ABSTRACT
SKAP2 (Src kinase-associated phosphoprotein 2), a substrate of Src family kinases, has been suggested to be involved in actin-mediated cellular processes. However, little is known about its role in mouse oocyte maturation. In this study, we thus investigated the expression, localization, and functions of SKAP2 during mouse oocyte asymmetric division. SKAP2 protein expression was detected at all developmental stages in mouse oocytes. Immunofluorescent staining showed that SKAP2 was mainly distributed at the cortex of the oocytes during maturation. Treatment with cytochalasin B in oocytes confirmed that SKAP2 was co-localized with actin. Depletion of SKAP2 by injection with specific short interfering RNA caused failure of spindle migration, polar body extrusion, and cytokinesis defects. Meanwhile, the staining of actin filaments at the oocyte membrane and in the cytoplasm was significantly reduced after these treatments. SKAP2 depletion also disrupted actin cap and cortical granule-free domain formation, and arrested a large proportion of oocytes at the telophase stage. Moreover, Arp2/3 complex and WAVE2 expression was decreased after the depletion of SKAP2 activity. Our results indicate that SKAP2 regulates the Arp2/3 complex and is essential for actin-mediated asymmetric cytokinesis by interacting with WAVE2 in mouse oocytes.
KEYWORDS: actin, cytokinesis, mouse oocyte meiosis, polar body extrusion, SKAP2
Introduction
Mammalian oocytes experience two successive asymmetric divisions that segregate homologous chromosomes and sister chromatids prior to fertilization.1 Oocytes finally generate a large haploid egg and two smaller polar bodies. Meiosis is an extreme event for retaining maternal components within the oocyte for early embryo development.2 Oocytes are arrested in the prophase of the first meiotic division within ovarian follicles. Meiosis resumes after hormonal stimulation, as indicated by nuclear envelope breakdown. Next, the first meiotic spindle gradually assembles around the centrally positioned metaphase chromosomes and then migrates to the cortex of the oocyte.3 When the spindle moves to the cortex, an actin cap, a cortical granule-free domain (CGFD), and microvillus-free regions are successfully formed over the spindle. Finally, cytokinesis occurs and the oocyte extrudes the first polar body, resulting in a highly polarized oocyte.4
Mouse oocyte asymmetric division is a highly coordinated process that results from oocyte polarization including spindle positioning and cortical reorganization.5 The formation of oocyte polarization is independent of any external ligand and the molecular signal is inherent in the oocytes.6 As the meiotic spindle in large mammalian oocytes lacks true centrosomes and astral microtubules, microtubule organization centers act as centrosomes. The off-center positioning of the spindle may not depend on microtubules as in mitosis but requires the involvement of dynamic filamentous actin instead,7 which is nucleated by the Arp2/3 complex,8–10 formin-2,11 and spire.12 Several nucleation-promoting factors (NPFs), including WAVE2,13 JMY,14 WHAMM,15 N-WASP,16 and WASH,17 share a conserved C-terminal WCA domain, which activate the Arp2/3 complex18 and help to regulate the branched actin filaments for mammalian oocyte asymmetric division.19 Although many molecular mechanisms were reported to regulate cortical polarity during mouse oocyte maturation, the detailed mechanisms that regulate oocyte polarization are yet to be investigated.
SKAP2 is a substrate of Src family kinases (SFKs)20 and highly homologous to SKAP55 (SKAP1).21 SFKs regulate different cellular processes including proliferation, adhesion, migration, and stress responses.22 SKAP2 protein is expressed ubiquitously23 and the amino acid sequence of mouse SKAP2 is 90% identical to that of human SKAP2, indicating the functional preservation of this protein. The structure of SKAP2 contains a pleckstrin homology (PH) domain, which can interact with lipids at membranes,24 and a carboxyl-terminal Src homology (SH) 3 domain.25 Moreover, in hematopoietic cells and immune cells, SKAP2 has been suggested to be essential for cell adhesion by associating with integrin and actin filamentous.26,27 Recent studies have demonstrated that SKAP2 plays a role in actin assembly by interacting with WAVE2 and cortactin.20 Meanwhile, WAVE2 and its activation of Arp2/3 complex are involved in actin polymerization and polar body emission during mouse oocyte maturation.13 Although SKAP2 has been reported to be involved in a variety of essential biological processes, much less is known about its role in mouse oocyte meiosis and there is no direct evidence linking SKAP2 to oocyte polarization.
In this study, we explored the expression, localization, and role of SKAP2 during meiosis. Our results illustrated that SKAP2 regulated Arp2/3 complex and was required for asymmetric cytokinesis and polar body extrusion by interacting with WAVE2 during mouse oocyte maturation.
Results
Expression and subcellular localization of SKAP2 during mouse oocyte meiotic maturation
To identify SKAP2 expression specifically in mouse oocytes, we here examined SKAP2 protein levels by western blotting. Oocytes were cultured for 0, 2, 6.5, 8.5, 10, and 12 h, representing stages at which most oocytes had reached the germinal vesicle (GV), germinal vesicle breakdown (GVBD), pro-metaphase I (Pro-MI), metaphase I (MI), anaphase-telophase I (ATI), and metaphase II (MII) stages. As shown in Fig. 1A, SKAP2 expression increased from the GV stage to the MI stage, while it slightly decreased at the MII stage. Next, immunofluorescent staining was performed on mouse oocytes to investigate the subcellular localization of SKAP2 at various stages of meiotic maturation. As shown in Fig. 1B, SKAP2 was distributed mainly at the cortex of the oocyte and co-localized with actin. Besides, during the late MI, ATI, and MII stages, SKAP2 was gradually accumulated in the cortical actin cap domain.
