Abstract
The Ca2+/calmodulin-dependent protein phosphatase calcineurin orchestrates sexual reproduction, stress responses, and virulence via branched downstream pathways in the opportunistic human fungal pathogen Cryptococcus neoformans. The calcineurin-binding protein Cbp1, the calcineurin temperature suppressor Cts1, the calcineurin-responsive zinc finger transcription factor Crz1, and the calcineurin targets Pbp1, Tif3, and Puf4, all function downstream of calcineurin to orchestrate distinct cellular processes. To elucidate how the calcineurin pathway regulatory network governs unisexual reproduction, stress responses, and virulence, we have analyzed the self-filamentous C. deneoformans strain, XL280α, and generated double mutants of these calcineurin downstream genes. We demonstrated that calcineurin governs unisexual reproduction at different sexual developmental stages, in which the initiation of the yeast–hyphal morphological transition is independent of Crz1, whereas the sporulation process is dependent on Crz1. Calcineurin-dependent unisexual reproduction is independent of the pheromone response pathway. Crz1 synergistically interacts with different calcineurin downstream targets in responding to ER, high-calcium, and cell wall stresses. We observed a widespread synergy suggesting that these proteins function in complex branched pathways downstream of calcineurin with some functional redundancy, which may allow efficient signaling network rewiring within the pathway for prompt adaptation to changing environments. Finally, we showed that deletion of PBP1 or TIF3 in the cna1∆ mutant background conferred a modest level of growth tolerance at 37°, but that the cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants were both avirulent, suggesting that calcineurin may control virulence via mechanisms beyond thermotolerance.
Keywords: calcineurin, Cryptococcus deneoformans, genetic interactions, unisexual reproduction, stress response, virulence
CALCINEURIN, the Ca2+/calmodulin-dependent protein phosphatase, is conserved from fungi to humans, and it plays central functions in governing key cellular processes (Cyert et al. 1991; Liu et al. 1991b; Sugiura et al. 2002; Aramburu et al. 2004). The phosphatase is composed of two subunits: a catalytic subunit, Cna1, and a regulatory subunit, Cnb1 (Klee et al. 1979). Calcineurin dephosphorylates and activates transcription factors, NF-AT in mammalian systems, and Crz1, the calcineurin-responsive zinc finger transcription factor 1, in fungi, which results in the translocation of NF-AT and Crz1 into the nucleus (Flanagan et al. 1991; Northrop et al. 1994; Beals et al. 1997; Stathopoulos-Gerontides et al. 1999; Park et al. 2016; Chow et al. 2017). Activation of NF-AT is crucial for skeletal muscle development, immune response, cardiac function, and many other important developmental processes, and dysregulation of the calcineurin pathway has been implicated in many human developmental and neurodegenerative diseases (Clipstone and Crabtree 1992; Steinbach et al. 2007; Al-Shanti and Stewart 2009; Furman and Norris 2014; Kipanyula et al. 2016; Dewenter et al. 2017). Calcineurin phosphatase activity is inhibited by two immunosuppressive drugs, cyclosporin A (CsA) and FK506, which target cyclophilin A and FKBP12, respectively (Stewart et al. 1982; Liu et al. 1991a; Blankenship et al. 2003a).
In many human fungal pathogens, calcineurin orchestrates fungal morphogenesis, stress responses, and virulence (Fox and Heitman 2002; Steinbach et al. 2007). In ascomycetes, calcineurin controls hyphal growth, drug tolerance, and virulence in multiple Candida species, including Candida albicans, Ca. glabrata, Ca. lusitaniae, Ca. tropicalis, and Ca. dubliniensis (Bader et al. 2003; Blankenship et al. 2003b; Sanglard et al. 2003; Chen et al. 2011, 2012, 2014; Zhang et al. 2012). In Ca. albicans, calcineurin is essential for survival during membrane stress and in serum, which promotes virulence (Cruz et al. 2002; Blankenship et al. 2003b; Blankenship and Heitman 2005). In another ascomycete, Aspergillus fumigatus, calcineurin is required for hyphal maturation and pathogenicity (Steinbach et al. 2006; Juvvadi et al. 2011, 2014). In the zygomycete, Mucor circinelloides, calcineurin is crucial for its dimorphic transition and virulence (Lee et al. 2013, 2015). The involvement of calcineurin in fungal virulence renders it a prominent drug target for antifungal treatments (Steinbach et al. 2007; Coelho and Casadevall 2016). Several studies have shown synergy between calcineurin inhibitors and other antifungal drugs, which provided new frontiers for novel antifungal therapies (Marchetti et al. 2000; Steinbach et al. 2004; Cowen and Lindquist 2005).
