316 (53.6) | 224 (52.1) | 0.14 |
| INH- or RMP-resistant | 84 (14.2) | 47 (10.9) | |
| MDR-TB | 190 (32.2) | 159 (37.0) |
DST = drug susceptibility testing; NRA = nitrate reductase assay; INH = isoniazid; RMP = rifampin; MDR-TB = multidrug-resistant tuberculosis.
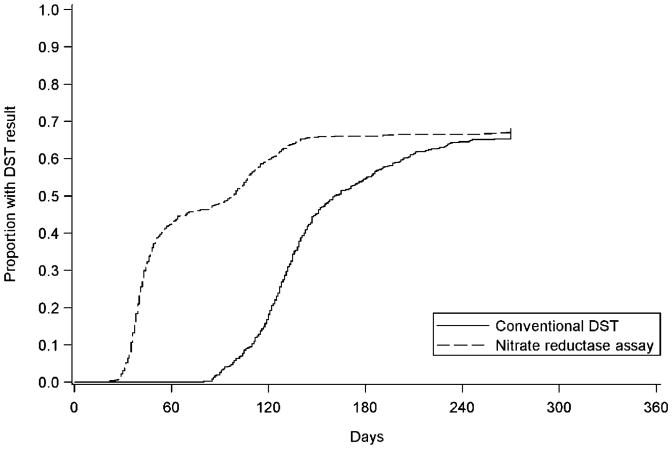
The median time to DST result (first-third quartiles) was 44 (37–83) days and 133 (118–160) days among those tested by NRA vs. conventional DST (unadjusted hazard ratio [HR] 0.65, 95%CI 0.57–0.74; Figure 2). The median time from NRA processing to result was 28 (26–30) days. The adjusted HR (aHR) for the effect of NRA on time to DST result was 0.64 (95%CI 0.56–0.73). Fifty-four individuals died while awaiting DST results: 24 (3.2%) awaiting NRA vs. 30 (4.8%) awaiting conventional DST (P = 0.16).
Figure 2.
Median time to DST (n = 1378). DST = drug susceptibility testing.
As shown in Table 4, 461 (62.6%) NRA referrals experienced positive treatment outcomes, compared with 363 (58.8%) of those referred for conventional DST (adjusted odds ratio [aOR] 1.13, 95%CI 0.91–1.41). Among individuals with drug-resistant TB, there was a similar non-significant trend of improved treatment outcomes with NRA (aOR 1.19, 95%CI 0.83–1.72). Among those referred with no prior treatment history (Group A in Table 1), favorable outcomes were observed in 56.5% of NRA referrals vs. 47.7% of conventional DST referrals (aOR 1.39, 95%CI 1.01–1.90).
Table 4. Effect of NRA on favorable treatment response.
| Cohort, n included in model | NRA n (%) | Conventional DST n (%) | Unadjusted OR (95%CI) | Adjusted OR (95%CI)* |
|---|---|---|---|---|
| Entire cohort (n = 1354) | 461 (62.6) | 363 (58.8) | 1.17 (0.94–1.46) | 1.13 (0.91–1.41) |
| Drug-resistant cohort (n = 474) | 136 (50.6) | 94 (45.9) | 1.27 (0.84–1.74) | 1.19 (0.83–1.72) |
| No prior treatment (n = 627) | 186 (56.5) | 142 (47.7) | 1.43 (1.04–1.96) | 1.39 (1.01–1.90) |
Controlling for differences in age and suspected Category I or II failures.
NRA = nitrate reductase assay; DST = drug susceptibility testing; OR = odds ratio; CI = confidence interval.
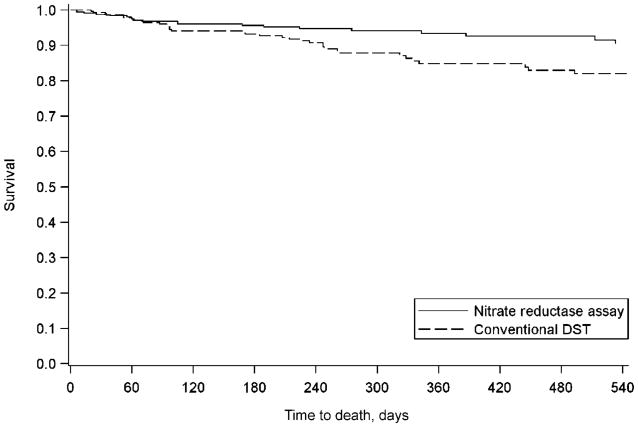
There was a trend toward reduced mortality among individuals tested by NRA vs. conventional DST (6.1% vs. 8.8%, P = 0.06), with a trend toward a protective effect on survival (aHR 0.77, 95%CI 0.52–1.15). This association was similar when limited to individuals with drug resistance (aHR 0.72, 95%CI 0.43–1.23). Among those referred with no prior treatment history, death occurred in 6.3% of NRA referrals compared with 13.0% of conventional DST referrals (P = 0.007). NRA was significantly associated with greater survival (aHR 0.53, 95%CI 0.31–0.90; Figure 3).