Figure 1.
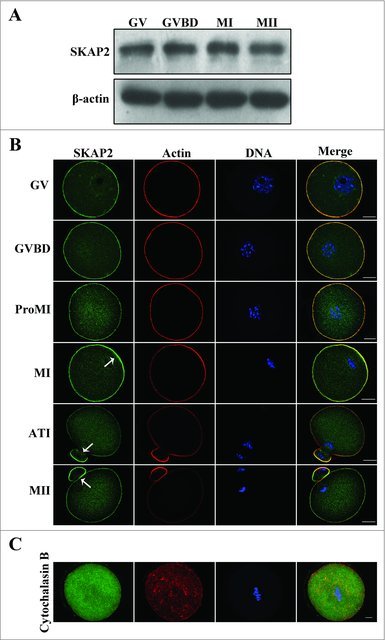
Expression and subcellular localization of SKAP2 during mouse oocyte meiotic maturation. (A) Representative images of SKAP2 protein level during mouse oocyte maturation. The expression of SKAP2 protein increased from GV stage to MI stage, but decreased at MII stage. A total of 200 oocytes were used in each sample. (B) Localization of SKAP2 during mouse oocyte meiosis. SKAP2 was distributed at the cortex of oocytes from GV stage to MII stage and the region near the cortex. (C) Localization of SKAP2 after CB treatment. Oocytes were cultured in M2 medium with 10 μg/ml CB for 8 h and then collected for immunostaining. SKAP2 was stably co-localized with F-actin. Green: SKAP2; red: actin; blue: chromatin. Bar = 20 μm.
Localization of SKAP2 and actin after cytochalasin B treatment
To further investigate the correlation between SKAP2 and actin, cytochalasin B (CB), as an actin-disrupting agent, was used to treat oocytes that were at the MI stage.28 As shown in Fig. 1C, the actin was disassembled after treatment. Meanwhile, SKAP2 exhibited a diffuse cytoplasmic distribution and no special signals were detected in MI stage oocytes.
SKAP2 RNAi caused failure of polar body extrusion in mouse oocytes
To further investigate the function of SKAP2 during mouse oocyte maturation, SKAP2 expression was down-regulated using siRNA injection, which successfully depleted its mRNA levels (1.02 ± 0.16 vs 0.51 ± 0.03, P < 0.05) (Fig. 2A). Then, we examined the expression of SKAP2 protein by western blotting. As shown in Fig. 2B, the protein level of SKAP2 was successfully reduced after SKAP2 siRNA injection. The relative level of SKAP2 protein in the SKAP2 siRNA-injected group was significantly decreased compared with that in the control (1.00 ± 0.08 vs 0.38 ± 0.04, n = 200, P < 0.05) (Fig. 2C).
Figure 2.
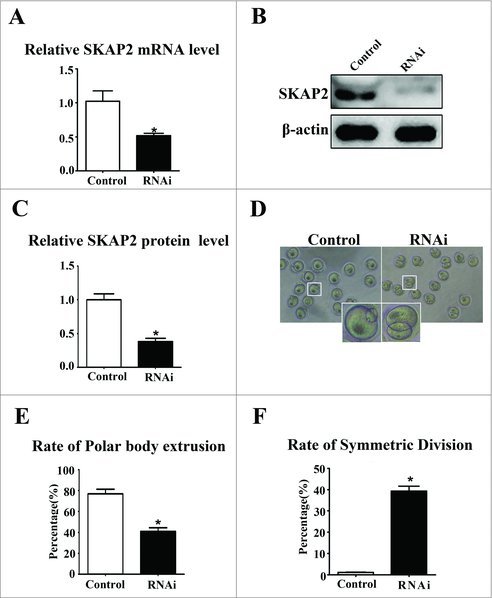
Effects of SKAP2 RNAi on mouse oocyte meiotic maturation. (A) SKAP2 mRNA levels after siRNA injection were significantly decreased. (B) Representative images of SKAP2 protein level in the SKAP2 siRNA-injected group and the control. SKAP2 protein expression was significantly reduced after siRNA injection. (C) Relative level of SKAP2 protein in the SKAP2 siRNA-injected group was lower than in the control. (D) Representative images of polar body in the SKAP2 siRNA-injected and control oocytes. (E) Rate of first polar body extrusion in the SKAP2 siRNA-injected group was lower than in the control. (F) Rate of symmetrical division in SKAP2 siRNA-injected oocytes was higher than in the control. Data are presented as mean ± SEM of three independent experiments, *: significant difference (P < 0.05).
After SKAP2 siRNA injection, oocytes were transformed to fresh M2 medium and cultured for 12 h. The normal oocytes extruded polar body and reached to MII stage. The rate of first polar body extrusion in the SKAP2 siRNA-injected group was decreased greatly compared with that in the control oocytes (77.05 ± 4.26%, n = 100 vs 40.92 ± 3.57%, n = 100, P < 0.05) (Fig. 2E). Nevertheless, we found that a large proportion of oocytes injected with SKAP2 siRNA failed to achieve asymmetric division and regularly extruded an abnormally large polar body (Fig. 2D). As shown in Fig. 2F, compared with the control group, the rate of symmetric division was significantly increased in the SKAP2 siRNA-injected group (1.23 ± 0.18%, n = 280 vs 39.34 ± 2.34%, n = 169, P < 0.05).
