Abstract
Colorectal cancer is the second leading cause of mortality in the West, and rectal cancer accounts for about 25% of the colon cancers. The concept of total mesothelial excision (TME) was the most important event in surgery for rectal cancer of the last two decades, because even without a curative approach, it reduced local recurrence and extended 5-year survival.
Keywords: rectal cancer, TME, mesorectum, CRM, Circumferential Resection Line
1. INTRODUCTION
Colorectal cancer is the second leading cause of mortality in the West, and rectal cancer accounts for about 25% of colon cancers. Carcinoma of the intraperitoneal rectum acts as a colon cancer, with regard to recurrence and prognosis, while the opposite, the extraperitoneal rectum, 10 to 12 cm in length, comprises the rectum from the oncological point of view. The concept of total mesothelial excision (TME) was the most important event in surgery for rectal cancer in the last two decades, because even without a curative approach, the local recurrence decreased to 6 to 12%, and 5-year survival improved by 53-87% (1–3).
The essence of the TME hypothesis is that lymph nodes are randomly distributed within the mesorectum, and that they are not all visible or palpable. The size of the normal mesorectum lymph nodes in about 80% of cases is<3mm. Most (54%) of mesorectum lymph nodes are located posteriorly, and 92% of the posterior lymph nodes lie within the upper half of the upper 2/3 of the rectum (4).
The intramural spread of cancer downward is very rare, but extramural spread appears both in distal and anterior directions, within the mesorectum fascia. It is believed that this is because the rectum and the mesorectum constitute an embryonic entity - the back colon inside its lymphatic vessel wrap.
2. TOTAL MESORECTAL EXCISION
TME consists of the complete removal of the rectum, together with the surrounding mesorectum lymphovascular fatty tissue (mesorectum), by a precise, sharp dissection along the visceral pelvis fasciation (Holy plane - Heald introduced the term “holy plane” to indicate an adjustable anatomic dissection plan) to minimize residual tumor (5).
The main goal of TME is to remove the rectal tumor with the pararectal lymph nodes, which are the first area of lymph drainage for tumor cells, and preservation of structures outside the rectal fasciation, particularly nerve fibers that supply the urinary bladder, prostate and vagina.
TME is a difficult surgery due to the complicated anatomy with multiple areas of surgical dissection in the narrow pelvis space.
Anatomically three space scan be distinguished around the rectum.
The inner space is surrounded by a visceral fascia on the posterior side, and Denonvillier’s fascia on the front of the rectum. These fascias are united on both lateral sides, at the site where the nerve plexus is located.
Intermediate space is limited by the parietal pelvis fascia on the posterior side and the internal iliac arteries and their branches on both lateral sides, and on the front. The outer space is localized outside the internal iliac arteries and their branches.
The mesorectum corresponds to the inner space and the holy plane with the visceral fascia. Thus, total mesorectum excision means removing the internal space with the visceral fasciation and Denon-Villiers fascia whilst preserving the pelvis nerve plexus on both lateral sides (Figure 1).
Figure 1. Lines of mesorectum excision.
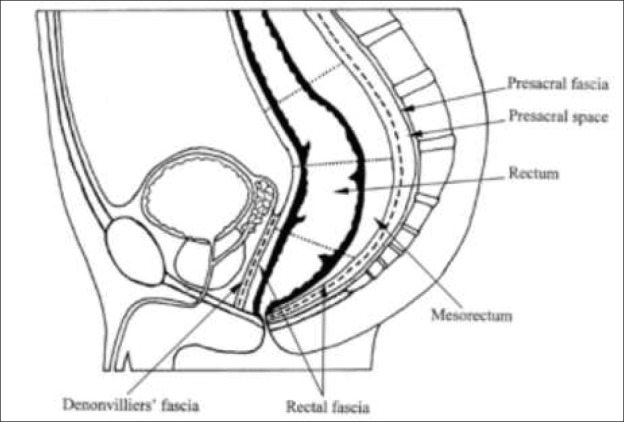
The key concept of modern rectal surgery is to remain on the mesorectum fascia. The posterior surgical plan lies between the fascias of the mesorectum (visceral fascias) and the transverse fascias (partial fascias) covering the sacrum, the coccigeum, the central sacral artery and the transverse vein (Figure 2). This potential space between the visceral fascia covering the mesorectum and the parietal fascias (endopelvic fascias) is the area, relatively speaking without blood vessels (Figure 2) of the TME dissection (holy plane).Inferior to this, at level S4, the visceral and parietal fascias are condensed and form a rectosacral fascia (Waldayers), which presents a thick fascial reflexion that runs anterior and inferior to the transverse fascias. The result of this fascial order is a relatively avascular tissue region between mesorectum fascias and parietal pelvis fascias.
