Abstract
The risk of developing tuberculosis (TB) may be related to nutritional status. To determine the impact of nutritional status on TB incidence, the authors analyzed data from the First National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Follow-up Study (NHEFS). NHANES I collected information on a probability sample of the US population in 1971–1975. Adults were followed up in 1982–1992. Incident TB cases were ascertained through interviews, medical records, and death certificates. TB incidences were compared across different levels of nutritional status after controlling for potential confounding using proportional hazards regression appropriate to the complex sample design. TB incidence among adults with normal body mass index was 24.7 per 100,000 person-years (95% confidence interval (CI): 13.0, 36.3). In contrast, among persons who were underweight, overweight, and obese, estimated TB incidence rates were 260.2 (95% CI: 98.6, 421.8), 8.9 (95% CI: 2.2, 15.6), and 5.1 (95% CI: 0.0, 10.5) per 100,000 person-years, respectively. Adjusted hazard ratios were 12.43 (95% CI: 5.75, 26.95), 0.28 (95% CI: 0.13, 0.63), and 0.20 (95% CI: 0.07, 0.62), respectively, after controlling for demographic, socioeconomic, and medical characteristics. A low serum albumin level also increased the risk of TB, but low vitamin A, thiamine, riboflavin, and iron status did not. A population’s nutritional profile is an important determinant of its TB incidence.
Keywords: body mass index, malnutrition, nutrition surveys, obesity, protein-energy malnutrition, subcutaneous fat, tuberculosis
Protein-energy undernutrition impairs T-lymphocyte-mediated immunologic defenses, increasing the risk of specific infectious diseases (1–6). Among these, tuberculosis (TB) is a leading cause of morbidity and mortality, especially in middle- and low-income countries. An estimated 2 billion people worldwide are infected with Mycobacterium tuberculosis, and 1 billion people are undernourished (7, 8). According to the World Health Organization, the population attributable incidence of TB due to protein-energy undernutrition may exceed that due to human immunodeficiency virus (HIV) infection, smoking, or diabetes mellitus (9).
The bulk of evidence relating TB to undernutrition in humans comes from long-term historical trends inversely correlating TB incidence with economic development, ecologic comparisons between high- and low-income countries, and acute changes in TB incidence during famines, wars, economic crises, and natural disasters. In these situations, the effects of undernutrition cannot be disentangled from broader circumstances (10–16). Because TB causes anorexia and weight loss, cross-sectional and case-control studies cannot separate cause and effect. Cohort studies carried out among US Navy recruits and in Norway have demonstrated increased TB risk in thin persons (17–19). In a study in Philadelphia, Pennsylvania, men with low vitamin A and C levels had a higher TB incidence than men with adequate levels (20). In New York City, a clinical trial demonstrated that use of multivitamin-mineral supplements decreased TB incidence among family members of active TB cases (21). These studies date from the 1940s and 1950s. Since that time, the impact of specific nutrients on TB risk has not been adequately studied.
Mounting evidence suggests that obesity may decrease the risk of TB (22, 23). In China, a study of more than 42,000 elderly persons found that TB incidence was significantly lower in overweight persons than in normal-weight controls (23).
To determine the TB risk associated with nutritional status and to identify specific nutrients involved, we analyzed data from a population-based, 20-year follow-up study of adults in the United States.
MATERIALS AND METHODS
Study population and data sources
The First National Health and Nutrition Examination Survey (NHANES I), carried out during 1971–1975, collected extensive data on a probability sample of the civilian, noninstitutionalized US population aged 1–74 years in the 48 contiguous states, excepting reservation lands of American Indians (n = 23,808). Details of the complex survey design, plan, operation, and results have been published previously (24–27). Subjects were asked specifically about their TB history, and a representative subset had tuberculin skin tests. Subjects who had TB before NHANES I were excluded from analysis.
NHANES I provided the baseline sample for the NHANES I Epidemiologic Follow-up Study (NHEFS). Details on the plan, operation, and results of the NHEFS have been published previously (28–32). The NHEFS included all 14,407 adults aged 25–74 years at their NHANES I examination. They were followed up 4 times from 1982 to 1992 to identify morbidity and mortality that could be related to their baseline characteristics. Follow-up data were obtained for 13,419 (93.1%) subjects from interviews, medical records, and death certificates: 13,155 (91.3%) subjects were interviewed at least once, 10,779 (74.8%) at least twice, and 8,949 (62.1%) at least thrice. Proxy informants were interviewed for deceased and incapacitated subjects. Respondents were asked about any overnight stays in hospitals and skilled nursing facilities; 11,025 (83.8% of 13,155) reported at least 1 stay in a health-care facility. Medical records were obtained for 10,765 (97.6% of 11,025) individuals and 48,737 facility stays; 2,487 (23.1%) persons had a single stay, 8,270 (76.8%) had ≥2 stays, and 6,825 (63.4%) had ≥3 stays. Death certificates were obtained for 4,482 (97.4%) of 4,604 deceased subjects (32.0% of the entire cohort of 14,407 subjects).
Outcome variables
Incident cases of TB were ascertained from interviews, medical records, and death certificates. The scripted interview did not ask about TB by name but included many questions probing for information about serious illnesses, health-care facility stays, and health-related conditions beyond those specified by name (28–31, 33). Key words and phrases were recorded verbatim, and the character strings were searched for “TB” or “tuberculosis.” For each mention, the full context was read so as to include only active TB. TB exposure, TB screening, and TB skin testing without active disease were not counted.
Medical records were abstracted by National Center for Health Statistics staff who had no knowledge of the present research. Trained personnel assigned International Classification of Diseases, Ninth Revision (ICD-9), codes on the basis of admission and discharge records. Death certificate data were obtained at both the individual level and the condition level, using multiple-cause methods to capture all information on each death certificate. Data from medical records and death certificates were searched for ICD-9 codes for TB (ICD-9 codes 010-018 and 137). TB exposure without disease (ICD-9 code V01.1), primary infection without disease (ICD-9 code 010.0), and tuberculin skin test positivity without disease (ICD-9 code 795.5) were excluded. To check the validity of the NHEFS-based population-estimated TB incidence, we compared it with actual reported incidence based on the Centers for Disease Control and Prevention’s National TB Surveillance System for this time period, taking into account the age structure of the cohort.
Nutritional status variables
Anthropometric indicators of nutritional status included body mass index (BMI), subcutaneous fat, and lean skeletal muscle. BMI (weight (kg)/height (m)2) was categorized as low (<18.5), normal (18.5– <25), overweight (25– <30), or obese (≥30) (34, 35). Subcutaneous fat was based on the sum of the right triceps and subscapular skinfold thicknesses (36). Skeletal muscle was based on the cross-sectional area of the right mid-upper arm muscle (cm2) using Frisancho’s method (37). Unlike the case for BMI, a fixed scale for classifying skinfold thickness and arm muscle area as low/normal/high has not been established. Therefore, we compared the mean skinfold thicknesses and arm muscle areas for persons who subsequently developed TB and those who did not. We also classified skinfold thickness and arm muscle area as low/normal/high on the basis of their sex-specific population distributions, defining low as <5th percentile, high as >60th percentile, and normal as values between those cutpoints.
Hemoglobin was measured for all examinees, while a representative subsample of 11,348 people was tested for serum albumin, iron, iron-binding capacity, transferrin saturation, vitamin A, thiamine, riboflavin, and creatinine, plus urinary thiamine, riboflavin, and creatinine (38, 39). Vitamin D levels were not measured. Laboratory values were dichotomized as normal/abnormal on the basis of standard reference values for the population at that time (40).
Covariates
Of the characteristics measured at the NHANES I examination, we analyzed sex, age, race, Hispanic ethnicity, foreign birth, income, urban/rural residence, residence in a federally designated poverty area, and medical history of diabetes mellitus, anemia, and cancer.
Analytic methods
Data were analyzed with SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and SUDAAN 10.0 (Research Triangle Institute, Research Triangle Park, North Carolina) software for population estimates, taking into account the complex sample design and sampling probabilities (27, 41). Statistical inferences are based on the population-estimated results. A P value less than 0.05 was considered significant, and all statistical tests were 2-sided.
TB incidences were compared among groups with different levels of baseline nutritional status. For continuous variables, the population-estimated distributions were compared using the t test or the Wilcoxon rank-sum test. For categorical measures of nutritional status, we compared cumulative TB incidence and incidence density per 100,000 person-years across levels of the nutritional indicator. For TB cases, follow-up time ended on the earliest date of diagnosis. Otherwise, follow-up time was censored on the date of the last observation. For each predictor variable, persons with normal values constituted the reference group. Groups were compared graphically by means of Kaplan-Meier plots and statistically by means of the log-rank test. Because BMI was correlated with both skinfold thickness and arm muscle area (BMI-skinfold thickness: r = 0.72 (P < 0.0001); BMI-arm muscle area: r = 0.51 (P < 0.0001)), these 3 anthropometric factors together were nearly collinear; skeletal muscle and subcutaneous adipose tissue are major components of body mass. Therefore, the adjusted hazard ratios for each were calculated in 3 separate main-effects models, each controlling for the same covariates.