Figure 3.
Time to death, no prior treatment risk factor (n = 563). DST = drug susceptibility testing.
Discussion
Direct NRA, a phenotypic method with low cost and low technological demand, performed robustly in a programmatic setting in district laboratories. Smear-positive patients referred for direct NRA benefited from a shorter time to DST result than conventional methods. We have previously reported the cost of this method to be approximately US$4.80 per sample (US$5.30 per sample including labor costs).19 The superior yield of direct NRA may be due to the lack of centrifugation of samples in the indirect conventional assay compared with the direct method, which involves centrifugation of all samples. Even in the context of a growing number of commercially available genotypic rapid methods, the NRA may still have utility in many resource-poor settings as a simple, inexpensive phenotypic method that can be performed in any laboratory with capacity for mycobacterial culture using solid media.
Under program conditions, the use of NRA had a significant clinical impact on TB treatment outcome and time to death, but not for the entire cohort. Individuals who have received prior treatment regimens have a high pre-test probability of MDR-TB and are likely to be started on empiric MDR-TB treatment. For these individuals, rapid DST may not provide significant benefit, except to pare back SLDs to spare toxicity and cost. On the other hand, for individuals with an ‘intermediate pre-test probability’ of MDR-TB, regimen changes are more likely to be deferred until DST results are obtained. Such risk groups would include individuals with epidemiologic risk factors (such as a previous episode of TB, household contact, diabetes mellitus, HIV, etc.) or poor early treatment response (i.e., smear or culture-positive after 2–4 months of FLD treatment). For these individuals, referral for NRA resulted in improved anti-tuberculosis treatment outcomes and increased survival.
Our study had several limitations. This study was designed to evaluate the impact of a programmatic intervention, but not through a randomized study design. Although patient characteristics were largely comparable among NRA vs. conventional groups and we adjusted for the few baseline differences in groups, we cannot rule out the potential confounding effect of unmeasured differences, including potential calendar bias. Furthermore, we did not obtain final anti-tuberculosis treatment outcomes for 8.6% of individuals; a proportion of these missing outcomes (22.0%) was due to censoring at the end of the observation period.
Nonetheless, to our knowledge, this is the first published report of a clinical benefit of rapid DST in terms of both treatment outcome and time to death. Programmatic strategies to treat MDR-TB often include both empiric treatment for suspected MDR-TB cases as well as rapid DST methods.26 Our findings provide insight into considerations for the use of empiric regimens and rapid DST, particularly in determining which populations might benefit from these strategies, either alone or combined.
Conclusions
The implementation of direct NRA within a comprehensive laboratory strengthening program in Lima, Peru, resulted in more and faster DST results. For those referred for DST without prior treatment history, NRA was associated with improved treatment outcome and survival. Our experience highlights the importance of implementing rapid DST methods within a larger infrastructure that can maximize the benefit of any diagnostic strategy with strong links to clinical services.
Acknowledgments
This study was supported by the Bill & Melinda Gates Foundation, the US Centers for Disease Control and Prevention, the US National Institute of Allergy and Infectious Diseases (K23 AI054591-01A2 to SSS), the Infectious Disease Society of America and the Heiser Foundation.
The opinions expressed by the authors contributing to this article do not necessarily reflect the opinions of the CDC or the institutions with which the authors are affiliated.