SKAP2 RNAi decreased actin filaments distribution in mouse oocytes
To investigate the cause of cytokinesis defects, immunofluorescent staining was performed on MI oocytes to examine the actin filaments. As shown in Fig. 3A, the chromosomes of oocytes had moved to the cortex and the actin cap had formed in the control group at the later MI stage. However, after SKAP2 siRNA injection, the spindle stayed near the center of oocytes and actin cap formation was disrupted. At MII stages, the chromosomes localized under the region of the cortex with an actin cap in the control oocytes. However, no typical actin cap was observed and oocytes exhibited abnormal MII morphology in the SKAP2 siRNA-injected group.
Figure 3.
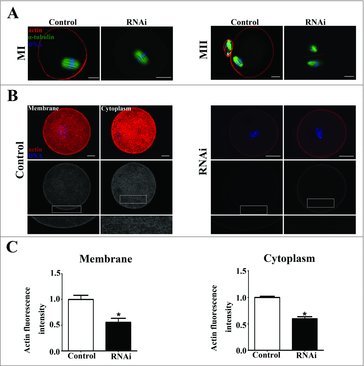
Effects of SKAP2 RNAi on actin expression. (A) Representative images of actin cap in the SKAP2 siRNA-injected and control groups. At MI and MII stages, actin cap formed in the control group, while no actin cap was detected in SKAP2 siRNA-injected group. Red: actin; green: spindle; blue: chromatin. Bar = 20 μm. (B) Actin filament distribution in oocyte membrane and cytoplasm after SKAP2 siRNA injection. The actin distribution was disrupted at both membrane and cytoplasm in the SKAP2 siRNA-injected group. Red: actin; blue: chromatin. Bar = 20 μm. (C) Average actin fluorescence intensities in oocyte membrane and cytoplasm were analyzed. Actin expression was reduced after SKAP2 siRNA injection. *: significant difference (P < 0.05).
We then investigated the average actin fluorescence intensity after the injection of SKAP2 siRNA. As shown in Fig. 3B, the distribution of actin filament in the SKAP2 siRNA-injected group was slightly smaller than that in the control group in both oocyte membrane and cytoplasm. In addition, the levels of actin fluorescence intensity in the control and siRNA-injected oocytes were analyzed. After depleting the SKAP2 activity, the average actin fluorescence intensity of membrane was significantly decreased compared with that in the control group (1 ± 0.08 vs 0.55 ± 0.07, P < 0.05) (Fig. 3C). The average actin fluorescence intensity of cytoplasm in the SKAP2 siRNA-injected group was also markedly reduced (1 ± 0.02 vs 0.59 ± 0.03, P < 0.05). Taken together, these results indicated that SKAP2 was involved in polar body extrusion through its effects on actin assembly.
SKAP2 RNAi caused failure of spindle migration
To further explore the function of SKAP2 during mouse oocyte meiosis, we investigated spindle migration, which played a vital role in polar body extrusion. As shown in Fig. 4A, after 9.5 h in culture, the spindle of oocytes in the control group had formed and moved to the cortex. In contrast, mouse oocytes in the SKAP2 siRNA-injected group exhibited a centrally localized spindle (22.03 ± 1.91%, n = 206 vs 46.90 ± 1.70%, n = 167; P < 0.05) (Fig. 4B).
Figure 4.
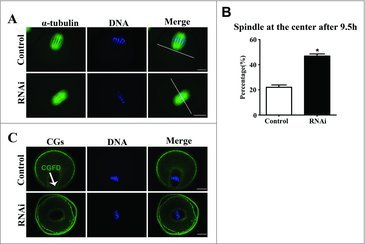
Effects of SKAP2 RNAi on spindle migration in mouse oocytes. (A) Spindle localization in mouse oocytes. In the control group, the spindles in most oocytes migrated to the cortex, whereas in the SKAP2 siRNA-injected group, they remained at the center of the cytoplasm. Green: α-tubulin; blue: chromatin. Bar = 20 μm. (B) Percentage of oocytes with spindles at the center in the SKAP2 siRNA-injected group was higher than in the control. *Significantly different (P < 0.05). (C) Cortical granule-free domain formation after SKAP2 siRNA injection in mouse oocytes. In the control group, cortical granules were absent at the cortex near the chromosomes at the MI stage. Cortical granules were distributed consistently across the entire cortex in the SKAP2 siRNA-injected group. Green: cortical granules; blue: chromatin. Bar = 20 μm.
CGFD formation is generally known to be a remarkable feature of mouse oocyte polarization, which results from spindle migration. As shown in Fig. 4C, CGFD had formed over the spindle in the control group at the MI stage. In contrast, no CGFD was observed in the oocytes injected with SKAP2 siRNA. This suggested that SKAP2 depletion disrupted oocyte polarization and also spindle migration.
SKAP2 RNAi caused cytokinesis defects in mouse oocyte meiosis
To investigate the role of SKAP2 in oocyte cycle procession, the oocytes were cultured for 12 h after siRNA microinjection. Then, immunostaining was performed on oocytes to assess oocyte stages. As shown in Fig. 5A, most oocytes successfully extruded a normal polar body and were arrested at the MII stage in the control group, while in the SKAP2 siRNA-injected group, a large proportion of oocytes were arrested at telophase I stage and failed to extrude the polar body. The rate of oocytes arrested at the telophase I stage in the SKAP2 siRNA-injected group was significantly increased compared with that in the control (14.16 ± 1.52%, n = 264 vs 24.98 ± 1.40%, n = 177; P < 0.05). The oocytes arrested at the MII stage were significantly decreased after SKAP2 siRNA injection (69.95 ± 4.56%, n = 264 vs 37.76 ± 3.40%, n = 177; P < 0.05) (Fig. 5B). These results indicated that the completion of cytokinesis and normal polar body extrusion were affected by SKAP2 depletion.