We controlled for potential confounding with multivariable Cox proportional hazards regression incorporating the complex survey design and sampling weights. Predictor variables based on fewer than 5 actual TB cases were not included in the multivariable model. Control variables were examined for effect modification and confounding by means of stratified analysis using Mantel-Haenszel methods and by means of proportional hazards regression incorporating appropriate interaction terms, ensuring that each variable satisfied the proportional hazards assumption.
RESULTS
Of the 14,407 members of the NHEFS cohort, 218 were excluded because they had TB before NHANES I, leaving 14,189 in the analysis cohort. Their characteristics reflect the design-based oversampling of persons living in poverty areas, the elderly, and women of childbearing age (Table 1).
Table 1.
Characteristics (Population-estimated Percentage) of Participants Who Later Developed Tuberculosis (n = 61) and Those Who Did Not (n = 14,128), NHANES I Epidemiologic Follow-up Study, 1971–1992a
| Characteristic | Persons Who Later Developed Tuberculosis |
Persons Who Did Not Develop Tuberculosis |
P Value | ||
|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | ||
| Male sex | 40.1 | 20.8, 59.4 | 52.7 | 51.5, 54.0 | 0.2 |
| Age group, years | 0.1 | ||||
| 25–34 | 12.6 | 0, 28.6 | 26.3 | 25.2, 27.4 | |
| 35–44 | 13.9 | 1.5, 28.6 | 21.4 | 20.4, 22.4 | |
| 45–54 | 37.5 | 17.5, 57.5 | 21.9 | 21.0, 22.9 | |
| 55–64 | 14.3 | 0, 29.8 | 18.1 | 17.0, 19.2 | |
| ≥65 | 21.8 | 11.6, 32.0 | 12.2 | 11.1, 13.4 | |
| Race | 0.5 | ||||
| White | 85.2 | 75.2, 95.2 | 89.2 | 87.9, 90.6 | |
| Black | 12.5 | 3.2, 21.9 | 9.7 | 8.4, 11.0 | |
| Other | 2.2 | 0, 5.9 | 1.1 | 0.8, 1.4 | |
| Hispanic ethnicity | 4.7 | 0, 9.5 | 4.2 | 3.1, 5.4 | 0.9 |
| Residence in an urban area | 0.8 | ||||
| Urban | 59.9 | 42.7, 77.1 | 55.0 | 51.6, 58.3 | |
| Suburban | 8.8 | 0, 17.7 | 12.6 | 10.5, 14.7 | |
| Rural | 31.3 | 13.4, 49.2 | 32.4 | 29.6, 35.2 | |
| Residence in a designated poverty areab |
32.3 | 16.0, 48.7 | 18.9 | 14.0, 23.9 | 0.07 |
| Immigrant to the United States |
6.3 | 0, 13.2 | 8.1 | 7.2, 9.0 | 0.6 |
| Annual incomec,d | 0.05 | ||||
| <$10,000 | 61.6 | 45.0, 78.1 | 49.3 | 46.8, 51.8 | |
| $10,000-$20,000 | 18.8 | 6.3, 31.4 | 38.4 | 36.7, 40.2 | |
| >$20,000 | 19.6 | 5.6, 33.6 | 12.3 | 10.8, 13.8 | |
| Alcohol | |||||
| Alcohol consumption of >7 drinks/weekd |
23.5 | 6.1, 40.9 | 19.6 | 18.0, 21.1 | 0.6 |
| Current smokingd | 79.0 | 65.5, 92.5 | 61.0 | 59.7, 62.4 | 0.03 |
| Medical history | |||||
| Anemia | 31.3 | 13.2, 49.4 | 21.2 | 20.3, 22.1 | 0.2 |
| Diabetes | 12.9 | 1.8, 24.0 | 3.8 | 3.4, 4.3 | 0.005 |
| Body mass indexe | <0.0001 | ||||
| Low (<18.5) | 31.7 | 15.3, 48.1 | 3.1 | 2.8, 3.5 | |
| Normal (18.5–<25) | 51.6 | 34.1, 69.1 | 47.0 | 45.7, 48.3 | |
| Overweight (25–<30) | 13.3 | 5.1, 21.6 | 34.0 | 33.0, 35.1 | |
| Obese (≥30) | 3.4 | 0, 7.1 | 15.8 | 14.9, 16.7 | |
| Skinfold thicknessf | <0.0001 | ||||
| Low | 32.6 | 13.7, 51.6 | 4.8 | 4.3, 5.3 | |
| Normal | 54.3 | 33.6, 75.0 | 55.3 | 53.9, 56.6 | |
| High | 13.1 | 4.1, 22.0 | 39.9 | 38.6, 41.3 | |
| Mid-upper arm muscle areaf | <0.0001 | ||||
| Low | 24.0 | 8.6, 39.5 | 4.9 | 4.5, 5.4 | |
| Normal | 60.0 | 42.0, 78.1 | 55.1 | 53.7, 56.4 | |
| High | 15.9 | 6.4, 25.5 | 40.0 | 38.6, 41.4 | |
| Anemiag | 11.4 | 0.1, 22.7 | 12.8 | 11.6, 14.1 | 0.8 |
| Hypoalbuminemiah | 11.2 | 0, 29.2 | 0.5 | 0.4, 0.7 | <0.0001 |
| Iron deficiencyi | Indeterminate | ||||
| Unlikely | 90.5 | 82.7, 98.4 | 89.0 | 88.1, 89.9 | |
| Possible | 9.5 | 1.6, 17.3 | 9.1 | 8.4, 9.8 | |
| Probable | 0 | 0 | 1.9 | 1.5, 2.2 | |
| Low serum vitamin A level,j μg/L | Indeterminate | ||||
| <20 | 0 | 0 | 0.1 | 0.1, 0.2 | |
| <30 | 4.0 | 0, 9.4 | 1.5 | 1.2, 1.7 | 0.13 |
| Low thiamine excretion | 0.9 | 0, 2.7 | 0.2 | 0.1, 0.4 | 0.2 |
| Low riboflavin excretion | 0 | 0 | 0.8 | 0.6, 1.0 | Indeterminate |
Abbreviations: CI, confidence interval; NHANES I, First National Health and Nutrition Examination Survey; NHEFS, NHANES I Epidemiologic Follow-up Study.
Data shown are population-estimated percentages for categorical variables incorporating survey design specifications (adjusted weights, stratification, multistage cluster sampling). Of 14,407 persons in the entire NHEFS cohort, 218 with a history of tuberculosis prior to the NHANES I baseline medical examination were excluded, leaving 14,189 persons in the analysis cohort.
Data on residence in a federally designated poverty area was recorded for the main Nutrition Survey (n = 11,348), carried out in 1971–1974 and comprised of 65 primary sampling units, but not the Augmentation Survey (n = 3,059), carried out in 1974–1975 and comprised of 35 additional primary sampling units. Both components, separately and together, were based on nationally representative samples.
An income of $10,000 in 1973 dollars is equivalent to an income of $38,783 in 2000 dollars and $49,111 in 2010 dollars (http://data.bls.gov/ cgi-bin/cpicalc.pl).
Data on income were missing for 3.8% of NHEFS cohort participants, data on alcohol consumption were missing for 13.5%, and data on smoking were missing for 9.2%.
Weight (kg)/height (m)2.
Skinfold thickness and arm muscle a “Low” was defined as <5th percentile, “hi rea were classifi gh” as >60th perc ed as low, normal, or high on the basis of their sex-specific population distributions. entile, and “normal” as values between those cutpoints.
Anemia was defined as a hemoglobin level less than 1 1.5 g/dL in females and less than 13.0 g/dL in males.
Hypoalbuminemia was defined as a s erum albumin lev el less than 3.5 g/dL.
Iron status and the likelihood of iron d as follows. “Iron deficiency probable” was “iron deficiency possible” was defined as abnormalities. eficiency were ba defined as low s any 2 of these 3 sed on serum iron level, percent transferrin saturation, and total iron-binding capacity, erum iron level and low percent transferrin saturation and high iron-binding capacity; abnormalities; and “iron deficiency unlikely” was defined as only 1 or none of these abnormalities.
Different criteria for vitamin A deficiency have been proposed for clinical purposes (<20 μg/L or <70 μmol/L) and epidemiologic purposes (<30 μg/L or <105 μmol/L).