Figure 2. Posterior dissection plan.
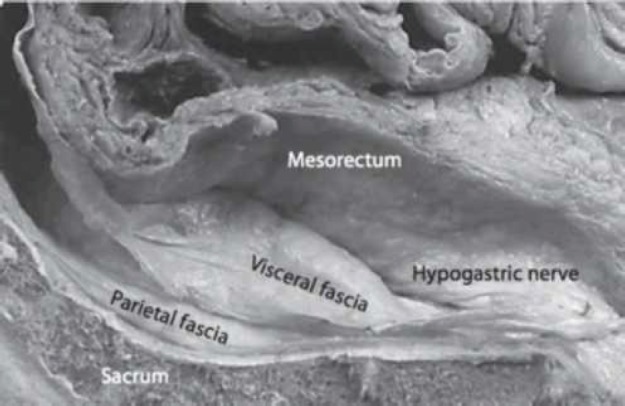
Posterior to the “holy plane” of rectal surgery is the presacral vein plexus, a structure with the risk of being damaged during surgical procedures.
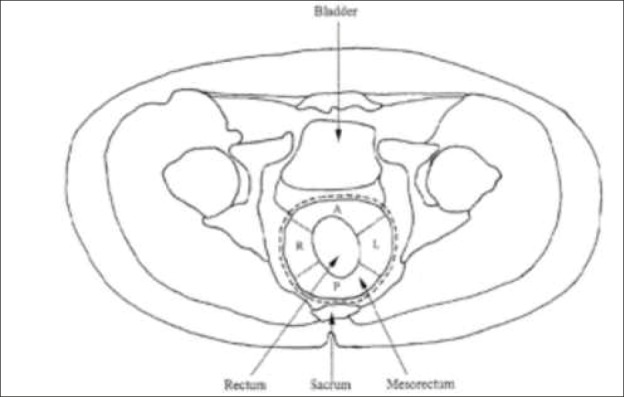
Distal condensation of the mesothelial fascias forms the lateral ligament of the rectum, which may contain branches of the middle rectal artery. These ligaments attach the rectum to the lateral sides of the pelvis. Lymphatic flow from the rectum flows mainly in the interior space, along the art. mesenterica inferior, to its origin. Some lymph nodes, especially in the lower rectum, penetrate the intermediate and outer spaces, through the lateral ligaments and descend along the art. iliaca interna. These lymph nodes are called lateral lymph nodes. The essence of the TME hypothesis is that the lymph nodes are randomly distributed within the mesorectum and not all visible or palpable, and that most nodes (92%) are located in the proximal two-thirds of the posterior mesorectum.. Extramural deposits appear distally and anteriorly within the mesorectum fascia. The TME concept has been gradually changed in order to be defined as the complete removal of circumferential fat tissue around the rectum, with the preservation of the autonomic nerves.. TME is therefore often referred to as “circumferential surgery” (Figure 3). However, when the nerves are preserved, there is a risk that the tumor cells will remain around the nerve, especially in an advanced carcinoma, Dukec C, when the rate of local recurrence is high. The local recurrence rate is 3 to 33%in conventional surgery, while TME results indicate a recurrence rate of <10% (6). It is important to note that the excellent results of a 5% local recurrence rate without adjuvant therapy by Heald were not achieved most rectal surgeons. However, it has been shown that careful observance of this procedure significantly reduces the rate of local recurrence of rectal cancer.