The basidiomycetous Cryptococcus species can cause life-threatening meningoencephalitis in immunocompromised patients, which contributes to almost 200,000 annual mortalities around the world (Park et al. 2009; Rajasingham et al. 2017). Calcineurin regulates Cryptococcus sexual reproduction, stress responses, and pathogenicity (Kozubowski et al. 2009). In the Cryptococcus species complex, the sister species Cryptococcus neoformans and C. deneoformans can undergo bisexual reproduction between MATa and MATα cells (Kwon-Chung 1976; Nielsen et al. 2003), while C. deneoformans also undergoes robust unisexual reproduction in the absence of an opposite mating type partner (Lin et al. 2005). During Cryptococcus sexual reproduction, yeast cells undergo a morphological transition to a hyphal state, and this process is regulated by the pheromone response pathway and the transcription factors Mat2 and Znf2 (Wang et al. 2012; Feretzaki and Heitman 2013). In the absence of CNA1 or CNB1, hyphal production is blocked during both unisexual and bisexual reproduction (Cruz et al. 2001), and this calcineurin-dependent filamentation is independent of the pheromone response pathway under some conditions (Gyawali et al. 2017). A multicopy suppressor screen identified a calcineurin temperature suppressor Cts1, which is also required for filamentation during Crytopcoccus sexual reproduction (Fox et al. 2003), and a yeast two-hybrid screen identified a calcineurin-binding protein Cbp1/Calcipressin, which is only required for filamentation during bisexual but not unisexual reproduction (Görlach et al. 2000; Fox and Heitman 2005). In a phosphoproteomic study, Park et al. (2016) identified 44 putative calcineurin targets and demonstrated that calcineurin regulates bisexual reproduction in C. neoformans through three Cna1 downstream targets: the poly(A)-binding protein-binding protein Pbp1 and the translation initiation factor 4B Tif3, which are both required for hyphal production, and the pumillio-family RNA-binding protein Puf4, which negatively regulates hyphal production (Park et al. 2016).
Growth at host temperature is a prerequisite for fungal virulence (Fox and Heitman 2002). In Cryptococcus, calcineurin is essential for growth at 37° and virulence in mice (Odom et al. 1997; Fox et al. 2001). The temperature-sensitive phenotype exhibited by the cna1∆ mutant is suppressed by a high copy number of the CTS1 gene (Fox et al. 2003). Cts1 is dephosphorylated by calcineurin at 37°, suggesting that Cts1 functions as an effector for the calcineurin pathway during high-temperature stress (Aboobakar et al. 2011). Cts1 is also required for high-temperature tolerance and virulence in Cryptococcus (Fox et al. 2003). The calcineurin-binding protein Cbp1 is not required for calcineurin-dependent high-temperature growth, and deletion of CBP1 only attenuates but does not abolish virulence (Görlach et al. 2000). Based on the phenotypes of cna1∆, cts1∆, and cbp1∆ mutants, virulence is correlated with the ability to grow at 37°. On the contrary, the calcineurin downstream targets Puf4 and Pbp1 regulate thermosensitivity and virulence in an opposite fashion in C. neoformans (Glazier et al. 2015; Park et al. 2016). Puf4 is required for high-temperature stress tolerance but not required for virulence (Glazier et al. 2015). Whereas deletion of PBP1 confers high-temperature resistance, the virulence of pbp1∆ mutants is, however, attenuated (Park et al. 2016). This opposite correlation between virulence and thermosensitivity in puf4 and pbp1 mutants suggests that virulence is a complex trait, impacted by many factors in addition to thermotolerance to host temperature.