TB incidence
Of the 14,189 participants, 13,211 (93.1%) had usable follow-up data totaling 209,013 person-years and averaging 15.8 years in duration (standard deviation, 5.5; range, <1– 22). Sixty-one (0.43%) incident TB cases were detected (crude incidence = 28.3/100,000 person-years). Incorporating sampling weights and design specifications, the population-estimated cumulative incidence was 372,332 (95% confidence interval (CI): 244,787, 499,877) TB cases over a 20-year period (1973–1992). For comparison, the actual TB incidence in the national TB surveillance system for the 48 states was 380,578 cases for the same time period and age group (42, 43). The NHEFS-based population-estimated average annual TB incidence density was 22.8 per 100,000 person-years (95% CI: 15.0, 30.6) for the 20-year period 1973–1992. In national surveillance data, annual TB incidence in 1971 was 25.8 per 100,000 person-years, decreasing to 14.2 per 100,000 in 1992 (i.e., bracketing the NHEFS-based estimated annual average).
Anthropometric indicators of nutritional status
Mean BMI, skinfold thickness, and arm muscle area were significantly lower in persons who subsequently developed TB than in those who did not, except for arm muscle area among females (Table 2). For each measure, there was an inverse gradient with TB incidence (Table 3). TB incidence among participants with BMI <18.5, representing approximately 3% of the population, was 260.2 per 100,000 person-years (95% CI: 98.6, 421.8), 11.7-fold higher than that among participants with normal BMI (24.7/100,000 person-years, 95% CI: 13.0, 36.3). In contrast, TB incidence was 2.8-fold lower (8.9/100,000 person-years, 95% CI: 2.2, 15.6) among those who were overweight and 4.8-fold lower (5.1/100,000 person-years, 95% CI: 0.0, 10.5) among those who were obese.
Table 2.
Characteristics (Population-estimated Mean and Median Values) of Participants Who Later Developed Tuberculosis (n = 61) and Those Who Did Not (n = 14,128), NHANES I Epidemiologic Follow-up Study, 1971–1992a
| Characteristic | Persons Who Later Developed Tuberculosis |
Persons Who Did Not Develop Tuberculosis |
P Value | ||
|---|---|---|---|---|---|
| Mean or Median | 95% CI | Mean or Median | 95% CI | ||
| Mean body mass indexb | |||||
| Total | 21.6 | 20.5, 22.7 | 25.5 | 25.5, 25.7 | <0.0001 |
| Men | 20.9 | 19.6, 22.1 | 25.8 | 25.7, 26.0 | <0.0001 |
| Women | 22.7 | 20.5, 24.9 | 25.4 | 25.3, 25.6 | 0.01 |
| Mean skinfold thickness, mm | |||||
| Total | 22.1 | 18.0, 26.3 | 36.8 | 36.3, 37.3 | <0.0001 |
| Men | 16.0 | 12.3, 19.6 | 29.1 | 28.6, 29.5 | <0.0001 |
| Women | 31.4 | 24.5, 38.3 | 43.8 | 43.2, 44.4 | 0.0006 |
| Mean mid-upper arm muscle area, cm2 | |||||
| Total | 46.9 | 43.2, 50.7 | 51.6 | 51.1, 52.1 | 0.02 |
| Men | 51.5 | 48.5, 54.4 | 63.7 | 63.3, 64.2 | <0.0001 |
| Women | 40.2 | 35.6, 40.9 | 40.8 | 40.4, 41.2 | 0.8 |
| Mean hemoglobin level, g/dL | |||||
| Total | 14.1 | 13.7, 14.6 | 14.6 | 14.5, 14.7 | 0.04 |
| Men | 14.7 | 14.2, 15.2 | 15.5 | 15.4, 15.6 | 0.001 |
| Women | 13.3 | 12.9, 13.8 | 13.7 | 13.6, 13.8 | 0.06 |
| Mean serum albumin level, g/dL | 4.1 | 3.9, 4.3 | 4.4 | 4.4, 4.4 | 0.006 |
| Mean serum vitamin A level, μg/L | 64.3 | 55.2, 73.4 | 59.9 | 59.0, 60.8 | 0.30 |
| Median urinary thiamine level, μg/g creatinine | 346 | 254, 576 | 302 | 284, 323 | 0.12 |
| Median urinary riboflavin level, μg/g creatinine | 286 | 175, 460 | 255 | 246, 266 | 0.29 |
Abbreviations: CI, confidence interval; NHANES I, First National Health and Nutrition Examination Survey; NHEFS, NHANES I Epidemiologic Follow-up Study.
Data shown are population-estimated mean and median values (as indicated) incorporating survey design specifications (adjusted weights, stratification, multistage cluster sampling). Of 14,407 persons in the entire NHEFS cohort, 218 with a history of tuberculosis prior to the NHANES I baseline medical examination were excluded, leaving 14,189 persons in the analysis cohort.
Weight (kg)/height (m)2.
Table 3.
Population-estimated Incidence of Tuberculosis According to Anthropometric and Laboratory Measures of Nutritional Status Among US Adults, NHANES I Epidemiologic Follow-up Study, 1971–1992
| Nutritional Status Indicator | Crude Frequency of TB in NHEFS Cohort |
Crude Distribution of Subjects in Each Category of NHEFS Cohorta |
No. of TB Cases, in Thousands (Population Estimate) |
Incidence Density/100,000 Person-Years (Population Estimate) |
Hazard Ratio |
95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| No. of Cases |
% of Category Total |
No. of Subjects |
% of NHEFS Total |
No. of Cases |
95% CI | No. of Cases |
95% CI | |||
| Total | 61 | 0.43 | 14,189 | 100 | 372.0 | 245, 500 | 22.8 | 15.0, 30.6 | ||
| Body mass indexb | ||||||||||
| <18.5 | 11 | 2.17 | 508 | 3.8 | 118.0 | 45, 191 | 260.2 | 98.6, 421.8 | 11.7 | 5.3, 25.9 |
| 18.5–25 | 32 | 0.48 | 6,639 | 46.8 | 192.0 | 101, 282 | 24.7 | 13.0, 36.3 | 1 | Reference |
| 25–30 | 14 | 0.30 | 4,647 | 32.8 | 50.0 | 12, 87 | 8.9 | 2.2, 15.6 | 0.37 | 0.17, 0.82 |
| >30 | 4 | 0.17 | 2,388 | 16.8 | 13.0 | 0, 26 | 5.1 | 0.0, 10.5 | 0.22 | 0.07, 0.69 |
| SFT,c mm | ||||||||||
| Low | 14 | 1.83 | 767 | 5.42 | 121.5 | 44.1, 198.9 | 170.6 | 59.6, 281.5 | 8.04 | 3.10, 20.87 |
| Normal | 34 | 0.43 | 7,846 | 55.44 | 202.2 | 96.2, 308.6 | 22.4 | 10.6, 34.2 | 1 | Reference |
| High | 13 | 0.23 | 5,538 | 39.14 | 48.7 | 12.6, 84.8 | 7.5 | 1.9, 13.0 | 0.34 | 0.14, 0.85 |
| AMA,c cm2 | ||||||||||
| Low | 13 | 1.55 | 839 | 5.92 | 89.5 | 32.1, 146.9 | 120.4 | 41.6, 199.1 | 5.09 | 2.00, 12.94 |
| Normal | 35 | 0.45 | 7,751 | 54.70 | 223.4 | 118.1, 328.7 | 24.8 | 13.0, 36.6 | 1 | Reference |
| High | 13 | 0.23 | 5,580 | 39.20 | 59.4 | 16.5, 102.3 | 9.1 | 2.5, 15.6 | 0.36 | 0.17, 0.79 |
| Both SFT and AMA | ||||||||||
| SFT low, AMA low | 7 | 5.07 | 138 | 0.98 | 59.6 | 18.0, 101.2 | 572.7 | 134.2, 1,011.2 | 27.87 | 10.26, 75.68 |
| SFT low, AMA normal | 7 | 1.35 | 519 | 3.67 | 61.8 | 0, 133.0 | 119.4 | 0, 239.7 | 2.98 | 0.63, 14.12 |
| SFT low, AMA high | 0 | 0 | 110 | 0.78 | 0 | 0 | 0 | |||
| SFT normal, AMA low | 6 | 1.11 | 540 | 3.82 | 29.9 | 0, 69.6 | 63.8 | 0, 128.1 | 5.54 | 1.34, 22.91 |
| SFT normal, AMA normal |
22 | 0.44 | 4,984 | 35.23 | 133.7 | 53.2, 214.3 | 23.2 | 9.1, 37.2 | 1 | Reference |
| SFT normal, AMA high | 6 | 0.26 | 2,321 | 16.41 | 38.6 | 0, 77.5 | 13.8 | 0, 27.7 | 0.46 | 0.13, 1.66 |
| SFT high, AMA low | 0 | 0 | 161 | 1.14 | 0 | 0 | 0 | |||
| SFT high, AMA normal | 6 | 0.26 | 2,246 | 15.88 | 27.8 | 0, 59.4 | 10.3 | 0, 20.6 | 0.59 | 0.23, 1.49 |
