Abstract
Purpose
To analyze corneal persistent epithelial defects (PED) that occurred at three to four weeks after −4.5 PRK in rabbits and apply this pathophysiology to the treatment of PED that occur after any corneal manipulations or diseases.
Methods
Two corneas out of 168 that had −4.5D PRK to study epithelial basement membrane regeneration developed spontaneous PED that did not heal at three weeks after PRK. These were studied with slit lamp photos, immunohistochemistry for the myofibroblast marker alpha-smooth muscle actin (α-SMA), and transmission electron microscopy.
Results
Myofibroblasts developed at the stromal surface within the PED and for a short distance peripheral to the leading-edge of the epithelium. No normal epithelial basement membrane (EBM) was detectible within the PED or for up to 0.3 mm behind the leading-edge of the epithelium, although EBM had normally regenerated in other areas of the excimer laser ablated zone where the epithelium healed promptly.
Conclusions
A PED in the cornea results in the development of myofibroblasts and disordered extracellular matrix produced by these cells that together cause opacity within, and a short distance beyond, the PED. Clinicians should treat PEDs within ten days of non-closure of the epithelium to facilitate epithelial healing to prevent long-term stromal scarring (fibrosis).
Introduction
It has been generally recognized by clinicians for decades that persistence of a corneal epithelial defect beyond ten days to three weeks after trauma, surgery, infection, or disease, usually leads to scarring of the corneal stroma underlying the epithelial defect, although little has been written on the etiology of this disorder. Research over the past decade on the critical role of the epithelial basement membrane (EBM) in the etiology of stromal scarring (late haze or fibrosis) after photorefractive keratectomy (PRK) or stromal scars after microbial keratitis has provided important insights into stromal opacities occurring after most trauma, surgery, infection, or toxic exposures to the cornea.1–7
During a series of studies contrasting healing of rabbit corneas after −4.5D PRK, where corneal transparency is nearly always maintained, and −9D PRK, where late haze (fibrosis) is noted in 100% of corneas, two corneas developed spontaneous persistent epithelial defects (Fig. 1A) that did not heal within the typical first 5 to 7 days after −4.5D PRK. Thus, these persistent epithelial defects occurred in two of 168 (1.2%) consecutive eyes that had −4.5D PRK. Both corneas had not healed fully within three weeks of surgery and developed severe anterior stromal scarring (Fig. 1B) characteristic of human corneas that develop persistent epithelial defects. Although the epithelium of one of these corneas subsequently closed at 4 weeks after surgery, stromal scarring persisted. These corneas were studied with immunohistochemistry and transmission electron microscopy (TEM) to characterize the stromal healing response that occurs with persistent epithelial defects.
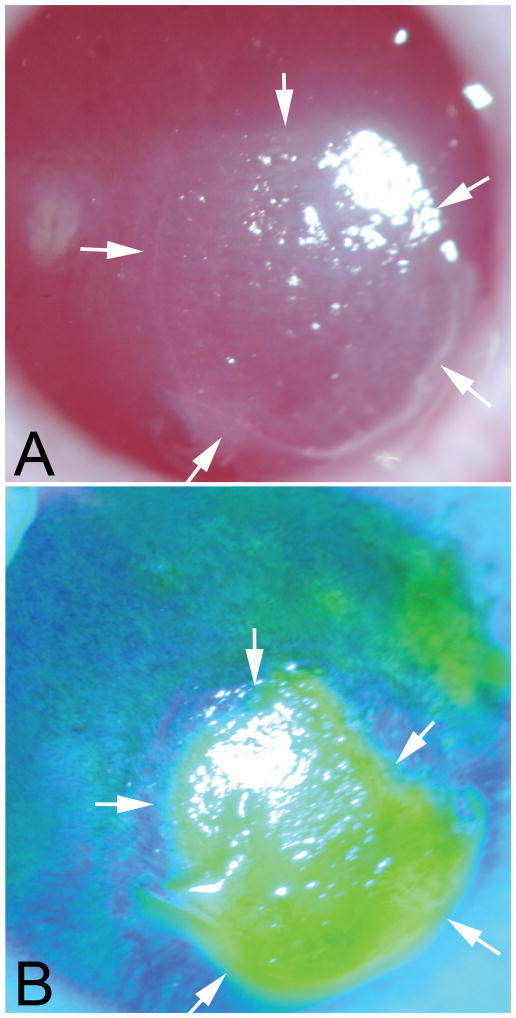
Fig. 1.