In Saccharomyces cerevisiae and Ca. albicans, calcineurin orchestrates stress responses via the calcineurin-responsive zinc finger transcription factor 1 (Crz1) (Stathopoulos and Cyert 1997; Yoshimoto et al. 2002; Onyewu et al. 2004; Santos and de Larrinoa 2005; Karababa et al. 2006). A Crz1 ortholog identified in C. neoformans regulates cell wall integrity (Lev et al. 2012; Moranova et al. 2014), and transcriptional profiling suggests the calcineurin-Crz1 regulatory network has been extensively rewired in C. neoformans compared to S. cerevisiae (Chow et al. 2017). Deletion of CRZ1 does not impact bisexual reproduction in C. neoformans, suggesting that calcineurin regulates mating through a Crz1-independent pathway (Park et al. 2016; Chow et al. 2017). crz1∆ mutants display similar but less-severe stress response phenotypes compared to cna1∆ mutants, and the virulence of crz1∆ mutants is only attenuated rather than abolished in contrast to cna1∆ mutants, indicating that Crz1 functions in a branched pathway downstream of calcineurin (Chow et al. 2017). Similarly, in Ca. albicans, Crz1 is involved in azole tolerance but not required for virulence (Onyewu et al. 2004), and in A. fumigatus, the Crz1 ortholog CrzA functions downstream of calcineurin and plays contributory but not essential roles in regulating hyphal growth, conidial germination, and virulence compared to calcineurin (Cramer et al. 2008). Thus, it is a common theme in fungal pathogens that calcineurin regulates morphogenesis, stress tolerance, and virulence through branched downstream pathways.
To better understand how the calcineurin pathway regulates sexual reproduction, stress responses, and virulence, we conducted genetic analyses in the self-filamentous C. deneoformans strain XL280α. We found that the yeast–hyphal morphological transition during unisexual reproduction is independent of Crz1, but that the sporulation process is dependent upon Crz1. We demonstrated that the calcineurin downstream targets synergistically interact with each other in regulating stress responses, and functional redundancy among these genes enables plasticity in the calcineurin pathway and allows rapid signaling network rewiring during evolution. Finally, we showed that high-temperature-tolerant cna1∆ pbp1∆ and cna1∆ tif3∆ mutants are avirulent, revealing that conferring thermotolerance to host temperature is not the only way in which the calcineurin pathway controls virulence.
Materials and Methods
Strains, media, and growth conditions
Strains used in this study are listed in Supplemental Material, Table S1. All gene deletion mutants were generated in the congenic strain pair MATα XL280 and MATa XL280 backgrounds (Lin et al. 2005; Zhai et al. 2013). The congenic strain pair MATα JEC21 and MATa JEC20 was used for wild-type bisexual mating (Kwon-Chung et al. 1992). Yeast cells were grown at 30° on Yeast extract Peptone Dextrose (YPD) medium. Strains harboring dominant selectable markers were grown on YPD medium supplemented with nourseothricin (NAT) or G418 (NEO) for selection. Mating assays were performed on either 5% V8 juice agar medium (pH 7.0) or Murashige and Skoog (MS) medium without sucrose (Sigma [Sigma Chemical], St. Louis, MO) in the dark at room temperature for the designated time period.
Bioinformatics
BLASTP searches using C. neoformans Cna1, Cnb1, Cbp1, Cts1, Crz1, Pbp1, Tif3, and Puf4 protein sequences against the C. deneoformans JEC21 genome on FungiDB (www.fungidb.org) identified the calcineurin pathway orthologous genes CNJ02230 (CNA1), CND00260 (CNB1), CNA07790 (CBP1), CNG01630 (CTS1), CNA01450 (CRZ1), CNE04890 (PBP1), CNK00210 (TIF3), and CNC04280 (PUF4) (Stajich et al. 2012; Park et al. 2016). To test whether the putative Cna1-targeted dephosphorylation sites in Pbp1, Tif3, and Puf4 are conserved between C. neoformans and C. deneoformans, protein sequences between these two species were aligned using Clustal ω (Sievers et al. 2011).