| SFT high, AMA high | 7 | 0.22 | 3,129 | 22.12 | 20.8 | 4.0, 37.6 | 5.7 | 1.1, 10.3 | 0.25 | 0.09, 0.67 |
| Blood hemoglobin level | ||||||||||
| Low (anemia)d | 10 | 0.48 | 2,087 | 15.32 | 42.2 | 0, 84.4 | 20.7 | 0, 41.5 | 0.88 | 0.29, 2.66 |
| Normal | 49 | 0.42 | 11,533 | 84.68 | 327.0 | 205.3, 448.6 | 23.7 | 14.8, 32.7 | 1 | Reference |
| Serum albumin level | ||||||||||
| Low (hypoalbuminemia)e | 3 | 1.86 | 155 | 1.39 | 44.8 | 0, 117.7 | 408.7 | 0, 1,085.2 | 12.96 | 2.50, 67.10 |
| Normal | 50 | 0.45 | 11,013 | 98.61 | 323.2 | 190.1, 456.3 | 264.4 | 89.2, 439.6 | 1 | Reference |
| Iron statusf | ||||||||||
| Deficiency unlikely | 44 | 0.48 | 9,258 | 87.8 | 325.3 | 181.1, 469.4 | 30.8 | 17.0, 44.5 | 1 | Reference |
| Deficiency possible | 7 | 0.67 | 1,046 | 9.9 | 34.0 | 5.5, 62.6 | 32.1 | 4.9, 59.3 | 1.04 | 0.29, 1.34 |
| Deficiency likely | 0 | 0 | 235 | 2.2 | 0 | 0 | Undefined | Undefined | ||
| Serum vitamin A level, μmol/L |
||||||||||
| <105 | 3 | 1.46 | 205 | 1.9 | 14.6 | 0, 34.1 | 80.9 | 0, 189.8 | 2.80 | 0.70, 11.40 |
| >105 | 48 | 0.45 | 10,584 | 98.10 | 348.5 | 199.0, 498.1 | 29.6 | 16.8, 42.4 | 1 | Reference |
| Low thiamine excretion | 1 | 4.0 | 25 | 0.2 | 3.3 | 0, 9.9 | 123.2 | 0, 381.0 | 3.70 | 0.50, 28.80 |
| Low riboflavin excretion | 0 | 0 | 95 | 0.9 | 0 | 0 | Undefined | Undefined | ||
Abbreviations: AMA, arm muscle area; CI, confidence interval; NHANES I, First National Health and Nutrition Examination Survey; NHEFS, NHANES I Epidemiologic Follow-up Study; SFT, skinfold thickness; TB, tuberculosis.
Excludes 218 subjects with a prior history of TB before NHANES I.
Weight (kg)/height (m)2.
SFT and AMA were classified as low, normal, or high on the basis of their sex-specific population distributions. “Low” was defined as <5th percentile, “high” as >60th percentile, and “normal” as values between those cutpoints.
Anemia was defined as a hemoglobin level less than 11.5 g/dL in females and less than 13.0 g/dL in males.
Hypoalbuminemia was defined as a serum albumin level less than 3.5 g/dL.
The likelihood of iron deficiency was based on serum iron level, percent transferrin saturation, and total iron-binding capacity, as follows. “Iron deficiency probable” was defined as low serum iron level and low percent transferrin saturation and high iron-binding capacity; “iron deficiency possible” was defined as any 2 of these 3 abnormalities; and “iron deficiency unlikely” was defined as only 1 or none of these abnormalities.
BMI does not distinguish between muscle, adipose tissue, bone, viscera, and water. To differentiate the effects of muscle from the effects of adipose tissue, we examined TB incidence by skinfold thickness, arm muscle area, and the interaction between the two. Mean skinfold thickness was 14.7 mm lower and arm muscle area was 4.7 cm2 lower among persons who developed TB than among those who did not (Table 2). Both fat and muscle were inversely associated with TB incidence. As skinfold thickness increased from low to normal to high, the TB incidence rate decreased from 170.6 per 100,000 person-years (95% CI: 59.6, 281.5) to 22.4 (95% CI: 10.6, 34.2) to 7.5 (95% CI: 1.9, 13.0). The pattern was similar with arm muscle area, but with a smaller amplitude (Table 3).
Subcutaneous fat and skeletal muscle had a synergistic effect. In the subset of people with both low skinfold thickness and low arm muscle area, TB incidence was 572.7 per 100,000 person-years (95% CI: 134.2, 1,011.2), more than 20-fold greater than the incidence in people with normal skinfold thickness and arm muscle area (Table 3). Among people with low values for either variable but not both, the risk of TB increased 3.0- to 5.5-fold. In contrast, among people whose arm muscle area and skinfold thickness were high, TB risk was significantly lower than among people with normal values (Table 3).
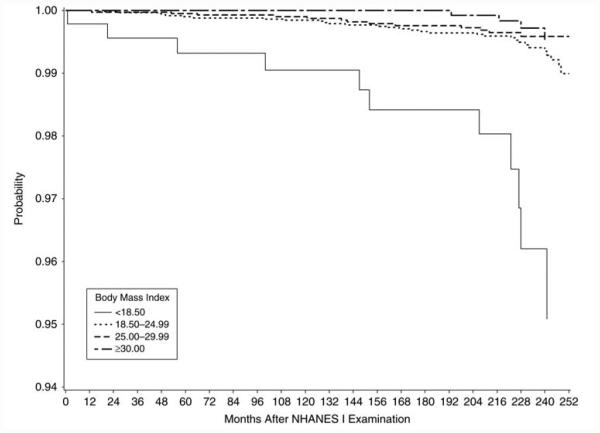
Kaplan-Meier plots (Figure 1 and Web Figure 1 (http://aje.oxfordjournals.org/)) display the TB incidence in undernourished, adequately nourished, and overweight groups over time. Undernourished participants developed TB significantly more quickly and obese participants developed TB significantly more slowly than those with normal nutritional status (P < 0.0001, log-rank test).
Figure 1.
Kaplan-Meier plot of the probability of remaining free of tuberculosis according to body mass index (weight (kg)/height (m)2), First National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Follow-up Study, 1971–1992.
Hemoglobin, serum proteins, and serum and urinary micronutrients
Compared with participants who did not develop TB, population-estimated mean hemoglobin levels (14.1 g/dL (95% CI: 13.7, 14.6) vs. 14.6 g/dL (95% CI: 14.5, 14.7); P = 0.04) and serum albumin levels (4.1 g/dL (95% CI: 3.9, 4.3) vs. 4.4 g/dL (95% CI: 4.4, 4.4); P = 0.006) were significantly lower among persons who later developed TB (Table 2). An abnormally low hemoglobin level (i.e., anemia) was observed in 2.5% of the population, but their TB incidence was not elevated. Hypoalbuminemia was observed in only 0.7% of the population, but their TB risk was 12.9-fold higher (95% CI: 2.5, 67.1) than among people with normal albumin levels (Table 3). Serum vitamin A, iron status, and urinary excretion of thiamine and riboflavin were not significantly associated with TB risk.
Multivariable analysis
Based on multivariable proportional hazards regression for complex sample surveys, the population-estimated hazard of developing TB for persons with low BMI was 12.4-fold (95% CI: 5.7, 26.9) greater than that for persons with normal BMI, after controlling for age, sex, race, Hispanic ethnicity, immigration, urban/rural residence, income, residence in a designated poverty area, excess alcohol consumption, smoking, and diabetes mellitus (Table 4). In contrast, among people who were overweight, it was nearly 4-fold lower (adjusted hazard ratio = 0.28, 95% CI: 0.13, 0.63); among people who were obese, it was 5-fold lower (adjusted hazard ratio = 0.20, 95% CI: 0.07, 0.62). Similarly, low skinfold thickness and low arm muscle area increased the hazard of developing TB 9.2-fold (95% CI: 3.2, 26.0) and 5.6-fold (95% CI: 2.2, 14.3), respectively. Anemia and iron status were not predictors of TB risk. There were too few TB cases among persons with low vitamin A, thiamine, riboflavin, or albumin levels to include those variables. Otherwise, only male sex, increasing age, smoking, and diabetes mellitus increased TB risk.
Table 4.