Figure 5.
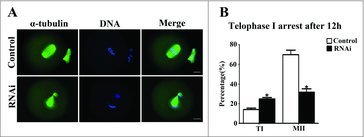
Effect of SKAP2 RNAi on mouse oocyte cytokinesis. (A) Oocytes in the SKAP2 siRNA-injected group were arrested at the TI stage after culture for 12 h, whereas oocytes in the control group had extruded the first polar body. Green: α-tubulin; blue: chromatin. Bar = 20 μm. (B) Percentages of oocytes at TI and MII stages in the SKAP2 siRNA-injected and control groups. In the SKAP2 siRNA-injected group, a large proportion of oocytes were arrested at the TI stage and fewer oocytes had progressed to the MII stage. *: significant difference (P < 0.05).
SKAP2 RNAi decreased ARP2 and WAVE2 expression
To explore the possible mechanism of SKAP2 in mouse oocyte asymmetric cytokinesis, we examined ARP2 expression by immunofluorescent staining after SKAP2 siRNA injection. ARP2, a major subunit of the Arp2/3 complex, functions as an ATP-binding component of the Arp2/3 complex, which is involved in mediating the formation of branched actin network and regulating actin polymerization. As shown in Fig. 6A, after culture for 9 h, the subcellular localization of ARP2 in the control group was specifically distributed in the oocyte cortex, while ARP2 was found to disperse to the cytoplasm and have no specific localization in the SKAP2 siRNA-injected oocytes. The fluorescence intensity of ARP2 in the SKAP2 siRNA-injected group was significantly lower than in the control group (0.99 ± 0.08 vs 0.38 ± 0.03, P < 0.05; Fig. 6C). Meanwhile, western blotting and quantitative analysis showed that the ARP2 expression in the SKAP2-injected group was also decreased (1 ± 0.08 vs 0.47 ± 0.03, P < 0.05; Fig. 6E). This indicated that SKAP2 was essential for ARP2 expression during mouse oocyte meiosis.
Figure 6.
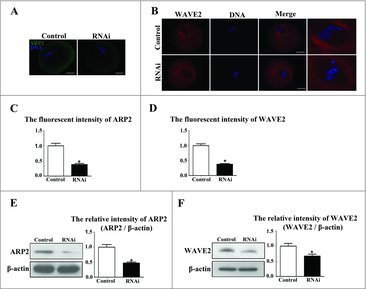
Effects of SKAP2 RNAi on ARP2 and WAVE2 expression. (A) Subcellular localization of ARP2 after SKAP2 siRNA injection. ARP2 was mainly distributed at the membrane in the control oocytes, whereas ARP2 expression was barely detectable in the siRNA-injected group. Green: ARP2; blue: chromatin. Bar = 20 μm. (B) Localization of WAVE2 after SKAP2 siRNA injection. WAVE2 was expressed around the spindle, whereas no specific localization of WAVE2 was observed around spindle in the siRNA-injected group. Red: WAVE2; blue: chromatin. Bar = 20 μm. (C) The fluorescence intensity of ARP2 in the SKAP2 siRNA-injected oocytes was decreased. (D) The fluorescence intensity of WAVE2 in SKAP2 siRNA-injected oocytes was significantly reduced. (E) ARP2 expression was reduced after SKAP2 siRNA injection by western blotting examination, as the relative intensity of ARP2 protein was significantly decreased. (F) WAVE2 expression was decreased after SKAP2 siRNA injection by western blotting analysis, as the relative intensity of WAVE2 protein was significantly reduced. *: significant difference (P < 0.05).
We then investigated the expression of WAVE2 in mouse oocytes meiosis. As shown in Fig. 6B, immunofluorescent staining showed that the localization of WAVE2 was around MI spindles in the control group, while no specific WAVE2 expression was detected in the SKAP2 siRNA-injected group. Meanwhile, the average WAVE2 fluorescence intensity in the SKAP2 siRNA-injected group was lower than in the control group (1 ± 0.05 vs 0.38 ± 0.02, P < 0.05; Fig. 6D). To confirm this, we assessed WAVE2 protein level by western blotting. WAVE2 expression level was significantly decreased in the SKAP2 siRNA-injected group. Analysis of the relative protein intensity also confirmed this decrease (1 ± 0.09 vs 0.67 ± 0.06, P < 0.05; Fig. 6F).
Discussion
In this study, we investigated the expression, localization patterns, and possible functions of SKAP2 during mouse oocyte meiosis. The results showed that depletion of SKAP2 activities had different effects on actin assembly, spindle migration, polar body extrusion, asymmetric cytokinesis, and ARP2 and WAVE2 expression.
Previous studies showed that SKAP2 was localized at the leading edge of the cell membrane;26,29 however, its functions and mechanisms during mouse oocyte maturation have remained unclear. Our results indicated that SKAP2 was mainly enriched in the cortical cortex through the whole oocyte meiosis. Additionally, the localization pattern of SKAP2 was similar to that of actin, which has been found to play a critical role in polar body extrusion during meiosis.30 Furthermore, CB treatment disrupted the specific localization of SKAP2 and dispersed it into the cytoplasm, further indicating the possible relationship between SKAP2 and actin. These results together suggest that SKAP2 may be related to actin dynamics and play a possible role in actin-mediated processes during mouse oocyte maturation.
