ABSTRACT
Pathogens subvert host defense systems including autophagy and apoptosis for their survival and proliferation. Legionella pneumophila is a Gram-negative bacterium that grows in alveolar macrophages and causes severe pneumonia. Early during infection Legionella secretes effector proteins that convert the plasma membrane-derived vacuole containing Legionella into an endoplasmic reticulum (ER)-like replicative vacuole. These vacuoles ultimately fuse with the ER, where the pathogen replicates. Recently, we showed that one of the effectors, Lpg1137, is a serine protease that targets the mitochondria-associated ER membrane (MAM) and degrades STX17 (syntaxin 17), a SNARE implicated in macroautophagy/autophagy as well as mitochondria dynamics and membrane trafficking in fed cells. Degradation of STX17 blocks autophagy and BAX-induced apoptosis.
KEYWORDS: apoptosis, autophagy, BAX, endoplasmic reticulum, Legionella, mitochondria, mitochondria-associated membrane, RavZ, STX17
STX17 was found as an ER-localized syntaxin in 1998. This syntaxin is unique in that it has a long C-terminal hydrophobic domain (CHD) consisting of 44 amino acids that is divided by Lys254. This CHD appears to form a hairpin-like or W-shaped structure, allowing the C-terminal region to face the cytoplasm. Along with the other 5 syntaxins, STX17 is one of the last eukaryotic common ancestors present in diverse eukaryotic organisms, but was lost in multiple lineages including yeast. STX17 attracted little attention for a long time after its discovery, but the situation dramatically changed when Mizushima and colleagues in 2012 discovered that STX17 mediates autophagosome fusion with endolysosomes in cooperation with SNAP29 and VAMP8. Soon after, Yoshimori and colleagues found that STX17 facilitates autophagosome formation by recruiting to the MAM the class III phosphatidylinositol (PtdIns) 3-kinase complex through interaction with ATG14, a subunit of the kinase complex. The latter finding can partly explain the observation of Lippincott-Schwartz and colleagues that mitochondria supply proteins and lipids for autophagosome biogenesis. Recently, we identified a role of STX17 in fed cells; STX17 at the MAM promotes mitochondrial division by defining the localization and activity of the mitochondrial fission factor DNM1L/DRP1. In this function as well as the recruitment of the PtdIns 3-kinase complex, STX17 does not act as a fusion machinery, but functions as a scaffold. Lys254 is critically important for the localization of STX17 to the MAM and the interaction with DNM1L. STX17 interacts with the PRKA/protein kinase A-anchoring protein RAB32 and interferes with PRKA-mediated phosphorylation and inactivation of DNM1L. This finding has provided a molecular explanation for the seminal discovery by Voeltz and colleagues that the ER marks the site for mitochondrial fission.
Very recently, we discovered that Legionella pneumophila degrades STX17 by means of Lpg1137, one of the effectors that are secreted by Legionella through the Dot/Icm system. Lpg1137 has a sequence (-Gly-Leu-Ser68-Gly-Gly-) that matches the consensus sequence for the active site of serine proteases (Gly-X-Ser-X-Gly/Ala, where X is any residue). Indeed, Lpg1137 exhibits proteolytic activity toward STX17 in vitro, whereas an active site mutant in which Ser68 is replaced by Ala does not. Expressed Lpg1137 localizes to the MAM and mitochondria, in addition to the cytosol, and binds to STX17. A STX17 mutant in which Lys254 is replaced by Cys, which does not localize to the ER-mitochondria interface, does not significantly bind to Lpg1137 and is not cleaved by Lpg1137.
As a consequence of STX17 cleavage, Legionella blocks starvation-induced autophagy (Fig. 1, lower left). Loss of STX17 due to Lgp1137 expression or knockdown by short interfering RNA blocks the formation of not only LC3-positive puncta but also puncta positive for ZFYVE1/DFCP1 and ATG14, implying the failure of PtdIns-3-phosphate (PtdIns3P) formation at the MAM/omegasome. This was confirmed by the finding that the PtdIns3P-binding protein RavZ fails to localize to ER puncta in the absence of STX17 during starvation. RavZ is a Legionella effector with protease activity that cleaves the bond between LC3 and phosphatidylethanolamine, thereby preventing autophagosome formation. Expression of RavZ interferes with the formation of LC3-positve structures, but expressed STX17 is still colocalized with RavZ, suggesting that STX17 binds to PtdIns3P-positive omegasomes without formation of autophagosomes decorated with LC3.
Figure 1.
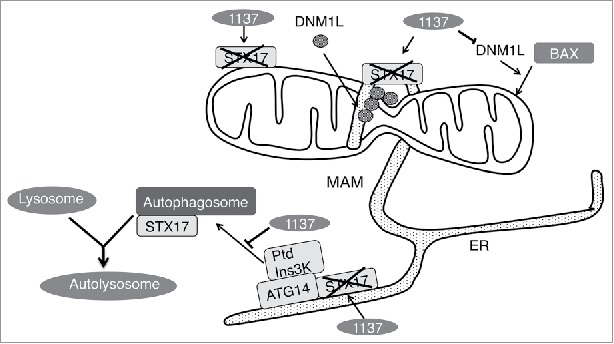
Lpg1137 cleaves STX17, and prevents autophagy and apoptosis. STX17 participates in mitochondrial division in fed cells, and autophagosome formation and fusion with lysosomes in starved cells. Lpg1137, which is delivered into the host cell cytosol, cleaves STX17 and thereby abrogates autophagy by preventing PtdIns3P formation at the MAM, and apoptosis by blocking the DNM1L-dependent translocation of the proapoptotic protein BAX to mitochondria.
Our findings confirmed the notion that STX17 participates in an early stage of autophagy, i.e., autophagosome formation. Perhaps STX17 dependency is different between autophagosome formation and autophagosome fusion. The previous work demonstrating that STX17 mediates autophagosome fusion with endolysosomes can be reconciled by the idea that autophagosome formation might be less sensitive to STX17 depletion than autophagosome-lysosome fusion. Our finding also highlighted a strategy of Legionella for avoiding elimination by the host cell defense system. Legionella appears to use multiple strategies to block autophagy. In addition to STX17 cleavage, it prevents autophagosome formation through degradation of the bond between LC3 and phosphatidylethanolamine by RavZ, and disturbance of sphingolipid metabolism by the Legionella effector sphingosine-1-phosphate lyase (LpSpl). Future work is required to reveal the interplay among multiple effectors (RavZ, Lpg1137 and LpSpl) that suppress autophagy, as well as the strategic advantage of the presence of multiple autophagy suppressors for infection by, and intracellular growth of, Legionella. Use of these proteins may provide new insight into the mechanism of autophagy.
Degradation of STX17 by Lpg1137 blocks not only autophagy but also BAX-dependent (staurosporine-induced) apoptosis (Fig. 1, upper right). There is intimate crosstalk between autophagy and apoptosis, which is mediated by several proteins with dual roles in autophagy and apoptosis. Our findings have revealed another layer of regulation in the crosstalk between these pathways.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (#17K19406 to M.T., and #26111520, #26713016, and #16H01206 to K.A.) and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (to M.T. and K.A.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.