Gene disruption and generation of double mutants
Table S2 lists the primers used in this study. To generate deletion mutants for the genes of interest, deletion constructs consisting of the 5′ and 3′ regions of the targeted genes flanking an appropriate selection marker (NAT or NEO cassette) were generated by overlap PCR as previously described (Davidson et al. 2002). The deletion constructs were introduced into the congenic strain pair XL280α and XL280a via biolistic transformation, as previously described (Toffaletti et al. 1993). Transformants were selected on YPD medium supplemented with NAT (100 mg/liter) or NEO (200 mg/liter). Gene replacements by homologous recombination were confirmed by PCR. Two independent deletion mutants were obtained for CNA1 (CF637 and CF645), CNB1 (CF1095 and CF1099), CBP1 (CF799 and CF802), CTS1 (CF787 and CF794), and CRZ1 (CF684 and CF688). Only one gene deletion mutant was obtained for PBP1 (CF1100), TIF3 (CF1113), and PUF4 (CF1112). Additional deletion mutants were obtained for PBP1 (CF1123 and CF1126), TIF3 (CF1219 and CF1220), and PUF4 (CF1194 and CF1195) by microscopically dissecting meiotic spores from bisexual crosses between, respectively, CF1100, CF1113, or CF1112 and the XL280 strain of the opposite mating type. A MATa crz1∆ mutant (CF863) was obtained from mating cross between strains CF688 and XL280a.
Double mutants were obtained from mating crosses between two single mutants of opposite mating types. Spore progeny of each mating cross were isolated by microscopic manipulation using a fiber optic needle spore dissection system, as previously described (Idnurm 2010). Gene deletions of the double mutants were verified by PCR. The crz1∆ cbp1∆ (CF1025 and CF1026), crz1∆ cts1∆ (CF998 and CF1001), and crz1∆ puf4∆ (CF1200 and CF1203) double mutants were generated from mating crosses of CF863 with CF802, CF787, or CF1112, respectively. The crz1∆ pbp1∆ (CF1143 and CF1145) and crz1∆ tif3∆ (CF1231 and CF1239) double mutants were generated from mating crosses of CF688 with CF1100 or CF1113, respectively. The cna1∆ pbp1∆ (CF1288 and CF1289) and cna1∆ tif3∆ (CF1252 and CF1256) double mutants were generated from mating crosses of CF637 with CF1100 or CF1113, respectively. The cbp1∆ pbp1∆ (CF1260 and CF1268) double mutants were generated from mating crosses of CF802 with CF1100.
Phenotypic assays
To test whether the constructed deletion mutants undergo unisexual reproduction, strains were grown overnight in liquid YPD medium at 30°, cells were washed twice with ddH2O, and then diluted to a final density of OD600 = 2. For each strain, 10 μl of the diluted culture was inoculated onto MS and V8 medium, and incubated at room temperature in the dark for 5 and 14 days. To test whether the deletion mutants undergo pheromone-independent unisexual reproduction, cells were inoculated onto V8 medium supplemented with 200 µM CuSO4. Hyphal growth on the edge of the mating patches, basidia, and spore chains were captured by using a Nikon (Garden City, NY) Eclipse E400 microscope equipped with a Nikon DXM1200F camera.
For spotting assays, strains were grown overnight in liquid YPD medium at 30°, and cells were washed twice with ddH2O and diluted to a final density of OD600 = 0.8. Five 10-fold serial dilutions for each strain were made and spot inoculated onto solid media. To test cell viability of the deletion mutants at high temperature, 5 μl of 10-fold serial dilutions were spotted on YPD medium and incubated at different temperatures (30, 37, or 39°) for 2–3 days. To test stress-related phenotypes, 3–5 μl of 10-fold serial dilutions were spotted on YPD medium supplemented with 20 mM DTT (Sigma), 0.35 M CaCl2, 0.5% calcofluor white (CFW) (Sigma), or 1% Congo red (CR) (Sigma), and incubated at 30° for 2–3 days. crz1∆ cbp1∆, crz1∆ pbp1∆, and cbp1∆ pbp1∆ double mutants were also tested on YPD medium supplemented with 0.75 and 1% CFW, and 1.5 and 2% CR. To test cell susceptibility to the calcineurin inhibitor FK506 at different temperatures, 5 μl of 10-fold serial dilutions were spotted on YPD medium supplemented with 5 ng/ml FK506 (Astella Pharma), and incubated at a range of temperatures (17, 25, 30, 35, 36, or 37°) for 2–3 days.