To confirm this hypothesis, siRNA microinjection was performed on oocytes to knock down the SKAP2 expression level in order to examine the role of SKAP2 during mouse oocyte meiosis. Our results demonstrated that the depletion of SKAP2 resulted in failure of oocyte polar body extrusion and an increase in the number of oocytes arrested at telophase I stage. Moreover, the oocytes failed to achieve asymmetric cytokinesis and extruded large polar body, indicating the failure of asymmetric cell division. Our results suggest that SKAP2 plays a vital role in polar body extrusion in mouse oocytes.
During mouse oocyte meiotic maturation, actin was required for spindle positioning and cortical reorganization, which have been reported to be directly involved in oocyte asymmetric division and polar body extrusion.30 It was reported that SKAP2 associated with NCK2/F-actin at membrane ruffles, which was involved in regulating cytoskeletal rearrangement and actin organization.31 Therefore, we hypothesized that SKAP2 might affect polar body extrusion through its regulation of actin during oocyte meiosis.
To further confirm this hypothesis, actin cap formation and actin filament distribution were examined during mouse oocyte maturation. Actin filament staining after disrupting SKAP2 activity was significantly decreased in oocyte cytoplasm and membrane, and actin cap formation was also disrupted. These results suggest that SKAP2 is probably involved in regulating actin assembly and may further affect polar body extrusion. The effects of SKAP2 on actin filaments were similar to those of the Arp2/3 complex28 and its NPFs, which were reported to regulate actin dynamics followed by polar body extrusion. Overall, our results suggest that SKAP2 is involved in mouse oocyte meiosis based on its regulation of actin assembly.
In mammalian oocytes, actin drove the spindle migration and mediated it moving to the cortex, directly promoting oocyte asymmetric division.32 In addition, several molecules, such as MLC2,33 ROCK,34 and FMNL1,35 were reported to regulate actin activity for spindle positioning in mouse oocytes. We further investigated whether SKAP2 modulated the spindle migration during oocyte cytokinesis. Interestingly, the spindle localized near the center of oocytes in the SKAP2-depleted group. Moreover, the formation of CGFD, a typical characteristic of oocyte polarization resulting from spindle migration, was disrupted after disrupting SKAP2 activity. Similar results were reported on Cdc42,36 Rho GTPase RhoA,37 Rac,38 and their downstream regulators, which were involved in polar body extrusion and cytokinesis based on regulating spindle migration during mouse oocyte meiosis. Thus, our results indicate that SKAP2 regulates spindle migration for asymmetric division and may interact with the proteins mentioned above.
To better identify the possible mechanism by which SKAP2 influences polar body extrusion, we investigated the expression of ARP2 and WAVE2. ARP2, an actin-related protein, is essential for Arp2/3 complex activity. As with actin, nucleotide binding and hydrolysis by ARP2 influence the structure and function of this complex.39 Meanwhile, the Arp2/3 complex is principally activated by engaging with NPFs for polar body extrusion during meiosis. The present study showed that ARP2 expression was decreased after SKAP2 depletion, which indirectly suggested that SKAP2 might regulate actin assembly through its effects on the Arp2/3 complex during mouse oocyte meiosis.
WAVE2, a known regulator of the Arp2/3 complex, regulates meiotic spindle migration, positioning, and polar body emission during mouse oocyte meiosis.13 Previous studies found that SKAP2 regulated actin assembly in conjunction with WAVE2 in fibroblasts and glioblastoma cells.20 This led us to hypothesize that SKAP2 might be involved in actin-mediated asymmetric cytokinesis by associating with WAVE2 in mouse oocytes. Our research also showed that SKAP2 depletion decreased WAVE2 expression. Thus, SKAP2 may affect actin assembly by interacting with WAVE2 and further regulate the maturation of mouse oocytes.
In conclusion, we investigated the essential roles of SKAP2 during mouse oocyte meiosis. Our results indicate that SKAP2 is required for actin assembly and spindle migration through interacting with WAVE2 and that the SKAP2-Arp2/3 complex-actin pathway is essential for asymmetric cytokinesis during mouse oocyte maturation.
Materials and methods
Antibodies
A rabbit polyclonal anti-SKAP2 antibody (Cat#: 12926-1-AP) was purchased from Proteintech Group (Chicago, CA, USA). Mouse monoclonal anti-α-tubulin-FITC antibody (Cat#: F2168) and phalloidin-TRITC (Cat#: D1951) were obtained from Sigma (St. Louis, MO, USA). Goat anti-rabbit FITC-conjugated IgG (Cat#: CW014) was from Comwin Biotech (Beijing, China). Rabbit polyclonal anti-ARP2 antibody (Cat#: ab128934) and mouse monoclonal anti-β-actin antibody (Cat#: ab6276) were purchased from Abcam (Cambridge, UK). Alexa Fluor 546-conjugated donkey anti-rabbit antibody (Cat#: A1040) was purchased from Invitrogen (Carlsbad, CA, USA). Rabbit polyclonal anti-WAVE2 antibody (Cat#: 3659) was from Santa Cruz (Santa Cruz, CA, USA).
Oocyte collection and culture
All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Studies Committee of Xiamen University, China (approval ID: XMUMC 2011-10-08). The GV stage oocytes were collected from the ovaries of female imprinting control region (ICR) mice that were 4 to 6 weeks old and then cultured in M2 medium (Sigma, MO, USA) covered with mineral oil at 37°C in 5% CO2. Oocytes were collected for immunostaining, microinjection, and real-time polymerase chain reaction (PCR) after culture at different stages.