Figure 3. TME is circumferential surgery.
3. THE PROXIMAL RESECTION LINE
Due to the relatively wide proximal resection boundary (the length of the resected colon is usually>15 cm, the proximal ligation of the mesenteric inferior artery), the proximal resection of the tumor line is extremely rare. The proximal resection line is determined by consideration of the flow, or the type of blood supply..
4. DISTAL RESECTION LINE
The distal resection line in rectal cancer is more critical, depending on the tumor localization (distance from the dentate line). We need to keeping mind three aspects of the spread: intramural, extramural, and lymphatic spread. Intramural extension of rectum cancer has been shown to be uncommon (7). In over 95% of cases, it is limited to about 1 to 2 cm distal from the Endoluminal visible tumor. The distal mural safe boundary in this range can be considered to be adequate in most cases (8).
For distal rectal carcinomas (<5 cm from the anal edge), the minimum distal bound length is 1 cm (9). But the edges of the resection should be measured on a fresh, fixed sample, as it shrinks up to 50% in formalin.
4. CIRCUMFERENTIAL RESECTION LINE
One important aspect of the growth of the rectal cancer is its tendency towards extramural spread into mesorectum lymphovascular fat tissue.
Therefore, the surgical excision of the mesorectum, with a sharp dissection in the plane, between the fascia propria and the transverse fascia, is indispensable. Radical removal of mesorectum tissue “en bloc” removes lymphatic, vascular, perineal tumor deposits. The significance of the en bloc removal of intact mesorectum has been confirmed by studies demonstrating tumor deposits separate from the primary tumor (10).
The CRM circumferential resection margin is the surgical area created by dissection during removal of the rectum from the surrounding tissue. It is a non peritonized, bare area of resection sample. The largest region is located posteriorly, and begins higher in relation to the front side, from the mesocolon of the sigmoidal column, and extends downward like a triangle increasing in size.
Below the peritoneal reflection, the triangle becomes the circumferential border, and spreads down to the bottom of the mesorectum, down to the skin.
CRM involvement is the most important factor for predicting the risk of local recurrence in patients with rectal cancer. A<2mm boundary between the tumor and the mesothelial fascia is considered positive, and is associated with a high rate of local recurrence.
Positive CRM is defined as a tumor extension (continuous or discontinuous) or the presence of positive lymph nodes <1 mm from the radial, non peritonealized soft tissue border (11–13). Patients who have an edge <1mm have an increased risk of distant metastases.
In patients with positive CRM, the rate of incomplete TME was 44%, while in patients with negative CRM, incomplete TME was only 11% (14). The percentage of local recurrences in CRM positive tumors was 22%, and only 5% in CRM negative cases (13). One of the major benefits of TME is the reduction in CRM, which is undoubtedly the main factor in reducing local recurrence. However, achieving CRM negative resection lines in most rectal tumors is technically challenging, especially for low carcinoma (6 cm from the anal edge). Involvement of the lateral margin is more likely to occur in advanced tumor stages, rather than in tumor-positive lymph nodes. Negative resection lines, unfortunately, do not depend on surgical technique alone, but also on the size of the tumor, and the tumor stage during the surgery. Therefore, adequate staging is mandatory
5. LEVEL OF VASCULAR PROXIMAL LIGATION
Proximal lymphovascular ligation at the source of the art. rectalis superior is adequate in most recurrent carcinomas. Adaptive lymphadenectomy is based on ligation of the major vascular branches. There is no proven advantage of high ligation of the art.mesenterica inferior at its origin. The available evidence suggests that in rectal carcinoma, without clinically suspicious lymph node involvement, the removal of lymph cortex up to the primary bloodstream origins is adequate. So, for rectal cancer this is the origin of the art. rectalis superior, just distal from the origin of the left colic artery.