Slit lamp photos of a persistent epithelial defect (PED) in the cornea of rabbit #1at three weeks after −4.5D PRK. A. The epithelial defect is delineated by arrows and can be noted to have a thickened epithelial leading edge around the perimeter of the PED. The bare stroma within the PED is opaque and the opacity in the stroma extends a small distance peripheral to the leading edge of the epithelial defect. B. Fluorescein staining of the same cornea shows the epithelial defect. Mag 10X
Methods
This study is both a case study of two laboratory cases of spontaneous persistent epithelial defects and an assessment of pathophysiology and treatment of persistent epithelial defects. Female New Zealand white rabbits (12 to 15-week-old) weighing 2.5 to 3.0 kg each had −4.5D PRK without mitomycin C using previously described methods.1 These eyes were treated with 0.5% moxifloxacin (Vigamox, Alcon, Ft. Worth, TX) four times a day following surgery and continued until the epithelium healed as detected by fluorescein staining with FUL-GLO® Fluorescein Sodium Strips USP, Akorn, Lake Forest, IL). In one rabbit, where the epithelium developed an epithelial defect that persisted for three weeks after surgery, the moxifloxacin drops were continued four times a day until obvious corneal scarring was seen in the stroma beneath the epithelial defect at the slit lamp (Fig. 1). At that point, the rabbit was euthanized with an intravenous injection of 100 mg/kg pentobarbital while the animal was under 30 mg/kg ketamine hydrochloride and 5 mg/kg xylazine IM general anesthesia. The corneoscleral rim was removed and bisected through the PED.1 Half of the corneoscleral rim was fixed in OCT compound (Sakura FineTek, Torrance, CA, USA) within a 24 mm × 24 mm × 5 mm mould (Fisher Scientific, Pittsburgh, PA, USA) for immunohistochemistry for the alpha-smooth muscle actin marker for myofibroblasts using previously detailed methods.1 The other half of the corneoscleral rim was fixed in fresh 2.5% glutaraldehyde and 4% paraformaldehyde with 0.2 M cacodylate buffer at 4°C and processed for TEM using previously detailed methods.1
In the second rabbit, the PED had not healed by three weeks after −4.5D PRK while the eye was being treated with 0.5% moxifloxacin four times a day following surgery. The area of the PED developed a dense scar, despite the epithelium then closing by four weeks after surgery. At four weeks after surgery the rabbit was euthanized and the cornea was removed and processed for TEM.
Results
In the first rabbit cornea with PED removed at three weeks after surgery, a rolled leading epithelial edge characteristic of PEDs was noted (Fig. 2A) in immunohistochemistry (IHC) for the alpha-smooth muscle actin (α-SMA) marker for myofibroblasts and 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) that stains the nuclei of all cells. It was not possible to cut sections including the leading-edge epithelium without this portion of the epithelium artifactually separating from the underlying stroma due to poor adhesion. There were α-SMA+ myofibroblasts present near the stromal surface even beyond the rolled leading edge (Fig. 1A). Greater numbers of α-SMA+ myofibroblasts were noted in the anterior stroma of the cornea (Fig. 2B) within the stroma underlying the prior epithelial defect.
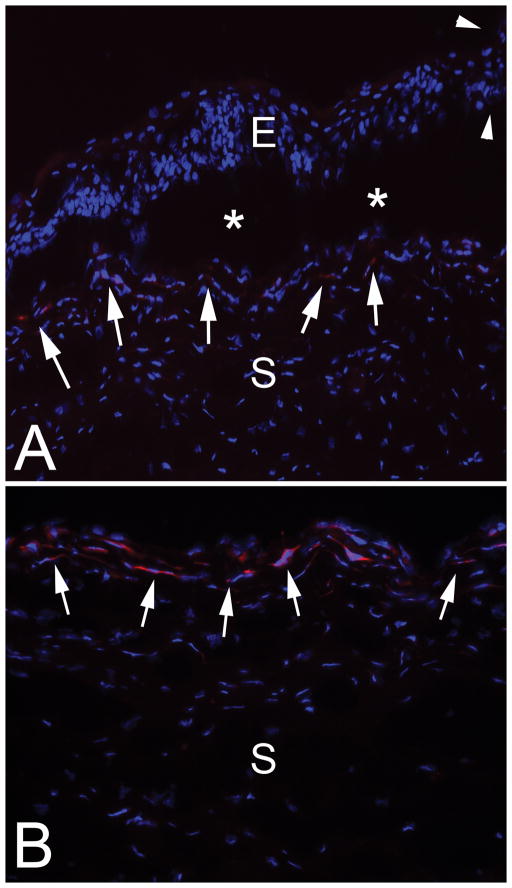
Fig. 2.