Real-time quantitative PCR analysis
SKAP2 gene expression was analyzed using real-time quantitative PCR and the ΔΔCT method, with normalization using β-actin. Total RNA was extracted from 50 oocytes using a Dynabead mRNA DIRECT kit (Life Technologies AS, Oslo, Norway). The first-strand cDNA was generated with a cDNA synthesis kit (Toyobo, Osaka, Japan), using Oligo (dT) 12–18 primers (Takara Bio Inc., Tokyo, Japan). The following specific primers were used to amplify the cDNA fragments of SKAP2 and β-actin:
SKAP2 forward, 5′- GCCATTGGCTTGGTGCCTA-3′;
SKAP2 reverse, 5′- TCCAAGGCACTTGCAGACAGA -3′;
β-actin forward, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′;
β-actin reverse, 5′-ATGGAGCCACCGATCCACA-3′.
A Step One Real-time PCR System (Applied Biosystems, Foster City, CA, USA) was used with the SYBR Green Real-time PCR Master Mix kit (Life Technologies, Carlsbad, CA, USA). The following PCR conditions were set: 50°C for 2 min, 95°C for 2 min, 40 cycles of 95°C for 15 s and 60°C for 1 min.
Western blotting
Each sample containing 200 oocytes was lysed in sodium dodecyl sulfate (SDS) loading buffer and heated for 5 min at 100°C. The proteins were separated by SDS-PAGE and then transferred onto a polyvinylidene fluoride (PVDF) membrane. Subsequently, the membrane was blocked in Tris Buffered Saline (TBS) containing 0.1% Tween and 5% skimmed milk powder for 1 h at room temperature to avoid nonspecific binding. Next, it was incubated overnight at 4°C with rabbit anti-SKAP2 antibody (1:1000), rabbit anti-WAVE2 antibody (1:1000), rabbit anti-ARP2 antibody (1:500), and mouse anti-β-actin antibody (1:1000). After washing three times in TBST for 10 min each, the membrane was incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (1:10,000) and HRP-conjugated mouse anti-rabbit IgG (1:10,000). Ultimately, the membrane was washed three times for 10 min each and then was visualized using enhanced chemiluminescence (ECL) detection substrate.
Cytochalasin B treatment
Cytochalasin B (Cat#: C6762, Sigma) stock was diluted in M2 medium to a final concentration of 10 μg/ml. The oocytes were cultured in this medium for 8 h, while the oocytes in the control group were cultured only in M2 medium. Oocytes were then collected for immunofluorescence microscopy.
SKAP2 siRNA injection
Each grown GV stage oocytes were microinjected with approximately 5–10 pl of 50 µM SKAP2 siRNA (5′-UCA GCA AAA CAG UGU UCU ATT-3′, 5′-UAG AAC ACU GUU UUG CUG ATT-3′) (GenePharma, Shanghai, China) or negative control siRNA (5′-UUC UCC GAA CGU GUC ACG UTT-3′, 5′-ACG UGA CAC GUU CGG AGA ATT-3′) (GenePharma, Shanghai, China). This process was accomplished using a Narishige MM0-202N hydraulic three-dimensional micromanipulator (Narishige Inc., Sea Cliff, NY, USA) equipped with a Nikon Diaphot ECLIPSE TE300 inverted microscope (Nikon UK Ltd., Kingston upon Thames, UK). After injection, the oocytes were cultured in M2 medium containing 2.5 µM milrinone for 24 h. After washing five times for 2 min each, the oocytes were then transferred to fresh M2 medium, cultured under paraffin oil in an incubator with an atmosphere of 5 % CO2, and maintained at 37°C in air. Oocytes were collected at different stages for further experiments.
Confocal microscopy
For staining of SKAP2, ARP2, WAVE2, CGs, or actin, the oocytes were fixed at room temperature in 4 % paraformaldehyde in phosphate-buffered saline (PBS) for 30 min. The oocytes were then permeabilized in PBS containing 0.5 % Triton X-100 at room temperature for 30 min. After 1 h in 1 % bovine serum albumin (BSA)- supplemented PBS, oocytes were incubated at room temperature for 1 h with phalloidin-TRITC (10 µg/ml) and for 30 min with lectin-FITC (100 µg/ml), or overnight at 4°C with rabbit anti-SKAP2 antibody (1:1000), rabbit anti-WAVE2 antibody (1:50), and rabbit anti-ARP2 antibody (1:50). After three washes in washing buffer (PBS containing 0.1 % Tween 20 and 0.01 % Triton X-100), the oocytes were labeled with Alexa Fluor 546-conjugated donkey anti-rabbit antibody (1:500) or goat anti-rabbit FITC-conjugated IgG (1:200) for 1 h at room temperature (this step was omitted for CGs and actin).
For double staining of SKAP2 and actin, after SKAP2 immunostaining, the oocytes were washed in washing buffer three times and then blocked again for 1 h in PBS supplemented with 1 % BSA, followed by immunostaining with phalloidin- TRITC for 30 min at room temperature.
For double staining of α-tubulin and actin, the oocytes were incubated with mouse FITC-conjugated anti-α-tubulin antibody (1:200) for 1 h at room temperature. After three washes, the oocytes were blocked again in PBS supplemented with 1 % BSA for 1 h at room temperature, and then stained with phalloidin-TRITC.
The samples were co-stained with DAPI for the immunostaining of DNA after three washes in washing buffer (0.1 % Tween 20 and 0.01 % Triton X-100). Oocytes were then mounted on glass slides with mounting medium and slices were examined using an FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan).
Fluorescence and western blotting band intensity analysis
The fluorescence intensity was assessed using Image J software (NIH). For fluorescence intensity analysis, samples of control and treated oocytes were mounted on the same glass slide. After immunofluorescent staining, the average fluorescence intensity per unit area within the region of interest (ROI) of immunofluorescence images was analyzed. The levels of fluorescence intensity of cell cytoplasm and membrane were measured independently using identically sized ROIs. When calculating the fluorescence intensity, oocytes with extremely strong or weak fluorescence were excluded. The average values of all measurements were used to compare the final average intensities of the control and treated oocytes.