Population-estimated Adjusted Hazard Ratios for the Relation of Tuberculosis Incidence With Selected Measures of Nutritional Status and Other Risk Factors Among US Adults, NHANES I Epidemiologic Follow-up Study, 1973–1992a
| Characteristic | Adjusted Hazard Ratio |
95% Confidence Interval |
|---|---|---|
| Sex | ||
| Female | 0.35 | 0.14, 0.86 |
| Male | 1 | Reference |
| Age group, years | ||
| 25–34 | 1 | Reference |
| Each 10-year increment |
1.62 | 1.16, 2.26 |
| Race | ||
| White | 1 | Reference |
| Nonwhite | 1.60 | 0.93, 2.76 |
| Annual incomeb | ||
| <$10,000 | 2.17 | 0.89, 5.25 |
| $10,000-$20,000 | 1.10 | 0.49, 2.45 |
| >$20,000 | 1 | Reference |
| Current smoking | ||
| Yes | 2.01 | 1.01, 4.03 |
| No | 1 | Reference |
| Body mass indexc | ||
| <18.5 | 12.43 | 5.75, 26.95 |
| 18.5-<25 | 1 | Reference |
| 25-<30 | 0.28 | 0.13, 0.63 |
| >30 | 0.20 | 0.07, 0.62 |
| Skinfold thicknessd | ||
| Low | 9.19 | 3.25, 25.98 |
| Normal | 1 | Reference |
| High | 0.30 | 0.12, 0.73 |
| Cross-sectional arm muscle aread | ||
| Low | 5.56 | 2.16, 14.30 |
| Normal | 1 | Reference |
| High | 0.31 | 0.15, 0.65 |
| Diabetes mellitus | 7.58 | 2.94, 9.49 |
| Anemiae | ||
| Yes | 0.69 | 0.22, 2.13 |
| No | 1 | Reference |
| Hypoalbuminemiaf | ||
| Yes | 0.93 | 0.36, 2.41 |
| No | 1 | Reference |
| Iron statusg | ||
| Iron deficiency unlikely | 1 | Reference |
| Iron deficiency possible |
0.82 | 0.30, 2.21 |
Abbreviation: NHANES I, First National Health and Nutrition Examination Survey.
Results were based on multivariable Cox proportional hazards regression analysis for complex survey data. Terms were included in the final model if they were statistically significant at the P < 0.05 level or if they had a substantial (>10%) effect on the hazard ratio for tuberculosis incidence.
An income of $10,000 in 1973 dollars is equivalent to an income of $38,783 in 2000 dollars and $49,111 in 2010 dollars (http://data.bls.gov/cgi-bin/cpicalc.pl).
Weight (kg)/height (m)2.
Skinfold thickness and arm muscle area were classified as low, normal, or high on the basis of their sex-specific population distributions. “Low’ was defined as <5th percentile, “high” as >60th percentile, and “normal’ as values between those cutpoints.
Anemia was defined as a hemoglobin level less than 11.5 g/dL in females and less than 13.0 g/dL in males.
Hypoalbuminemia was defined as a serum albumin level less than 3.5 g/dL.
Iron status and the likelihood of iron deficiency were based on serum iron level, percent transferrin saturation, and total iron-binding capacity, as follows. “Iron deficiency probable” was defined as low serum iron level and low percent transferrin saturation and high iron-binding capacity; “iron deficiency possible” was defined as any 2 of these 3 abnormalities; and “iron deficiency unlikely” was defined as only 1 or none of these abnormalities.
Internal validation
To exclude the possibility that the observed association between TB and nutrition was due to the risk of becoming infected rather than (or in addition to) the risk of developing active disease, we analyzed nutritional status and TB incidence in relation to tuberculin skin test results. Of 1,470 persons tested, tuberculin skin tests were negative in 948 (64.5%); 249 (16.9%) had induration of 1–9 mm, and 273 (18.6%) had induration of ≥10 mm. There was no difference in the distribution of tuberculin skin test results by BMI.
If undernutrition led to hospitalization or death, the observed risk may have increased due to increased ascertainment of TB from medical records or death certificates rather than an actual increased risk of TB. However, the proportions of persons with 1 or more stays at a health-care facility were identical (71.9%) in people with low BMI and people with normal BMI, and the proportion was higher among overweight (76.8%) and obese (78.6%) people—opposite the relation between TB incidence and BMI. Mortality was higher in people who were overweight (34.7%) and obese (38.0%) than in people with normal BMI (27.1%).
DISCUSSION
Protein-energy nutrition was strongly associated with TB incidence in US adults during the period 1973–1992, independent of demographic, socioeconomic, and medical factors. Persons with low BMI, little subcutaneous fat, or low skeletal muscle had 5.5- to 12.5-fold higher risks of TB than persons with normal nutritional status. One-third of TB cases occurring among US adults during this 20-year period arose from this small (≤5%) fraction of the population. Low levels of serum albumin and serum transferrin, both markers of protein nutritional status, were strongly associated with an increased risk of TB. We did not include NHANES I dietary measures of nutritional status in this report because specific nutrient intakes were based on a single 24-hour dietary recall, which is not an adequate baseline measurement for a long-term follow-up study. With that caveat, however, it was interesting in this context that protein intake less than 50% of normal was the only dietary measure strongly associated with increased TB incidence (data not shown) (44–46). Thus, the consistency of the evidence suggests that protein undernutrition is important in host defenses against TB. Amino acids play key physiologic roles as precursors of molecules that are important to host defense, such as tryptophan (niacin, serotonin), arginine (nitric oxide), and methionine (S-adenosylmethionine). When energy intake and reserves are inadequate, somatic and dietary proteins are prioritized for energy. The negative impact of protein undernutrition on cell-mediated immunity is well-documented (1–7, 10, 11). In populations where protein insufficiency is common, it may contribute substantially to TB incidence. However, nutrition-related deficits in cell-mediated immunity are rapidly reversible with appropriate nutrient intake (47, 48).
Persons who were overweight, had thick fat, or had large muscles (approximately 40% of the population) had only one-third to one-fifth the risk of TB as people with normal values for these measures, consistent with previous studies (17–19, 23). Adipose tissue may be a reservoir for nonreplicating M. tuberculosis (49, 50). Such bacilli accumulate triglycerides in cytoplasmic lipid bodies and proliferate slowly in vitro (51, 52). A molecular genetic basis for these phenotypic characteristics has been identified (53–58). Thus, the decreased incidence of TB associated with increased BMI, especially with increased adipose tissue, may be related to development of a nonreplicating “persister” phenotype of M. tuberculosis in a lipid-rich environment. If these observations are confirmed, policies regarding isoniazid preventive treatment could be revisited, because the potential benefit would be much lower among obese persons with low risk of developing active TB. Given the prevalence of obesity in the United States, the cost savings to TB control programs could be substantial, because the majority of persons being treated in public TB control programs are being treated for latent infection, not active TB disease.
This analysis had important limitations, especially the potential for biased ascertainment of TB incidence. Because follow-up interviews did not ask specifically about TB, the data may not have captured every incident case of TB, especially cases not resulting in hospitalization or death. This potential bias, however, does not explain the observed association. First, the NHEFS-based estimated TB incidence was remarkably close to actual TB incidence according to national surveillance data, reflecting internal and external validity and lending credibility to the results (42, 43). The US TB surveillance system has been shown to be highly complete (59–63). Second, ascertainment of TB incidence was neither biased toward underweight persons nor biased against overweight persons. Third, in the 1970s, hospitalization of TB patients was the norm; the practice decreased gradually over time. The proportion of TB patients hospitalized was very high throughout the 1970s, gradually trending downward to approximately 50% by the late 1990s (64–73). Twenty-one of the 61 TB cases were ascertained through interviews, not ICD-9 codes.
Misclassification may have resulted from the use of ICD-9 codes, but in this study ICD-9 codes were assigned by trained National Center for Health Statistics staff, not by each facility’s billing department. We further mitigated this potential source of error by excluding ICD-9 codes for primary TB infection, TB exposure, and tuberculin skin test positivity in the absence of active TB.
A second limitation is the fact that the nutritional status of the US population has changed. Even though obesity was not widespread in the 1970s, many people are skeptical that undernutrition was ever a substantial problem in the United States. Indeed, only 2.2% of the cohort had low BMI. Nevertheless, the historical record speaks for itself (74–78). Large-scale nutrition surveys conducted in the 1960s found such widespread hunger and malnutrition among impoverished groups in the United States (79–85) that in a 1969 address to Congress, President Richard Nixon stated, “In the past few years, we have awakened to the distressing fact that despite our material abundance and agricultural wealth … there can be no doubt that hunger and malnutrition exist in America, and that some millions are affected” (86). The 1969 White House Conference on Food, Nutrition, and Hunger was a turning point (79–81), leading to large-scale government initiatives to combat undernutrition in the 1970s: food stamps; Aid to Families with Dependent Children (AFDC); the Supplemental Nutrition Program for Women, Infants, and Children (WIC); school breakfast programs; and Meals on Wheels for the elderly (79–82, 87). Undernutrition was mitigated in the United States only after these programs had been implemented throughout the 1970s. NHANES I itself was implemented to monitor the impact of these programs; hence, people living in poverty and people at high risk of undernutrition were heavily oversampled in NHANES I.