Immunohistochemistry for the alpha-smooth muscle actin (α-SMA) marker for myofibroblasts in the cornea of rabbit #1. Blue stain is DAPI that stains all cell nuclei. A. The epithelial leading edge (arrowheads) is rolled and the epithelium for up to 0.3 mm posterior to the leading edge showed poor adhesion and repeated artifactual dissociation (*) from the underlying stroma in all sections cut with a cryostat. α-SMA+ myofibroblasts are present at the stromal surface all along the dissociated epithelium (arrows) and, therefore, peripheral to the leading-edge of the epithelium in the PED. E is epithelium and S is stroma. Magnification 400X. B. In the center of the PED the stromal surface has prominent α-SMA+ myofibroblasts (arrows). S is stroma. Mag. 400X
Transmission electron microscopy confirmed myofibroblasts in the anterior stroma underlying the epithelial defects (Fig. 3A and 3B) and a distance of approximately 0.3 mm beyond the leading-edge of the epithelial defect but not in the adjacent cornea where the epithelium had closed normally within the PRK ablated zone. Within the epithelial defects and approximately 0.3 mm peripheral there was no epithelial basement membrane lamina lucida and lamina densa detectible by TEM with magnifications up to 43,000X (lamina lucida and lamina densa, when present, are clearly seen at 20,000X magnification1). Normal lamina lucida and lamina densa was noted in the areas within the PRK-ablated zone where the epithelium healed normally within five to 10 days after surgery (Fig. 3C).
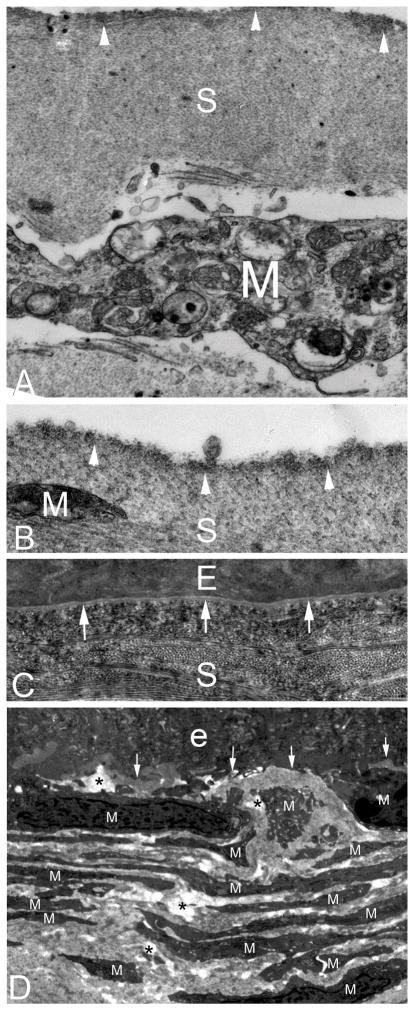
Fig. 3.