For quantification of the western blot results, Image J was used to measure the intensity values of bands. Three different replicates were used for the analysis.
Statistical analysis
Each experiment used at least three replicates. The results are presented as mean ± standard error of the mean (SEM). Statistical comparisons were performed by ANOVA using Graph-Pad Prism software (v.5; La Jolla, CA, USA), followed by Student's t test. Differences at P < 0.05 were considered to be significant.
Funding Statement
This work was supported by grants from the The National Key Research and Development Program of China under Grant No.2017YFD0501905, The National Natural Science Foundation of China under Grant No. 31672248 and No.31772407, The Fundamental Research Funds for the Central Universities under Grant No. 20720150055.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Xiao-Hong Huang and Jin-Ru Huang for providing technical assistance.
References
- [1].Petronczki M, Siomos MF, Nasmyth K. Un menage a quatre: The molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–40. doi: 10.1016/S0092-8674(03)00083-7. PMID:12600308 [DOI] [PubMed] [Google Scholar]
- [2].Maro B, Verlhac MH. Polar body formation: New rules for asymmetric divisions. Nat Cell Biol. 2002;4:E281–3. doi: 10.1038/ncb1202-e281. PMID:12461532 [DOI] [PubMed] [Google Scholar]
- [3].Longo FJ, Chen DY. Development of cortical polarity in mouse eggs–involvement of the meiotic apparatus. Dev Biol. 1985;107:382–94. doi: 10.1016/0012-1606(85)90320-3. PMID:4038667 [DOI] [PubMed] [Google Scholar]
- [4].Deng MQ, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ. Chromatin-mediated cortical granule redistribution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev Biol. 2003;257:166–76. doi: 10.1016/S0012-1606(03)00045-9. PMID:12710965 [DOI] [PubMed] [Google Scholar]
- [5].Brunet S, Verlhac MH. Positioning to get out of meiosis: The asymmetry of division. Hum Reprod Update. 2011;17:68–75. doi: 10.1093/humupd/dmq044. PMID:20833637 [DOI] [PubMed] [Google Scholar]
- [6].Kutsuna H, Suzuki K, Kamata N, Kato T, Hato F, Mizuno K, Kobayashi H, Ishii M, Kitagawa S. Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: The role of MAP kinases. Am J Physiol Cell Physiol. 2004;286:C55–C64. doi: 10.1152/ajpcell.00131.2003. PMID:12954601 [DOI] [PubMed] [Google Scholar]
- [7].Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–9. doi: 10.1016/j.cub.2008.08.044. PMID:18848445 [DOI] [PubMed] [Google Scholar]
- [8].Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA, Pollard TD. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;404:1007–11. doi: 10.1038/35010008. PMID:10801131 [DOI] [PubMed] [Google Scholar]
- [9].Cooper JA, Schafer DA. Control of actin assembly and disassembly at filament ends. Curr Opin Cell Biol. 2000;12:97–103. doi: 10.1016/S0955-0674(99)00062-9. PMID:10679358 [DOI] [PubMed] [Google Scholar]
- [10].Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–76. doi: 10.1146/annurev.biophys.29.1.545. PMID:10940259 [DOI] [PubMed] [Google Scholar]
- [11].Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol. 2002;4:921–8. doi: 10.1038/ncb880. PMID:12447394 [DOI] [PubMed] [Google Scholar]
- [12].Sitar T, Gallinger J, Ducka AM, Ikonen TP, Wohlhoefler M, Schmoller KM, Bausch AR, Joel P, Trybus KM, Noegel AA, et al. Molecular architecture of the spire-actin nucleus and its implication for actin filament assembly. Proc Natl Acad Sci U S A. 2011;108:19575–80. doi: 10.1073/pnas.1115465108. PMID:22106272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sun SC, Xu YN, Li YH, Lee SE, Jin YX, Cui XS, Kim NH. WAVE2 regulates meiotic spindle stability, peripheral positioning and polar body emission in mouse oocytes. Cell Cycle. 2011;10:1853–60. doi: 10.4161/cc.10.11.15796. PMID:21543895 [DOI] [PubMed] [Google Scholar]
- [14].Sun SC, Sun QY, Kim NH. JMY is required for asymmetric division and cytokinesis in mouse oocytes. Mol Hum Reprod. 2011;17:296–304. doi: 10.1093/molehr/gar006. PMID:21266449 [DOI] [PubMed] [Google Scholar]
- [15].Huang X, Ding L, Pan R, Ma PF, Cheng PP, Zhang CH, Shen YT, Xu L, Liu Y, He XQ, et al. WHAMM is required for meiotic spindle migration and asymmetric cytokinesis in mouse oocytes. Histochem Cell Biol. 2013;139:525–34. doi: 10.1007/s00418-012-1051-z. PMID:23160625 [DOI] [PubMed] [Google Scholar]
- [16].Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol. 2011;13:934–U400. doi: 10.1038/ncb2290. PMID:21785420 [DOI] [PubMed] [Google Scholar]
