ABSTRACT
Lipid droplets (LDs) are neutral lipid storage organelles that provide a rapidly accessible source of fatty acids (FAs) for energy during periods of nutrient deprivation. Surprisingly, lipids released by the macroautophagic/autophagic breakdown of membranous organelles are packaged and stored in new LDs during periods of prolonged starvation. Why cells would store FAs during an energy crisis was unknown. In our recent study, we demonstrated that FAs released during MTORC1-regulated autophagy are selectively channeled by DGAT1 (diacylglycerol O-acyltransferase 1) into triacylglycerol (TAG)-rich LDs. These DGAT1-dependent LDs sequester FAs and prevent the accumulation of acylcarnitines, which otherwise directly disrupt mitochondrial integrity. Our findings establish LD biogenesis as a general cellular response to periods of high autophagic flux that provide a lipid buffering system to mitigate lipotoxic cellular damage.
KEYWORDS: acylcarnitine, autophagy, DGAT1, lipid droplets, lipotoxicity, mitochondria, MTORC1
LDs are conserved, endoplasmic reticulum (ER)-derived organelles found in nearly every cell type. LDs consist of a core of neutral lipids (TAGs and cholesteryl esters) encircled by a phospholipid monolayer that is decorated with integrally and peripherally associated regulatory proteins. This specialized ultrastructure enables LDs to function as dynamic lipid storage depots that are highly responsive to the cellular metabolic state. During starvation, LD-associated neutral lipid lipases (e.g., PNPLA2/ATGL [patatin like phospholipase domain containing 2]) mediate the breakdown of LDs, liberating FAs from stored TAGs for the generation of energy in mitochondria via β-oxidation. Unexpectedly, during periods of prolonged starvation, it was found that FAs released by the autophagic breakdown of membranous organelles are packaged into new LDs. However, why cells would expend energy to synthesize and store TAGs in LDs during an energy crisis was a mystery.
Consistent with the previous report, starvation of mouse embryonic fibroblasts in Hank's balanced salt solution (HBSS) leads to an increase in LDs. These LDs are highly clustered and in close proximity to mitochondria, potentially forming membrane contact sites to facilitate LD-mitochondrial FA transfer. Starvation-induced increase in LDs is observed in multiple cell lines (U2OS, HeLa, Huh7) and can be blocked by inhibitors of autophagy and lysosomal function (e.g., bafilomycin A1 and 3-methyladenine), but not by inhibitors of FASN (fatty acid synthase; e.g., TVB-3166). Mechanistic target of rapamycin complex 1 (MTORC1) is a master regulator of cell growth that inhibits autophagy in the presence of growth factors and adequate nutrients. Inhibition of MTORC1 with the small molecule inhibitor torin1 or by deletion of the gene encoding the Ragulator subunit LAMTOR1/p18 is sufficient to increase autophagic flux and autophagy-dependent LD biogenesis, even in nutrient-rich complete medium (CM). Conversely, constitutive activation of MTORC1 by deletion of the gene encoding NPRL2, a subunit of the GATOR1 complex, blocks starvation-induced LD biogenesis. Thus, MTORC1-regulated autophagy is sufficient to induce LD biogenesis and is necessary for LD biogenesis during starvation. These data suggest that LD biogenesis is a general cellular response to periods of high autophagic flux.
To understand the function of LDs during starvation, we first examined the enzymes required for LD biogenesis during periods of nutrient excess (i.e., oleate-treated) and nutrient deprivation (i.e., HBSS). The final and committed step in TAG synthesis is mediated by the diacylglycerol acyltransferase enzymes DGAT1 and DGAT2. In the presence of oleate, LD biogenesis is only partially blocked by DGAT1 or DGAT2 inhibition alone, and complete blockade of LD biogenesis requires simultaneous inhibition of both enzymes. In contrast, inhibition of DGAT1, but not DGAT2, is sufficient to prevent LD biogenesis under starvation conditions. The inhibition of DGAT1 does not affect LC3 degradation kinetics or autophagic flux, indicating that the impairment of LD biogenesis is not due to disruption of autophagy (i.e., the source of FAs). Although DGAT1 and DGAT2 share TAG synthesis activity, our results demonstrate that the functions of these 2 enzymes are not redundant and that DGAT1 is specifically required for LD biogenesis during starvation.