Transmission electron microscopy of rabbit corneas that developed PED after −4.5D PRK. The cornea in rabbit #1 that had not fully healed by three weeks after PRK (also shown in Fig. 1 and Fig. 2) is imaged by TEM in panels A–C. A. A high magnification image of the stromal surface within the PED showing an irregular stromal surface with dense material (arrowheads) at and beneath the surface. A large myofibroblast (M) is noted beneath the surface. S is stroma. Mag. 21,000X. B. A higher magnification image of the stromal surface within the PED again shows the layer of increased density at the stromal surface (arrowheads). A small portion of the end of a myofibroblast (M) is present. Mag. 42,000X. C. The corneal surface of the same rabbit #1 cornea in the periphery of the excimer laser ablated PRK zone two mm outside of the PED. Note the fully-regenerated epithelial basement membrane with lamina lucida and lamina densa (arrows) at three weeks after PRK. Also note that the distribution of the collagen fibrils in this area of the stroma (S) is much more compact and distinct than within the PED in panel A. E is epithelium. Mag. 21,000X. D. TEM of the cornea from rabbit #2 that had not healed by three weeks after −4.5D PRK, which developed scarring, despite the epithelium subsequently closing by four weeks after surgery. Within the area of scar, where the PED had persisted for over three weeks, the anterior stroma was populated by stacks of myofibroblasts (M) and the disordered extracellular matrix between them. No EBM was detectible at its normal location beneath the epithelium (e) (area indicated by the arrows), even with magnifications up to 40,000X. * indicates artifactual breaks in tissue that occur during cutting of the section due to weakening of the stroma by disordered extracellular matrix laid down by myofibroblasts. Mag. 15000X.
In the second rabbit with a PED at three weeks after −4.5D PRK that subsequently closed by four weeks after surgery, TEM at four weeks after PRK (Fig. 3D) showed layers of myofibroblasts with large amounts of rough endoplasmic reticulum within the area of the cornea where the PED had been located. No EBM lamina lucida and lamina densa were detected within this area even with magnifications up to 55,000X.
Discussion
Persistent corneal epithelial defects (PED) that do not close within ten days to two weeks after trauma, surgery, or infection, and many other etiologies, commonly develop scarring of the anterior corneal stroma underlying the epithelial defect, even if the epithelium subsequently heals. Two rabbit corneas were evaluated in this study after a spontaneous PED did not heal for at least three weeks after −4.5 D PRK, where late haze does not occur when the epithelium closes in the typical five to eight days after surgery.1,4 These corneas demonstrated that the underlying mechanism of scarring is myofibroblast-mediated fibrosis—similar to that noted after −9D PRK1 or pseudomonas keratitis7 in rabbits. In those studies, the epithelium healed within 10 days after surgery or sterilization of the ulcer with antibiotics, respectively, but normal mature EBM did not regenerate for two months—likely due to deficient keratocyte contributions of components such as laminins and nidogens to the nascent EBM.4,5,8 In the case of a PED that never heals, no epithelial basement membrane could be present within the area of the epithelial defect since even nascent EBM is not produced without overlying epithelium. Absence of EBM on the stromal surface within the PED of cornea #1 in this study was confirmed by TEM (Fig. 3). In rabbit #2 of this study, even when the epithelium closed between three and four weeks after PRK, no EBM could be detected with TEM within the area of the prior PED (Fig. 3D).
An important function of intact mature EBM is to modulate the passage of activated epithelium-derived transforming growth factor beta (TGFβ) and platelet-derived growth factor (PDGF) into the stroma.1,2,4,9 If the regenerated EBM is defective, these growth factors penetrate into the stroma from the overlying epithelium and drive the development of fibrosis-producing myofibroblasts from both keratocyte-derived and bone marrow cell-derived precursor cells.4,9 In corneas with a PED, where the epithelium never closes, these pro-fibrotic growth factors likely pass into the exposed stroma from the tears10,11 after release from corneal epithelial cells peripheral to the epithelial defect,12,13 and perhaps from conjunctival epithelium14 and lacrimal gland.15 Thus, these growth factors bind the corresponding receptors on the stromal myofibroblast precursor cells and trigger the development of vimentin+ α-smooth muscle actin+ desmin+ (V+A+D+) mature myofibroblasts.16 Once the myofibroblasts develop in the anterior stroma, opacity is caused by the opaque myofibroblasts themselves17,18 and the disordered extracellular matrix these myofibroblasts produce.4 Note for the PED shown in Figure 1, TEM (Fig. 3) showed that myofibroblasts and fibrosis development at only three weeks after surgery. Thus, some α-SMA+ myofibroblasts developed but the surrounding stroma was just beginning to show a disorder of the normal arrangement of the collagen fibrils if one compares the sharpness of the collagen fibrils in the area of the cornea outside the PED (Fig 3C), where no myofibroblasts have developed, to that within the PED (Fig. 3A and 3B), where myofibroblasts are present and excreting excessive disorganized collagen type 1,19 as well as collagen type 37 and other extracellular matrix materials, not normally present in the corneal stroma.20 These newly deposited collagens disrupt the precise organization of the fibrils responsible for stromal transparency.20 The longer the PED persists, the more myofibroblasts will be generated and greater the amounts of disordered extracellular matrix secreted by these cells and the greater the disruption of the normal corneal stromal structure within the stroma underlying the PED. In the #2 rabbit, even though the epithelium eventually closed at four weeks after PRK, many subepithelial myofibroblasts persisted in the area of the former PED because normal overlying EBM did not regenerate. This points to the importance of facilitating timely PED closure to promote regeneration of normal EBM and resulting apoptosis of myofibroblasts to allow keratocytes to repopulate the anterior stroma and reabsorb extrinsic extracellular matrix material. This process can ultimately lead to the restoration of corneal transparency.