References
- 1.World Health Organization. WHO/HTM/TB/2010.3. Geneva, Switzerland: WHO; 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. [Google Scholar]
- 2.World Health Organization. Stop TB Partnership The Global Plan to Stop TB, 2011–2015. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 3.Golyshevskaia VI, Korneev AA, Chernousova LN, et al. New microbiological techniques in diagnosis of tuberculosis. Probl Tuberk. 1996;(6):22–25. Russian. [PubMed] [Google Scholar]
- 4.Angeby KA, Klintz L, Hoffner SE. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J Clin Microbiol. 2002;40:553–555. doi: 10.1128/JCM.40.2.553-555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A, Montoro E, Lemus D, et al. Multicenter evaluation of the nitrate reductase assay for drug resistance detection of Mycobacterium tuberculosis. J Microbiol Methods. 2005;63:145–150. doi: 10.1016/j.mimet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Solis LA, Shin SS, Han LL, Llanos F, Stowell M, Sloutsky A. Validation of a rapid method for detection of M. tuberculosis resistance to isoniazid and rifampin in Lima, Peru. Int J Tuberc Lung Dis. 2005;9:760–764. [PMC free article] [PubMed] [Google Scholar]
- 7.Musa HR, Ambroggi M, Souto A, Angeby KA. Drug susceptibility testing of Mycobacterium tuberculosis by a nitrate reductase assay applied directly on microscopy-positive sputum samples. J Clin Microbiol. 2005;43:3159–3161. doi: 10.1128/JCM.43.7.3159-3161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemus D, Montoro E, Echemendia M, Martin A, Portaels F, Palomino JC. Nitrate reductase assay for detection of drug resistance in Mycobacterium tuberculosis: simple and inexpensive method for low-resource laboratories. J Med Microbiol. 2006;55(Pt 7):861–863. doi: 10.1099/jmm.0.46540-0. [DOI] [PubMed] [Google Scholar]
- 9.Martin A, Panaiotov S, Portaels F, Hoffner S, Palomino JC, Angeby K. The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2008;62:56–64. doi: 10.1093/jac/dkn139. [DOI] [PubMed] [Google Scholar]
- 10.Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Palomino JC. Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2005;55:500–505. doi: 10.1093/jac/dki023. [DOI] [PubMed] [Google Scholar]
- 11.Bwanga F, Joloba ML, Haile M, Hoffner S. Evaluation of seven tests for the rapid detection of multidrug-resistant tuberculosis in Uganda. Int J Tuberc Lung Dis. 2010;14:890–895. [PubMed] [Google Scholar]
- 12.Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multidrug-resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67. doi: 10.1186/1471-2334-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bwanga F, Haile M, Joloba ML, Ochom E, Hoffner S. Direct nitrate reductase assay versus microscopic observation drug susceptibility test for rapid detection of MDR-TB in Uganda. PLoS ONE. 2011;6:e19565. doi: 10.1371/journal.pone.0019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin SS, Yagui M, Ascencios L, et al. Scale-up of multidrug-resistant tuberculosis laboratory services, Peru. Emerg Infect Dis. 2008;14:701–708. doi: 10.3201/eid1405.070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaya JA, Shin SS, Yagui M, et al. Implementing and evaluating a laboratory information system to optimize the treatment of tuberculosis patients. Int J Tuberc Lung Dis. 2006;10(Suppl 1):S58–S59. Abstract. [Google Scholar]
- 16.Banerjee R, Allen J, Lin SY, et al. Rapid drug susceptibility testing with a molecular beacon assay is associated with earlier diagnosis and treatment of multidrug-resistant tuberculosis in California. J Clin Microbiol. 2010;48:3779–3781. doi: 10.1128/JCM.01236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 18.Velasquez GE, Yagui M, Cegielski JP, et al. Targeted drug-resistance testing strategy for multidrug-resistant tuberculosis detection, Lima, Peru, 2005–2008. Emerg Infect Dis. 2011;17:432–440. doi: 10.3201/eid1703.101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asencios L, Yale G, Yagui M, et al. Programmatic implementation of rapid DST for Mycobacterium tuberculosis in Peru. Int J Tuberc Lung Dis. 2008;12:743–749. [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO/HTM/TB/2011.6. Geneva, Switzerland: WHO; 2008. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update. [PubMed] [Google Scholar]
- 21.World Health Organization. Management of MDR-TB: a field guide A companion document to Guidelines for Programmatic Management of Drug-resistant Tuberculosis. Geneva, Switzerland: WHO; 2009. [PubMed] [Google Scholar]
- 22.Heifets L. Drug susceptibility testing. Clin Lab Med. 1996;16:641–656. [PubMed] [Google Scholar]
- 23.Blaya J, Fraser HS. Development, implementation and preliminary study of a PDA-based tuberculosis result collection system. AMIA Annu Symp Proc. 2006:41–45. [PMC free article] [PubMed] [Google Scholar]
- 24.Blaya JA, Shin SS, Yagui MJ, et al. A web-based laboratory information system to improve quality of care of tuberculosis patients in Peru: functional requirements, implementation and usage statistics. BMC Med Inform Decis Mak. 2007;7:33. doi: 10.1186/1472-6947-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–645. [PubMed] [Google Scholar]
- 26.Mitnick CD, Appleton SC, Shin SS. Epidemiology and treatment of multidrug resistant tuberculosis. Semin Respir Crit Care Med. 2008;29:499–524. doi: 10.1055/s-0028-1085702. [DOI] [PMC free article] [PubMed] [Google Scholar]