In patients who have clinically suspected lymph node involvement, it is recommended to remove all suspicious nodes up to the source of art. mesenterica inferior.
High ligation of the inferior mesentery artery may be useful because it allows additional left-column mobility in the case of low colorectal anastomosis.
6. DISSECTION OF THE LATERAL LYMPH NODE
TME removes lymph nodes within the mesorectum en bloc. The incidence of lateral pelvis lymph node involvement, in patients with locally advanced carcinomas, is between 10 and 30%.
In Europe and North America, metastases in the lateral lymph nodes are considered distant metastases, and the introduction of neoadjuvant therapy leads to a local recurrence rate of less than 10%. In Asia, the involvement of lateral lymph nodes is considered to be the involvement of regional lymph nodes, and their dissection is standard. However, the role of this dissection is questionable. There is not enough evidence to support routine expanded lateral lymphadenectomy, as a supplement to mesorectum excision. Clinically suspect lymph nodes in the lateral walls of the pelvis should be removed, if it is technically feasible, or biopsied for staging purposes.
Coning refers to the tendency of the surgeon to dissect the rectum wall downward during distal dissection, resulting in the tapered appearance of the surgical resection sample. Surgeons should stay outside of the visceral mesothelial fascia.
7. MACROSCOPIC ASSESSMENT OF MESORECTAL EXCISION
In view of its circumferential appearance, the optimal/complete mesorectum excision is characterized by a good mesorectum mass, with a smooth, lipoma-like surface with few or minor defects/incisions (not deeper than 5mm). Suboptimal/nearly complete excision has a moderate mesorectum mass with a slightly irregular surface, boundary defects, and probably with a minor degree of taper. Incomplete mesorectum excision of poor quality is characterized by a small mesorectum mass with a highly irregular surface, large defects (>1 cm2), or deeper incisions down to the muscularis propria and/or a prominent taper (Table 1 and Figures 4, 5 and 6) (14, 15).
Table 1. Mesorectumexcision quality according to M.E.R.C.U.R.Y. criteria (16).
| Mesorectum excision quality according to M.E.R.C.U.R.Y. criteria | |||
|---|---|---|---|
| M.E.R.C.U.R.Y. I° | Complete | Mesorectum Defects Cone CRM |
Smooth, intact No less than 5 mm No cone Smooth, regular |
| M.E.R.C.U.R.Y. II° | Almost complete | Mesorectum Defects Cone CRM |
Moderate volume There is no visible muscularis propria Moderate irregular |
| M.E.R.C.U.R.Y. IIP | Incomplete | Mesorectum Defects Cone CRM |
Small volume Up to the muscularis propria Yes irregular |
Figure 4. Complete TME.
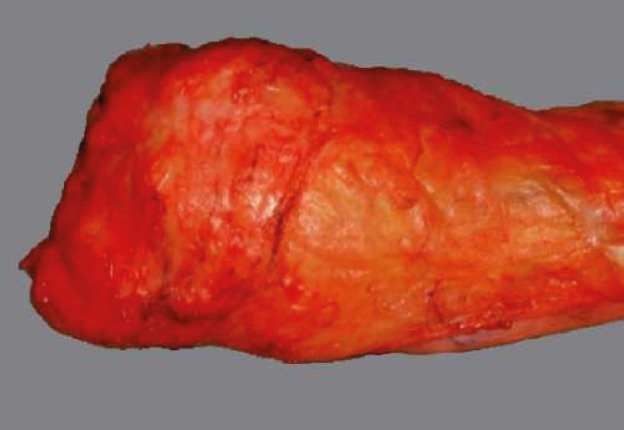
Figure 5. Almost complete TME.