A detailed discussion of the different etiologies and treatments for PED is beyond the scope of this article. However, regardless of the underlying etiology, most PED should initially be approached with standard treatment modalities such as ocular surface lubrication, bandage contact lenses, tarsorrhaphy, autologous serum drops, and/or amniotic membranes,21,22,23,24 with increasing aggressiveness beyond 8 to 10 days after surgery, trauma, or infection. The Table lists some of the most common conditions associated with PED and references articles with specific treatment strategies. However, general comments regarding measures to reduce scarring (fibrosis) in the stroma underlying the PED are of interest. The leading edge of the epithelium at the PED is commonly “rolled” and “stalled”, impeding subsequent growth of epithelium across the defect (Fig. 2A). “Freshening the edges” with a scalpel blade can facilitate epithelial healing.21 In addition, the stromal surface within the PED is typically irregular and may bind molecules from the tear film that further impede epithelial healing (Fig 3B). This surface irregularity may also retard epithelial closure, and even if the epithelial defect subsequently closes, may interfere with regeneration of normal EBM and increase haze (fibrosis, Fig. 3D).40 In our experience, to address this problem, it has been useful to freshen the edges of the PED for approximately 1 mm peripheral with a scalpel blade and possibly perform limited phototherapeutic keratectomy and masking-smoothing41 with the excimer laser using a beam diameter that is the size of the augmented epithelial defect. Amniotic membranes have a special role in potentially reducing or preventing fibrosis in PED. Amniotic membranes can downregulate TGFβ signaling.42 Thus, in addition to promoting epithelial closure in eyes with PED, amniotic membranes may reduce fibrosis by interfering with TGFβ modulation of myofibroblast development from precursor cells. Recently, morselized formulations of amniotic membranes have been described that can be applied topically to eyes with PED43 and these formulations could also potentially downregulate TGFβ-driven myofibroblast development in the stroma underlying a PED. Finally, the clinician should always consider the possibility that herpes simplex virus (HSV) infection underlies the PED.44 Therefore, when epithelial defects remain at 10 days after PRK, therapeutic debridement, UV-riboflavin crosslinking, traumatic or spontaneous abrasions or any other intervention or disease, we typically add empirical oral anti-HSV therapy to other modalities to promote timely epithelial healing.
Table.
Disorders commonly leading to persistent epithelial defects and treatments typically given beyond lubricants, serum drops, tarsorrhaphy, bandage contact lenses, amniotic membranes.