In addition to an increase in obesity, the incidence of diabetes mellitus has increased, the prevalence of smoking has decreased, and TB incidence among US-born persons has decreased, so the majority of TB cases now occur among foreign-born persons. The HIV epidemic also began in the 1980s. Apart from specific measures of nutritional status, diabetes and smoking were strong risk factors for TB in these data. While overweight persons in general had a lower risk of developing TB, the risk among overweight persons with diabetes was still elevated. HIV infection caused much TB morbidity from the mid-1980s through the late 1990s, when highly effective antiretroviral treatment became widely available. In the NHEFS cohort, 22 persons were identified as having HIV infection based on ICD-9 codes in the follow-up data, but none of them had TB. Thus, there were too few HIV-infected persons to control for HIV infection.
Because the population’s nutritional profile has changed, the results presented here do not reflect the population attributable risk after 1992, but they remain valid estimates of TB risk relative to nutritional indicators measured on a fixed scale, such as BMI categories and standard laboratory criteria. In addition, risk ratios based on absolute measures of nutritional status may be used to estimate attributable risk in populations with known, corresponding measures of nutritional status.
This study had important strengths. First, NHANES I and NHEFS have been documented extensively, with assessments of internal and external validity (24–32, 88–92). Second, standardized, validated indicators of nutritional status were based on body measurements and laboratory data. Third, multivariable statistical methods were based on the complex study design (27, 44). Fourth, to our knowledge, this is the only longitudinal cohort study of nutritional status and incident TB based on a nationally representative sample. Few observational studies have measured nutritional status and TB incidence among individuals in the appropriate temporal sequence, and none have excluded previous TB to ensure that only incident cases were included among the outcomes. In only 1 other study have investigators controlled for known risk factors using multivariable methods (23). Fifth, we separated the risks of TB in relation to adipose tissue, somatic protein, and individual micronutrients. Three older cohort studies demonstrated an association between TB incidence and weight-height indices, but they focused on “body build” as the operative concept rather than nutritional status (17–19, 93). However, both fat mass and muscle mass vary with nutritional intake and physical activity.
The absence of association with iron status could not have been predicted. Iron is critical to the immune response but also to the metabolism and replication of Mycobacterium. Data are insufficient to predict whether iron deficiency favors the microbe more than the host.
The absence of an association with vitamin A was not surprising, because vitamin A protects against infection mainly through epithelial integrity and humoral, not cellular, immune responses (94). Overt vitamin A deficiency was uncommon in the study cohort. Vitamin D has been associated with cellular immune defenses against TB, but vitamin D levels were not measured.
This study has implications beyond TB incidence in the US population. Changes in the prevalence of undernutrition in groups at risk for TB may affect TB incidence, as modeled by Lönnroth et al. (9, 22) and as noted by other investigators (95–97). Economic contractions and surging commodity food prices may affect TB incidence if they affect the nutritional status of populations; consider the popular term “belt-tightening” to describe economic hardship. Even modest nutritional deficits adversely affect cell-mediated immunity. Where undernutrition and TB are prevalent, the attributable proportion of TB cases may be substantial. In 1988, the US Surgeon General cited undernutrition as the leading cause of acquired immune system dysfunction worldwide (87). Most importantly, immune function is rapidly restored with nutritional repletion, suggesting that the provision of nutritional support to families and contacts of persons with TB may decrease TB incidence, in addition to the numerous other benefits of adequate nutrition.
ACKNOWLEDGMENTS
No external funding was provided for this study.
The authors are indebted to the late Dr. George Comstock for his thoughtful critique of an earlier version of this manuscript, Dr. Emily Bloss for her thoughtful review, Sara Shepherd and Dr. Carla Winston for their assistance with SUDAAN programming, Dr. Alice Tang for her assistance with the NHANES I tuberculin skin test data, and Hannah Oh and Sarah Smith for their assistance with historical data on tuberculosis incidence in the United States from the 1970s to the 1980s.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HIV
human immunodeficiency virus
- ICD-9
International Classification of Diseases, Ninth Revision
- NHANES I
First National Health and Nutrition Examination Survey
- NHEFS
NHANES I Epidemiologic Follow-up Study
- TB
tuberculosis
Footnotes
The conclusions and data interpretations presented in this report are solely those of the authors and do not necessarily represent the official position of the US government or the authors’ institutions.
Conflict of interest: none declared.
Contributor Information
J. Peter Cegielski, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia.
Lenore Arab, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California.
Joan Cornoni-Huntley, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
REFERENCES
- 1.Chandra RK, Gupta S, Singh H. Inducer and suppressor T cell subsets in protein-energy malnutrition. Analysis by monoclonal antibodies. Nutr Res. 1982;2(1):21–26. [Google Scholar]
- 2.Chandra RK. Numerical and functional deficiency in T helper cells in protein energy malnutrition. Clin Exp Immunol. 1983;51(1):126–132. [PMC free article] [PubMed] [Google Scholar]
- 3.Chandra RK. 1990 McCollum Award Lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr. 1991;53(5):1087–1101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- 4.Myrvik QN. Immunology and nutrition. In: Shils ME, Olson JA, Shike M, editors. Modern Nutrition in Health and Disease. 8th Vol. 1. Lea & Febiger; Philadelphia, PA: 1994. pp. 623–662. [Google Scholar]
- 5.Scrimshaw NS, Taylor CE, Gordon JE. Effect of malnutrition on resistance to infection. In: Scrimshaw NS, Taylor CE, Gordon JE, editors. Interactions of Nutrition and Infection. World Health Organization; Geneva, Switzerland: 1968. pp. 60–142. [Google Scholar]
- 6.Keusch GT. Nutrition and infection. In: Shils ME, Olson JA, Shike M, editors. Modern Nutrition in Health and Disease. 8th Vol. 1. Lea & Febiger; Philadelphia, PA: 1994. pp. 1241–1258. [Google Scholar]
- 7.Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin Chest Med. 2005;26(2):167–182. doi: 10.1016/j.ccm.2005.02.009. v. [DOI] [PubMed] [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations . The State of Food Insecurity in the World 2010. Food and Agriculture Organization of the United Nations; Rome, Italy: 2010. [Google Scholar]
- 9.Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375(9728):1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 10.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–298. [PubMed] [Google Scholar]
- 11.McMurray DN, Cegielski JP. HIV/AIDS, TB, and Nutrition: Scientific Inquiry Into the Nutritional Influences on Human Immunity With Special Reference to HIV Infection and Active TB in South Africa. Academy of Science of South Africa; Pretoria, South Africa: 2007. The influence of nutrition on the risk and outcomes of tuberculosis. In: Academy of Science of South Africa Consensus Panel on Nutrition, HIV/AIDS, and TB, ed; pp. 153–169. [Google Scholar]
- 12.Faber K. TB and nutrition. Acta Tuberc Scand. 1938;12(4):287–335. [Google Scholar]
- 13.Munro WT, Leitch I. Diet and tuberculosis. Proc Nutr Soc. 1945;3:155–164. doi: 10.1079/pns19450006. [DOI] [PubMed] [Google Scholar]
- 14.Marche J, Gounelle H. The relation of protein scarcity and modification of blood protein to tuberculosis among undernourished subjects. Milbank Mem Fund Q. 1950;28(2):114–126. [PubMed] [Google Scholar]
- 15.Cochrane AL. Tuberculosis among prisoners of war in Germany. Br Med J. 1945;2(4427):656–658. doi: 10.1136/bmj.2.4427.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winick M, editor. Hunger Disease: Studies by the Jewish Physicians in the Warsaw Ghetto. John Wiley & Sons, Inc; New York, NY: 1979. [Google Scholar]