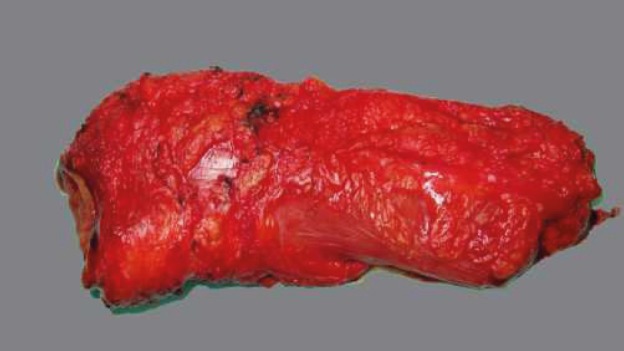
Figure 6. Incomplete TME.
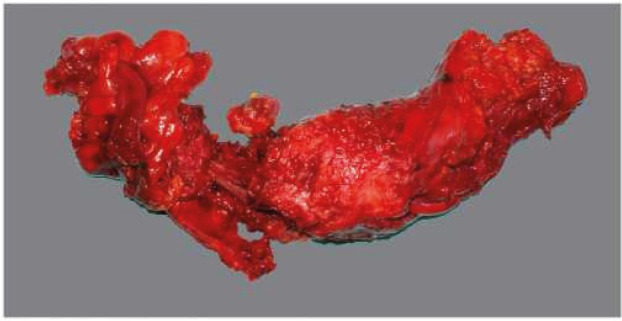
Only 50% of resected samples have optimal TME quality (14, 18) -year follow-up, overall recurrence in patients with complete or almost complete TME was 21.5% vs. 35.6% for patients with incomplete TME.
8. EN BLOC RESECTION OF ADHERENT TUMOR
En bloc resection with clean margins, including the neighboring organs affected by local invasion, can achieve a similar survival rate to that in patients without involvement of neighboring organs (19–21).
9. IMPORTANCE OF TME AND CRM COMPLETENESS
TME (which can be defined as complete excision of visceral mesorectum to the level of the levator, with the preservation of the pelvic nerves) is the gold standard for the treatment of rectal cancers and the middle lower third of the rectum. Defects in the mesorectum fascia on a tissue sample are associated with pelvic recurrence (22). However, when the edges were positive for a tumor, 78% of the patients had recurrence, compared to 10% of those whose edges were negative (23). Of patients with positive edges, 40% developed distant metastases, compared to 12% of those with negative edges (13).
TME can eradicate lymphatic spread in high-grade carcinomas (more than 5 cm above the dentate line), but it cannot achieve complete removal of the lymphatic spread of lower rectal neoplasms (less than 5 cm from the dentate line).For low-located carcinomas (below 5 cm), the incidence of lateral nodal involvement is 16.21%. In such patients, lateral node dissection can result in a 5-year survival of 42.4%. Some surgeons also believe that TME should be performed in cases of rectal tumors of the upper third, since the lymph nodes need to be removed below the tumor level. This is not a viable approach, because many surgeons emphasize that adequate mesorectum excision also presupposes enough mesorectum for adequate rectal function. Thus, in all upper and most medial rectal tumors, adequate rectal and mesorectum tissue is easily adequately preserved.
This technique can be called wide (Wide - WME) mesorectum excision, which keeps the distal rectum and improves the postoperative anal function. Thus, the rule is that TME for all tumors is 8 cm or less from the anterior anal edge, for tumors above 8 cm, WME should be performed (24). TME is probably not necessary for the upper third of the rectum, since pathological examinations of mesorectum samples have not shown metastases in the lymph nodes nor tumor deposits in the mesorectum more than 5 cm below the lower mural margin of the tumor. Therefore, subtotal ME 5 cm from the distal is probably sufficient to remove all rectal lymph nodes that potentially contain metastases.
For tumors in the upper third of the rectum a “Partial mesorectum excision” may be performed. In this case, the mesorectum dissection is performed 5 cm distal from the lower edge of the tumor, in a plane of 90% on the rectal wall, with a sharp mesorectum dissection (as opposed to the conventional blunt digital dissection previously performed in the case of frontal resection). Heald reported a 5-year local recurrence of 2.7% and survival of 87% when using TME (25). MCAnena and Heald then cited 3.5% recurrence rate and 5-year survival of 81% (26). MacFarlane, in an external review of Heald, cites a 4% recurrence rate after the curative TME (27).