| Disorder leading to PED | Treatments beyond lubricants, serum drops, tarsorrhaphy, bandage contact lenses, amniotic membrane |
|---|---|
| Neurotropic cornea25 | Topical nerve growth factor |
| Diabetes mellitus26 | Optimization diabetic control |
| Herpetic keratitis27,28 | Antiviral treatment, judicious corticosteroids |
| Corneal dystrophies29,30 | PTK+MS, DALK, PKP |
| Salzmann’s nodular degeneration31 | Mechanical stripping of fibrous tissue, PTK+MS |
| Limbal stem cell deficiency32,33 | Limbal stem cell transplantation, scleral contact lenses |
| Chemical injuries34 | Limbal stem cell transplantation, DALK |
| Dry eye and Sjogren’s syndrome35 | Topical cyclosporine A, systemic treatment for Sjogren’s disease |
| Anesthetic abuse36 | Discontinue anesthetic |
| Medicamentosa | Discontinue offending medication |
| Graft vs host disease37 | Systemic immunosuppression, topical cyclosporine A |
| Stevens-Johnson syndrome38 | Corticosteroids, other immunosuppressives |
| Atopic keratoconjunctivits39 | Topical corticosteroids, antihistamines |
PTK+MS (phototherapeutic keratectomy with masking smoothing; DALK (deep anterior lamellar keratoplasty); PKP (penetrating keratoplasty)
More research should be focused on other pharmacologic agents to modulate TGFβ-mediated scarring in PED. Whatever therapeutic modalities are used for treatment of PED, if the epithelium can be triggered to close, then the EBM can eventually regenerate. This, in turn, can lead to myofibroblasts apoptosis and disordered extracellular matrix reabsorption by repopulating keratocytes so that stromal transparency is restored over a period of months to years, as it can be after PRK6 and microbial corneal ulcers.7
Acknowledgments
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY.
Footnotes
None of the authors has any financial or proprietary interests in the topics of this manuscript.
References
- 1.Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophth Vis Sci. 2013;54:4026–33. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torricelli AAM, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: Structure, function and disease. Invest Ophth Vis Sci. 2013;54:6390–400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp Eye Res. 2015;134:33–8. doi: 10.1016/j.exer.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–8. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santhanam A, Marino GK, Torricelli AAM, Wilson SE. Epithelial basement membrane (EBM) regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol Vision. 2017;23:39–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Marino GK, Santhiago MR, Santhanam A, Dibbin LL, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE. Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J Ref Surg. 2017;33:337–346. doi: 10.3928/1081597X-20170126-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marino GK, Santhiago MR, Santhanam A, Dibbin LL, Thangavadivel S, Tam KP, Wilson SE. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res. doi: 10.1016/j.exer.2017.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp Eye Res. 2015;134:33–8. doi: 10.1016/j.exer.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh V, Jaini R, Torricelli AA, Santhiago M, Singh N, Ambati BK, Wilson SE. TGFβ and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp Eye Res. 2014;121:35–40. doi: 10.1016/j.exer.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Monroy D, Ji Z, Yoshino K, Huang A, Pflugfelder SC. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996 Jun;15:605–14. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- 11.Zheng X, De Paiva CS, Rao K, Li DQ, Farley WJ, Stern M, Pflugfelder SC. Evaluation of the transforming growth factor-beta activity in normal and dry eye human tears by CCL-185 cell bioassay. Cornea. 2010;29:1048–54. doi: 10.1097/ICO.0b013e3181cf98ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp Eye Res. 1994;59:63–71. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SE, Lloyd SA, He YG. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1987–95. [PubMed] [Google Scholar]
- 14.Bianchi E, Scarinci F, Grande C, Plateroti R, Plateroti P, Plateroti AM, Fumagalli L, Capozzi P, Feher J, Artico M. Immunohistochemical profile of VEGF, TGF-β and PGE2 in human pterygium and normal conjunctiva: experimental study and review of the literature. Int J Immunopathol Pharmacol. 2012;25:607–15. doi: 10.1177/039463201202500307. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen DH, Beuerman RW, Thompson HW, DiLoreto DA. Growth factor and neurotrophic factor mRNA in human lacrimal gland. Cornea. 1997;16:192–9. [PubMed] [Google Scholar]