- [17].Wang F, Zhang L, Zhang GL, Wang ZB, Cui XS, Kim NH, Sun SC. WASH complex regulates Arp2/3 complex for actin-based polar body extrusion in mouse oocytes. Sci Rep. 2014;4:5596. doi: 10.1038/srep05596. PMID:24998208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu J, Wang QC, Wang F, Duan X, Dai XX, Wang T, Liu HL, Cui XS, Kim NH, Sun SC. Nucleation promoting factors regulate the expression and localization of Arp2/3 complex during meiosis of mouse oocytes. Plos One. 2012;7(12):e52277. doi: 10.1371/journal.pone.0052277. PMID:23272233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Campellone KG, Welch MD. A nucleator arms race: Cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–51. doi: 10.1038/nrm2867. PMID:20237478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shimamura S, Sasaki K, Tanaka M. The Src substrate SKAP2 regulates actin assembly by interacting with WAVE2 and cortactin proteins. J Biol Chem. 2013;288:1171–83. doi: 10.1074/jbc.M112.386722. PMID:23161539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kouroku Y, Soyama A, Fujita E, Urase K, Tsukahara T, Momoi T. RA70 is a src kinase-associated protein expressed ubiquitously. Biochem Biophys Res Commun. 1998;252:738–42. doi: 10.1006/bbrc.1998.9637. PMID:9837776 [DOI] [PubMed] [Google Scholar]
- [22].Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–46. doi: 10.1038/sj.onc.1208080. PMID:15489911 [DOI] [PubMed] [Google Scholar]
- [23].Marie-Cardine A, Verhagen AM, Eckerskorn C, Schraven B. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. Febs Lett. 1998;435:55–60. doi: 10.1016/S0014-5793(98)01040-0. PMID:9755858 [DOI] [PubMed] [Google Scholar]
- [24].Swanson KD, Tang Y, Ceccarelli DF, Poy F, Sliwa JP, Neel BG, Eck MJ. The Skap-hom dimerization and PH domains comprise a 3 '-phosphoinositide- gated molecular switch. Mol Cell. 2008;32:564–75. doi: 10.1016/j.molcel.2008.09.022. PMID:19026786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Macias MJ, Wiesner S, Sudol M. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. Febs Lett. 2002;513:30–7. doi: 10.1016/S0014-5793(01)03290-2. PMID:11911877 [DOI] [PubMed] [Google Scholar]
- [26].Alenghat FJ, Baca QJ, Rubin NT, Pao LI, Matozaki T, Lowell CA, Golan DE, Neel BG, Swanson KD. Macrophages require Skap2 and Sirp alpha for integrin-stimulated cytoskeletal rearrangement. J Cell Sci. 2012;125:5535–45. doi: 10.1242/jcs.111260. PMID:22976304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reinhold A, Reimann S, Reinhold D, Schraven B, Togni M. Expression of SKAP-HOM in DCs is required for an optimal immune response in vivo. J Leukocyte Biol. 2009;86:61–71. doi: 10.1189/jlb.0608344. PMID:19369640 [DOI] [PubMed] [Google Scholar]
- [28].Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, Kim NH. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. Plos One. 2011;6(4):e18392. doi: 10.1371/journal.pone.0018392. PMID:21494665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alenghat FJ, Hyatt D, Swanson KD, Golan DE. An intermediary for Sirp alpha/Skap2-dependent integrin-stimulated cytoskeletal rearrangement in macrophages, and the implications for atherosclerosis. Circulation. 2014;130:2. [Google Scholar]
- [30].Bezanilla M, Wadsworth P. Spindle positioning: Actin mediates pushing and pulling. Curr Biol. 2009;19:R168–R9. doi: 10.1016/j.cub.2008.12.026. PMID:19243693: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou L, Zhang ZG, Zheng YF, Zhu YF, Wei ZJ, Xu H, Tang Q, Kong X, Hu L. SKAP2, a novel target of HSF4b, associates with NCK2/F-actin at membrane ruffles and regulates actin reorganization in lens cell. J Cell Mol Med. 2011;15:783–95. doi: 10.1111/j.1582-4934.2010.01048.x. PMID:20219016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yi KX, Li R. Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation. Cytoskeleton. 2012;69:727–37. doi: 10.1002/cm.21048. PMID:22753278 [DOI] [PubMed] [Google Scholar]
- [33].Duan X, Liu J, Zhu CC, Wang QC, Cui XS, Kim NH, Xiong B, Sun SC. RhoA-mediated MLC2 regulates actin dynamics for cytokinesis in meiosis. Cell Cycle. 2016;15:471–7. doi: 10.1080/15384101.2015.1128590. PMID:26701676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee SR, Xu YN, Jo YJ, Namgoong S, Kim NH. The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol Reprod Dev. 2015;82:849–58. doi: 10.1002/mrd.22524. PMID:26175189 [DOI] [PubMed] [Google Scholar]
- [35].Yin S, Sun QY. FMNL1, a key regulator for asymmetric cell division. Cell Cycle. 2015;14:2879–80. doi: 10.1080/15384101.2015.1046793. PMID:26301502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dehapiot B, Carriere V, Carroll J, Halet G. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev Biol. 2013;377:202–12. doi: 10.1016/j.ydbio.2013.01.029. PMID:23384564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang F, Zhang L, Duan X, Zhang GL, Wang ZB, Wang Q, Xiong B, Sun SC. RhoA-mediated FMNL1 regulates GM130 for actin assembly and phosphorylates MAPK for spindle formation in mouse oocyte meiosis. Cell Cycle. 2015;14:2835–43. doi: 10.1080/15384101.2015.1031438. PMID:26083584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–17. doi: 10.1016/j.devcel.2006.12.010. PMID:17276347 [DOI] [PubMed] [Google Scholar]
- [39].Goley ED, Welch MD. The ARP2/3 complex: An actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–26. doi: 10.1038/nrm2026. PMID:16990851 [DOI] [PubMed] [Google Scholar]