- 17.Palmer CE, Jablon S, Edwards PQ. Tuberculosis morbidity of young men in relation to tuberculin sensitivity and body build. Am Rev Tuberc. 1957;76(4):517–539. doi: 10.1164/artpd.1957.76.4.517. [DOI] [PubMed] [Google Scholar]
- 18.Edwards LB, Livesay VT, Acquaviva FA, et al. Height, weight, tuberculous infection, and tuberculous disease. Arch Environ Health. 1971;22(1):106–112. doi: 10.1080/00039896.1971.10665820. [DOI] [PubMed] [Google Scholar]
- 19.Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis. 1986;69(5):355–362. [PubMed] [Google Scholar]
- 20.Getz HR, Long ER, Henderson HJ. A study of the relation of nutrition to the development of tuberculosis; influence of ascorbic acid and vitamin A. Am Rev Tuberc. 1951;64(4):381–393. doi: 10.1164/art.1951.64.4.381. [DOI] [PubMed] [Google Scholar]
- 21.Downes J. An experiment in the control of tuberculosis among Negroes. Milbank Mem Fund Q. 1950;28(2):127–159. [PubMed] [Google Scholar]
- 22.Lönnroth K, Williams BG, Cegielski P, et al. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 23.Leung CC, Lam TH, Chan WM, et al. Lower risk of tuberculosis in obesity. Arch Intern Med. 2007;167(12):1297–1304. doi: 10.1001/archinte.167.12.1297. [DOI] [PubMed] [Google Scholar]
- 24.Miller HW. Plan and operation of the Health and Nutrition Examination Survey. United States—1971–1973. Vital Health Stat 1. 1973;(10a):1–46. DHEW publication no. (PHS) 79-1310. [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. Plan and operation of the Health and Nutrition Examination Survey: United States—1971–1973 Vital Health Stat 1. 1973;(10b):1–77. DHEW publication no. (HRA) 77-1310. [PubMed] [Google Scholar]
- 26.Engel A, Murphy RS, Maurer K, et al. Plan and operation of the HANES I augmentation survey of adults 25–74 years United States, 1974–1975. Vital Health Stat 1. 1978;(14):1–110. DHEW publication no. (PHS) 78-1314. [PubMed] [Google Scholar]
- 27.Landis JR, Lepkowski JM, Eklund SA, et al. A statistical methodology for analyzing data from a complex survey: the first National Health and Nutrition Examination Survey. Vital Health Stat 2. 1982;(92):1–52. DHHS publication no. (PHS) 82-1366. [PubMed] [Google Scholar]
- 28.Cohen BB, Barbano HE, Cox CS, et al. Plan and operation of the NHANES I Epidemiologic Follow-up Study: 1982–84. Vital Health Stat 1. 1987;(22):1–142. DHHS publication no. (PHS) 87-1324. [PubMed] [Google Scholar]
- 29.Finucane FF, Freid VM, Madans JH, et al. Plan and operation of the NHANES I Epidemiologic Follow-up Study, 1986. Vital Health Stat 1. 1990;(25):1–154. DHHS publication no. (PHS) 90-1307. [PubMed] [Google Scholar]
- 30.Cox CS, Rothwell ST, Madans JH, et al. Plan and operation of the NHANES I Epidemiologic Follow-up Study, 1987. Vital Health Stat 1. 1992;(27):1–90. DHHS publication no. (PHS) 92-1303. [PubMed] [Google Scholar]
- 31.Cox CS, Mussolino ME, Rothwell ST, et al. Plan and operation of the NHANES I Epidemiologic Follow-up Study, 1992. Vital Health Stat 1. 1997;(35):1–231. DHHS publication no. (PHS) 98-1311. [PubMed] [Google Scholar]
- 32.Cornoni-Huntley JC, Huntley RR, Feldman JJ, editors. Health Status and Well-Being of the Elderly: National Health and Nutrition Examination I—Epidemiologic Follow-up Survey. Oxford University Press; New York, NY: 1990. [Google Scholar]
- 33.National Center for Health Statistics. National Health and Nutrition Examination Survey . NHANES I Epidemiologic Followup Study: Public Use Data Files and Documentation [44 data files] National Center for Health Statistics; Hyattsville, MD: 2010. www.cdc.gov/nchs/nhanes/nhefs/nhefspuf.htm Accessed January 15, 2011. [Google Scholar]
- 34.National Center for Health Statistics . National Health and Nutrition Examination Survey: NHANES I [40 data files] National Center for Health Statistics; Hyattsville, MD: 2010. www.cdc.gov/nchs/nhanes/nhanesi.htm Accessed January 15, 2011. [Google Scholar]
- 35.National Center for Health Statistics . Instruction Manual: HANES Examination Staff Procedures Manual for the Health and Nutrition Examination Survey, 1971–1973, Part 15a. National Center for Health Statistics; Hyattsville, MD: 1971. [Google Scholar]
- 36.Gibson R. Principles of Nutritional Assessment. New York, NY: Oxford University Press: 1990. p. 180. [Google Scholar]
- 37.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34(11):2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 38.National Center for Health Statistics . HANES-1 Hematology and Clinical Chemistry Procedures Developed or Utilized by the Center for Disease Control Bureau of Laboratories 1971–1975. National Center for Health Statistics; Hyattsville, MD: 1975. [Google Scholar]
- 39.National Center for Health Statistics . Public Use Data Tape Documentation: Biochemistry, Serology, Hematology, Blood Slides, Urine Dipst. Tape Number 4800. National Health and Nutrition Examination Survey, 1971–76. National Center for Health Statistics; Hyattsville, MD: 1991. [Google Scholar]
- 40.Tilkian SM, Conover MB, Tilkian AG. Clinical Implications of Laboratory Tests. 3rd CV Mosby Company; St. Louis, MO: 1983. [Google Scholar]
- 41.Ingram DD, Makuc DM. Statistical issues in analyzing the NHANES I Epidemiologic Follow-up Study. Series 2: data evaluation and methods research. Vital Health Stat 2. 1994;(121):1–30. DHHS publication no. (PHS) 94-13950. [PubMed] [Google Scholar]
- 42.Centers for Disease Control . United States Summary of Morbidity and Mortality. Tuberculosis Statistics: States & Cities, 1985. Centers for Disease Control; Atlanta, GA: 1986. pp. 64–65. [Google Scholar]
- 43.Centers for Disease Control . Appendix. In: Tuberculosis in the United States. Centers for Disease Control; Atlanta, GA: 1976. pp. 36–37. [Google Scholar]
- 44.National Center for Health Statistics . Public Use Data Tape Documentation: Dietary Frequency and Adequacy Ages 1–74. Tape Number 4701. National Health and Nutrition Examination Survey, 1971–1975. National Center for Health Statistics; Hyattsville, MD: 1981. [Google Scholar]
- 45.National Center for Health Statistics . Public Use Data Tape Documentation: 24-Hour Food Consumption Intake Ages 1–74. Tape Number 4704. National Health and Nutrition Examination Survey, 1971–1975. National Center for Health Statistics; Hyattsville, MD: 1985. [Google Scholar]
- 46.National Center for Health Statistics . Public Use Data Tape Documentation: Model Gram and Nutrient Composition. Tape Numbers 4702 and 4703. National Health and Nutrition Examination Survey, 1971–1975. National Center for Health Statistics; Hyattsville, MD: 1985. [Google Scholar]
- 47.Koster FT, Palmer DL, Chakraborty J, et al. Cellular immune competence and diarrheal morbidity in malnourished Bangladeshi children: a prospective field study. Am J Clin Nutr. 1987;46(1):115–120. doi: 10.1093/ajcn/46.1.115. [DOI] [PubMed] [Google Scholar]
- 48.Neumann CG, Lawlor GJ, Jr, Stiehm ER, et al. Immunologic responses in malnourished children. Am J Clin Nutr. 1975;28(2):89–104. doi: 10.1093/ajcn/28.2.89. [DOI] [PubMed] [Google Scholar]
- 49.Ulrichs T, Lefmann M, Reich M, et al. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J Pathol. 2005;205(5):633–640. doi: 10.1002/path.1728. [DOI] [PubMed] [Google Scholar]
- 50.Neyrolles O, Hernández-Pando R, Pietri-Rouxel F, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One. 2006;1(1):e43. doi: 10.1371/journal.pone.0000043. doi:10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garton NJ, Christensen H, Minnikin DE, et al. Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiology. 2002;148(10):2951–2958. doi: 10.1099/00221287-148-10-2951. [DOI] [PubMed] [Google Scholar]
- 52.Garton NJ, Waddell SJ, Sherratt AL, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5(4):e75. doi: 10.1371/journal.pmed.0050075. doi:10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniel J, Deb C, Dubey VS, et al. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol. 2004;186(15):5017–5030. doi: 10.1128/JB.186.15.5017-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sirakova TD, Dubey VS, Deb C, et al. Identification of a diacylglycerol acyltransferase gene involved in accumulation of triacylglycerol in Mycobacterium tuberculosis under stress. Microbiology. 2006;152(9):2717–2725. doi: 10.1099/mic.0.28993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muttucumaru DG, Roberts G, Hinds J, et al. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb) 2004;84(3-4):239–246. doi: 10.1016/j.tube.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Schnappinger D, Ehrt S, Voskuil MI, et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi L, Sohaskey CD, Kana BD, et al. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A. 2005;102(43):15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKinney JD, Höner zu, Bentrup K, Muñoz-Elías EJ, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406(6797):735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 59.Curtis AB, McCray E, McKenna M, et al. Completeness and timeliness of tuberculosis case reporting. A multistate study. Am J Prev Med. 2001;20(2):108–112. doi: 10.1016/s0749-3797(00)00284-1. [DOI] [PubMed] [Google Scholar]