- 16.Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89:133–9. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999;112(Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 18.Jester JV, Brown D, Pappa A, Vasiliou V. Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering. Invest Ophthalmol Vis Sci. 2012;53:770–778. doi: 10.1167/iovs.11-9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poobalarahi F, Baicu CF, Bradshaw AD. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Physiol Heart Circ Physiol. 2006;291:H2924–32. [Google Scholar]
- 20.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–35. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzman LR, Jeng Management strategies for persistent epithelial defects of the cornea. Saudi J Ophthalmol. 2014;28:168–172. doi: 10.1016/j.sjopt.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrader S, Wedel T, Moll R, Geerling G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006 doi: 10.1007/s00417-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 23.Jeng BH, Dupps WJ., Jr Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–8. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- 24.Tseng SC. Amniotic membrane transplantation for persistent corneal epithelial defect. Br J Ophthalmol. 2001;85:1400–1. doi: 10.1136/bjo.85.12.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Thorin J. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998 38-19-36. [PubMed] [Google Scholar]
- 27.Fukuda M, Deai T, Higaki S, Hayashi K, Shimomura Y. Presence of a large amount of herpes simplex virus genome in the tear fluid of herpetic stromal keratitis and persistent epithelial defect patients. Semin Ophthalmol. 2008;23:217–20. doi: 10.1080/08820530802111366. [DOI] [PubMed] [Google Scholar]
- 28.Fukuada M, Deai T, Hibino T, Higaki S, Hayashi K, Shimomura Y. Quantitative analysis of herpes simplex virus genome in tears from patients with herpetic keratitis. Cornea. 2003;22(Suppl):S55–60. doi: 10.1097/00003226-200310001-00008. [DOI] [PubMed] [Google Scholar]
- 29.Sridhar MS, Rapuano CJ, Cosar CB, Cohen EJ, Laibson PR. Phototherapeutic keratectomy versus diamond burr polishing of Bowman’s membrane in the treatment of recurrent corneal erosions associated with anterior basement membrane dystrophy. Ophthalmology. 2002;109:674–9. doi: 10.1016/s0161-6420(01)01027-2. [DOI] [PubMed] [Google Scholar]
- 30.Foerster CG, Langenbucher A, Cursiefen C, Kruse FE, Seitz B. Delayed epithelial healing after keratoplasty for lattice corneal dystrophy. Cornea. 2007;26:1182–3. doi: 10.1097/ICO.0b013e318151f8cc. [DOI] [PubMed] [Google Scholar]
- 31.Maharana PK, Sharma N, Das S, Agarwal T, Sen S, Prakash G, Vajpayee RB. Salzmann’s Nodular Degeneration. Ocul Surf. 2016;14:20–30. doi: 10.1016/j.jtos.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 33.Atallah MR, Palioura S, Perez VL, Amescua G. Limbal stem cell transplantation: current perspectives. Clin Ophthalmol. 2016;10:593–602. doi: 10.2147/OPTH.S83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burcu A, Yalniz-Akkaya Z, Ozdemir MF, Erdem E, Onat MM, Ornek F. Surgical rehabilitation following ocular chemical injury. Cutan Ocul Toxicol. 2014;33:42–8. doi: 10.3109/15569527.2013.796477. [DOI] [PubMed] [Google Scholar]
- 35.Poon AC, Geerling G, Dart JKG, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85:1188–1197. doi: 10.1136/bjo.85.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagci A, Bozkurt B, Egrilmez S, Palamar M, Ozturk BT, Pekel H. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea. 2011;30:571–5. doi: 10.1097/ico.0b013e3182000af9. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22(Suppl):S19–27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- 38.Saeed HN, Chodosh J. Ocular manifestations of Stevens-Johnson syndrome and their management. Curr Opin Ophthalmol. 2016;27:522–529. doi: 10.1097/ICU.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 39.Akova YA, Rodriguez A, Foster CS. Atopic keratoconjunctivitis. 1994;2:125–44. doi: 10.3109/09273949409057069. [DOI] [PubMed] [Google Scholar]
- 40.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–97. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson SE, Marino G, Medeiros C, Santhiago MR. Phototherapeutic keratectomy (PTK): Science and Art. J Ref Surg J Refract Surg. 2017;33:203–210. doi: 10.3928/1081597X-20161123-01. [DOI] [PubMed] [Google Scholar]
- 42.Kawakita T, Espana EM, He H, Hornia A, Yeh LK, Ouyang J, Liu CY, Tseng SC. Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling. J Biol Chem. 2005;280:27085–92. doi: 10.1074/jbc.M409567200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng AM, Chua L, Casas V, Tseng SC. Morselized Amniotic Membrane Tissue for Refractory Corneal Epithelial Defects in Cicatricial Ocular Surface Diseases. Transl Vis Sci Technol. 2016;5:9. doi: 10.1167/tvst.5.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavan-Langston D. Herpes simplex virus ocular infections: current concepts of acute, latent and reactivated disease. Trans Am Ophthalmol Soc. 1990;88:727–796. [PMC free article] [PubMed] [Google Scholar]