The ability to block LD biogenesis with an inhibitor of DGAT1 provides a useful tool to probe the functional importance of LDs during starvation conditions. Employing single reaction monitoring (SRM)-based LC-MS steady-state lipidomic profiling and isotopic palmitate tracing, we found that DGAT1 inhibition has little effect on the cellular lipid landscape in CM. Indeed, TAG levels are unaffected by DGAT1 inhibition, indicating that DGAT1 is not necessary to maintain TAG pools in CM. In sharp contrast, DGAT1 inhibition during HBSS starvation results in a dramatic reduction in nearly all TAG species as well as a concomitant increase in the levels of acylcarnitines, which are important FA-conjugates that are formed at the mitochondria and are required for FA transport into the mitochondrial matrix for β-oxidation.
The accumulation of acylcarnitine suggests that the loss of LDs could affect mitochondrial function. Indeed, our results revealed that DGAT1 inhibition during starvation significantly reduces basal mitochondrial oxygen consumption rates, mitochondrial membrane potential, and cellular viability. The morphology and overall abundance of mitochondria is unaltered by DGAT1 inhibition, indicating that the decrease in mitochondrial functional parameters is not due to increased mitophagy or reduced mitochondrial biogenesis. Surprisingly, reducing acylcarnitine levels with the CPT1 inhibitor etomoxir rescues mitochondrial membrane potential in starved cells treated with DGAT1 inhibitor. Furthermore, treating isolated mitochondria with palmitoylcarnitine is sufficient to depolarize the mitochondria. These data implicate acylcarnitine as the lipotoxic culprit that directly disrupts mitochondrial function in the absence of LDs. We did not observe other lipotoxic lipidomic signatures, such as the accumulation of ceramide or an alteration in the ratio of phosphatidylethanoamine to phosphatidylcholine, and there is no observable activation of the ER unfolded protein response. Thus, our data suggest that DGAT1-dependent LD biogenesis during starvation is required to prevent the accumulation of acylcarnitines, which directly cause selective lipotoxic mitochondrial dysfunction.
In conclusion, our findings establish a novel function for LDs in preventing lipotoxic cellular damage during autophagy (Figure 1). An important future goal will be to understand the importance of LDs and lipotoxicity in physiological conditions associated with high autophagic flux (e.g., cancer and neurodegeneration).
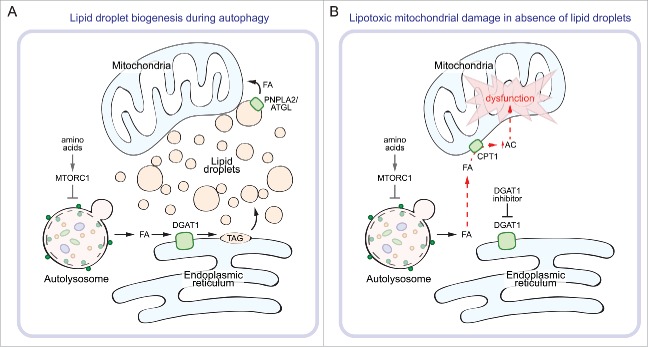
Figure 1.
Lipid droplets protect against lipotoxicity during autophagy. During prolonged starvation (e.g., amino acid depletion), MTORC1 is inactivated and autophagy is upregulated. (A) Autophagic breakdown of membranous organelles releases FAs that are channeled into DGAT1-dependent LDs. The FAs can be liberated from LDs by LD-associated lipases (e.g., PNPLA2/ATGL) and transferred to mitochondria for β-oxidation. (B) In the absence of LD biogenesis, such as during inhibition of DGAT1, autophagy-released FAs can no longer be sequestered as TAGs and instead accumulate as acylcarnitine (AC), which disrupts mitochondrial membrane integrity and impairs mitochondrial function.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by grants from the National Institutes of Health (R00DK095921 and R01GM112948 to J.A.O) and from the American Heart Association (16GRNT30870005 to J.A.O.). T.B.N was supported by a training grant from the NIH (T32DK061918).