- 60.Sprinson JE, Lawton ES, Porco TC, et al. Assessing the validity of tuberculosis surveillance data in California. BMC Public Health. 2006;6:217. doi: 10.1186/1471-2458-6-217. doi:10.1186/1471-2458-6-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloch AB, Cauthen GM, Simone PM, et al. Completion of tuberculosis therapy for patients reported in the United States in 1993. Int J Tuberc Lung Dis. 1999;3(4):273–280. [PubMed] [Google Scholar]
- 62.Trepka MJ, Beyer TO, Proctor ME, et al. An evaluation of the completeness of tuberculosis case reporting using hospital billing and laboratory data; Wisconsin, 1995. Ann Epidemiol. 1999;9(7):419–423. doi: 10.1016/s1047-2797(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 63.Yokoe DS, Coon SW, Dokholyan R, et al. Pharmacy data for tuberculosis surveillance and assessment of patient management. Emerg Infect Dis. 2004;10(8):1426–1431. doi: 10.3201/eid1008.031075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White MR. Hospitalization rates of tuberculosis in U.S. Navy enlisted personnel: a 15-year perspective. Mil Med. 1998;163(2):71–75. [PubMed] [Google Scholar]
- 65.Dandoy S. Current status of general hospital use for patients with tuberculosis in the United States: eight-year update. Am Rev Respir Dis. 1982;126(2):270–273. doi: 10.1164/arrd.1982.126.2.270. [DOI] [PubMed] [Google Scholar]
- 66.Powell KE, Brown ED, Seggerson JJ, et al. Evaluation of tuberculosis control programs: some national trends. Am J Public Health. 1984;74(4):344–348. doi: 10.2105/ajph.74.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown RE, Palmer CS, Simpson K. Estimate of Identifiable Direct Costs of Tuberculosis in the United States in 1991. Batelle; Washington, DC: 1993. pp. 13–19. 30–42. [Google Scholar]
- 68.Marks SM, Taylor Z, Burrows NR, et al. Hospitalization of homeless persons with tuberculosis in the United States. Am J Public Health. 2000;90(3):435–438. doi: 10.2105/ajph.90.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor Z, Marks SM, Ríos Burrows NM, et al. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis. 2000;4(10):931–939. [PMC free article] [PubMed] [Google Scholar]
- 70.Brown RE, Miller B, Taylor WR, et al. Health-care expenditures for tuberculosis in the United States. Arch Intern Med. 1995;155(15):1595–1600. [PubMed] [Google Scholar]
- 71.Rosenblum LS, Castro KG, Dooley S, et al. Effect of HIV infection and tuberculosis on hospitalizations and cost of care for young adults in the United States, 1985 to 1990. Ann Intern Med. 1994;121(10):786–792. doi: 10.7326/0003-4819-121-10-199411150-00009. [DOI] [PubMed] [Google Scholar]
- 72.Marks SM, Taylor Z, Miller BI. Tuberculosis prevention versus hospitalization: taxpayers save with prevention. J Health Care Poor Underserved. 2002;13(3):392–401. doi: 10.1353/hpu.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aitken ML, Anderson KM, Albert RK. Is the tuberculosis screening program of hospital employees still required? Am Rev Respir Dis. 1987;136(4):805–807. doi: 10.1164/ajrccm/136.4.805. [DOI] [PubMed] [Google Scholar]
- 74.Orshansky M. Counting the poor: another look at the poverty profile. 1965. Soc Secur Bull. 1988;51(10):25–51. [PubMed] [Google Scholar]
- 75.Orshansky M. Who’s who among the poor: a demographic view of poverty. Soc Secur Bull. 1965;28(7):3–32. [Google Scholar]
- 76.Bureau of the Census, US Department of Commerce . Poverty in the United States: 1985. US GPO; Washington, DC: 1987. Current Population Reports, series P-60, no. 158. [Google Scholar]
- 77.Bureau of the Census, US Department of Commerce . Trends in Relative Income: 1964 to 1989. US GPO; Washington, DC: 1991. Current Population Reports, series P-60, no. 177. [Google Scholar]
- 78.Bureau of the Census, US Department of Commerce . Poverty in the United States, 1992. US GPO; Washington, DC: 1993. Current Population Reports, series P-60, no. 185. [Google Scholar]
- 79.Mayer J. A report on the White House Conference on Food, Nutrition, Health, December 3–4, 1969, Washington, DC. Nutr Rev. 1969;27(9):247–251. [Google Scholar]
- 80.Mayer J. Hunger and undernutrition in the United States. J Nutr. 1990;120(8):919–923. doi: 10.1093/jn/120.8.919. [DOI] [PubMed] [Google Scholar]
- 81.Nestle M, Guttmacher S. Hunger in the United States: rationale, methods, and policy implications of state hunger surveys. J Nutr Educ. 1992;24(1):18S–22S. doi: 10.1111/j.1753-4887.1992.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 82.Physician Task Force on Hunger in America . Hunger in America. Wesleyan University Press; Middletown, CT: 1985. [Google Scholar]
- 83.Nord M, Andrews M, Carlson S. Household Food Security in the United States, 2004. Economic Research Service, US Department of Agriculture; Alexandria, VA: 2005. [Google Scholar]
- 84.Holben DH. Position of the American Dietetic Association: food insecurity and hunger in the United States. J Am Diet Assoc. 2006;106(3):446–458. doi: 10.1016/j.jada.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 85.Panel to Review U.S. Department of Agriculture’s Measurement of Food Insecurity and Hunger, National Research Council . Food Insecurity and Hunger in the United States: An Assessment of the Measure. National Academies Press; 2006; Washington, DC: http://www.nap.edu/catalog/11578.html Accessed January 14, 2011. [Google Scholar]
- 86.Nixon RM. Special message to the Congress recommending a program to end hunger in America, May 6, 1969. In: Peters G, Woolley JT, editors. The American Presidency Project [Web archive] University of California, Santa Barbara; Santa Barbara, CA: 1999. http://www.presidency.ucsb.edu/ws/?pid=2038 Accessed May 30, 2012. [Google Scholar]
- 87.Office of the Surgeon General, US Public Health Service . The Surgeon General’s Report on Nutrition and Health. US Public Health Service; Washington, DC: 1988. pp. 427–463. DHHS publication no. (PHS) 88-50210. [Google Scholar]
- 88.Madans JH, Kleinman JC, Cox CS, et al. 10 years after NHANES I: report of initial follow-up, 1982–84. Public Health Rep. 1986;101(5):465–473. [PMC free article] [PubMed] [Google Scholar]
- 89.Madans JH, Cox CS, Kleinman JC, et al. 10 years after NHANES I: mortality experience at initial follow-up, 1982–84. Public Health Rep. 1986;101(5):474–481. [PMC free article] [PubMed] [Google Scholar]
- 90.Madans JH, Reuben CA, Rothwell ST, et al. Differences in morbidity measures and risk factor identification using multiple data sources: the case of coronary heart disease. Stat Med. 1995;14(5–7):643–653. doi: 10.1002/sim.4780140521. [DOI] [PubMed] [Google Scholar]
- 91.Bryant EE, Kovar MG, Miller H. A study of the effect of remuneration upon response in the Health and Nutrition Examination Survey, United States. Vital Health Stat 2. 1975;(67):1–23. DHEW publication no. (HRA) 76-1341. [PubMed] [Google Scholar]
- 92.Russell LB, Milan E, Jagannathan R. Comparison of two surveys of hospitalization: the National Hospital Discharge Survey and the NHANES I Epidemiologic Follow-up Study. Vital Health Stat 2. 1997;(123):i–iii. 1–6. (DHHS publication no. (PHS) 97-1307. [PubMed] [Google Scholar]
- 93.Snider DE Jr. Tuberculosis and body build [editorial] JAMA. 1987;258(22):3299. [PubMed] [Google Scholar]
- 94.Sommer A. Vitamin A, infectious disease, and childhood mortality: a 2¢ solution? J Infect Dis. 1993;167(5):1003–1007. doi: 10.1093/infdis/167.5.1003. [DOI] [PubMed] [Google Scholar]
- 95.Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, et al. Poverty, obesity, and malnutrition: an international perspective recognizing the paradox. J Am Diet Assoc. 2007;107(11):1966–1972. doi: 10.1016/j.jada.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 96.Kennedy ET. The global face of nutrition: what can governments and industry do? J Nutr. 2005;135(4):913–915. doi: 10.1093/jn/135.4.913. [DOI] [PubMed] [Google Scholar]
- 97.Struble MB, Aomari LL. Position of the American Dietetic Association: addressing world hunger, malnutrition, and food insecurity. J Am Diet Assoc. 2003;103(8):1046–1057. doi: 10.1016/s0002-8223(03)00973-8. [DOI] [PubMed] [Google Scholar]