It is particularly vital to emphasize the importance of preoperative irradiation. The Swedish Rectal group reported that the local recurrence rate was 11% with preoperative radiation, compared to 27% with conventional surgical procedure (28) and the Dutch Colorectal Canicus Group reported that local recurrence is 2.4% for preoperative irradiation + TME, and 8.2% for TME alone (29).
10. PRESERVATION OF THE ANAL SPHINCTER AND UROGENITAL FUNCTIONS WITH TME
After the introduction of TME in Sweden, there was a significant reduction in abdominoperineal resection, from 60% to 27% (30). Since most rectal cancers do not spread out of the mesorectum at the time of surgery, a nerve preserving technique is part of the TME and it improves the functional outcome (31).
11. TME COMPLICATIONS
TME is associated with an increased risk of dehiscence of the anastomosis and problematic anorectal dysfunction. The structure of the lower anastomosis on the exposed rectal remains on the anorectal joint is associated with increased anastomotic leakage.
Dehiscence after TME ranges from 15-20% compared to 5% or less for the round and intraperitoneal rectal anastomoses. The risk factors for leakage of low anastomoses are male sex, preoperative irradiation. It seems reasonable to consider a protective stoma in all cases where the anastomosis is lower than 6 cm (32).
Table 2. The quality of mesorectum excision according to Procare criteria (17).
| The quality of mesorectum excision in TME samples proposed by the PROCARE guide. Samplesand whole (fresh) and transverse cross-sections (after fixation) should be examined to adequately evaluate mesorectum excision. | |
| Smooth, regular | Intact mesorectum with only minor irregularities on a smooth mesorectum surface No defects deeper than 5 mm There is no cone at the distal margin of the sample Smooth circumferential resection limit on the incision |
| Slightly irregular | Moderate volume of mesorectum, but irregularity of the mesorectumsurface Moderate coning of the sample Muscular propira is not visible atall places, with the exception of the levator insertion |
| Very irregular | A small volume of mesorectum with defects down to muscularis propria and/or very irregular circumferentialresection margin on the incision |
Conflict of interest
none declared.
REFERENCES
- 1.Hill GL, Rafique M. Extrafascial excision of the rectum for rectal cancer. Br J Surg. 1998;85:809–12. doi: 10.1046/j.1365-2168.1998.00735.x. [DOI] [PubMed] [Google Scholar]
- 2.Ross A, Rusnak C, Weinerman B, et al. Recurrence and survival after surgical management of rectal cancer. am J Surg. 1999;177:392–5. doi: 10.1016/s0002-9610(99)00080-x. [DOI] [PubMed] [Google Scholar]
- 3.Bjerkeset T, Edna TH. Rectal cancer: the influence of type of operation on local recurrence and survival. Eur J Surg. 1996;162(8):643–8. [PubMed] [Google Scholar]
- 4.Topor B, Acland R, Kolodko V, Galandiuk S. Mesorectal lymph nodes: their location and distribution within the mesorectum. Dis Colon Rectum. 2003;46:779–85. doi: 10.1007/s10350-004-6656-4. [DOI] [PubMed] [Google Scholar]
- 5.Heald RJ. The ‘Holy Plane’ of rectal surgery. J R Soc Med. 1988;81(9):503–8. doi: 10.1177/014107688808100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecil TD, Sexton R, Moran BJ, Heald RJ. Total mesorectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis Colon Rectum. 2004;47:1145–9. doi: 10.1007/s10350-004-0086-6. [DOI] [PubMed] [Google Scholar]
- 7.Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76:388–92. doi: 10.1002/1097-0142(19950801)76:3<388::aid-cncr2820760307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Maurer CA, Renzulli P, Meyer JD, Buchler MW. Rectal carcinoma. Optimizing therapy by partial or total mesorectum removal] Zentralbl Chir. 1999;124:428–35. (in German) [PubMed] [Google Scholar]
- 9.Shirouzu K, Ogata Y, Araki Y. Oncologic and functional results of total mesorectum excision and autonomic nerve-preserving operation for advanced lower rectal cancer. Dis Colon Rectum. 2004;47:1442–7. doi: 10.1007/s10350-004-0618-8. [DOI] [PubMed] [Google Scholar]
- 10.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? Br J Surg. 1982;69:613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 11.Quirke P, Durdey P, Dixon MF, Williams NS. Local re-currence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;2:996–9. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 12.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 13.Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, et al. Prognostic significance of the circumferential resection margin following total mesorectum ex-cision for rectal cancer. Br J Surg. 2002;89:327–34. doi: 10.1046/j.0007-1323.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- 14.Nagtegaal ID, van de velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection spec-imen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–34. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Hermanek P, Hohenberger W, Klimpfinger M, et al. The pathological assessment of mesorectum excision: implications for further treatment and quality management. Int J colorectal Dis. 2003;18:335–41. doi: 10.1007/s00384-002-0468-6. [DOI] [PubMed] [Google Scholar]
- 16.M.E.R.C.U.R.Y. (Magnetic Resonance Imaging and Rectal cancer European Equivalence Study) Hampshire, uk: Basingstoke; 2002. Studycoordina-tor Daniels, I., Pelican centre, North Hampshire Hospital. http://www.pelican-cancer.org/researchprojects. [Google Scholar]
- 17.PROCARE: multidisciplinary Belgian PROject on CAncer of the Rectum. Multidisciplinary guidelines for the treatment of rectal cancer. www.kankerregister.be (menu: procare) or www.registreducancer.be (menu: procare)
- 18.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 19.Bonfanti G, Bozzetti F, Doci R, Baticci F, Marolda R, Bignami P, Gennari L. Results of extended surgery for cancer of the rectum and sigmoid. Br J Surg. 1982;69:305–7. doi: 10.1002/bjs.1800690603. [DOI] [PubMed] [Google Scholar]
- 20.Sugarbaker PH, Corlew S. Influence of surgical techniques on survival in patients with colorectal cancer. Dis Colon Rectum. 1982;25:545–57. doi: 10.1007/BF02564164. [DOI] [PubMed] [Google Scholar]
- 21.Talamonti MS, Shumate CR, Carlson GW, Curley SA. Locally advanced carcinoma of the colon and rectum in-volving the urinary bladder. Surg Gynecol Obstet. 1993 Nov;177(5):481–7. [PubMed] [Google Scholar]
- 22.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–9. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 23.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin in-volvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 24.Seow-Choen F. Wide or total mesorectum excision. 2011.
- 25.Heald RJ, Ryall RD. Recurrence and survival after total mesorectum excision for rectal cancer. Lancet. 1986;1:1479–82. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 26.McAnena OJ, Heald RJ, Lockhart-Mummery HE. Operative and functional results of total mesorectum excision with ultra-low anterior resection in the management of carcinoma of the lower one-third of the rectum. Surg Gynecol Obstet. 1990;170:517–21. [PubMed] [Google Scholar]
- 27.Mcforlane JK, Ryall RD, Heald RJ. Mesorectal excision of rectal cancer. Lancet. 1993;341:457–60. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 28.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–7. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 29.Dutch Colorectal Cancer Group. 2001.
- 30.Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet. 2000;356:93–6. doi: 10.1016/s0140-6736(00)02469-7. [DOI] [PubMed] [Google Scholar]
- 31.Enker WE. Potency, cure, and local control in the operative treatment of rectal cancer. Arch Surg. 1992;127:1396–1401. doi: 10.1001/archsurg.1992.01420120030005. [DOI] [PubMed] [Google Scholar]
- 32.Karanjia ND, Corder AP, Bearn P, Heald RJ. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg. 1994;81:1224–6. doi: 10.1002/bjs.1800810850. [DOI] [PubMed] [Google Scholar]