RNA extraction and real-time PCR
For unisexual reproduction, wild-type XL280α and the deletion mutants (cna1∆, cnb1∆, cbp1∆, cts1∆, and crz1∆) were grown overnight in liquid YPD medium. Cells were washed twice with ddH2O and diluted to OD600 = 2, and 250 μl of the diluted cells were spotted on V8 or YPD agar medium. For bisexual reproduction, wild-type JEC20a and JEC21α were prepared the same way as described above, and then 250 μl of an equal-volume-mixture of cells were spotted on V8 or YPD agar medium. Mating patches were harvested after incubation at room temperature in the dark for 36 hr, and flash frozen in liquid nitrogen. RNA was extracted using TRIzol reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. RNA was treated with Turbo DNAse (Ambion), and single-stranded cDNA was synthesized by AffinityScript Reverse Transcriptase (RT)-RNAse (Stratagene, La Jolla, CA). For each sample, cDNA synthesized without the RT-RNAse block enzyme mixture was used as a “no RT control” to exclude genomic DNA contamination. The relative expression level of target genes was measured by quantitative real-time PCR using Brilliant III ultrafast SYBR green QPCR mix (Stratagene) in an Applied Biosystems 7500 real-time PCR System. For each target, a no template control was performed to analyze melting curves to exclude primer artifacts. Technical triplicates and biological triplicates were performed for each sample. Gene expression levels were normalized using the endogenous reference gene GPD1 and determined by using the comparative ∆∆Ct method. The primers used for real-time PCR are listed in Table S2. The Student’s t-test was used to determine if the relative gene expression levels between different strains exhibited statistically significant differences (P < 0.05).
Virulence studies
An intranasal inhalation mouse model of systemic cryptococcosis was used, as described previously, to study the virulence of pbp1∆ and tif3∆ deletion mutants (Ni et al. 2013; Zhai et al. 2013). For each gene of interest, a wild-type XL280α, a cna1∆ mutant (CF637), tif3 or pbp1 single mutants (CF1219 and CF1220 for tif3∆, and CF1123 for pbp1∆), and two double mutants (CF1252 and CF1256 for cna1∆ tif3∆, and CF1288 and CF1289 for cna1∆ pbp1∆) were tested as a group. For the tif3∆ group, mice were sedated with Nembutal (sodium pentobarbital) (37.5 mg/kg). For the pbp1∆ group, mice were sedated with isoflurane, a less invasive inhalation anesthetic, in compliance with animal welfare protocols to limit the use of a controlled substance. For each strain, 9 or 10 8- to 10-week-old female A/J mice (Jackson Laboratory) were inoculated with 5 × 106 cells in 50 μl PBS. After infection, mice were weighed daily and monitored twice a day. Moribund mice were killed. On day 20, four or five mice per strain were killed and dissected to study the fungal burden in the lung and the brain, and the remaining five mice for each strain were monitored for up to 60 days for survival studies. For the PBP1 group, mice that had survived cryptococcosis when inoculated with the cna1∆ mutant (CF637), the pbp1∆ mutant (CF1123), and the two cna1∆ pbp1∆ mutants (CF1288 and CF1289) were dissected at the end of the experiment for fungal burden studies. The dissected organ tissues were homogenized in 2 ml PBS, serially diluted, and plated on YPD agar medium supplemented with 50 μg/ml ampicillin and 30 μg/ml chloramphenicol. After incubation at 30° for 2–3 days, colonies were counted to calculate CFUs for each organ.
Statistical analysis
Survival curves were plotted according to the Kaplan–Meier method, and statistical significance between two survival curves was assessed with the log-rank test, with a P-value < 0.05 considered statistically significant. Welch’s t-test was used to determine if the fungal burden between different strains exhibited statistically significant differences (P < 0.05). All statistical analyses were performed using the Graphpad Prism 7 program.
Ethics statement
All experiments and animal care were conducted in accordance with the ethical guidelines of the Institutional Animal Care and Use Committee (IACUC) of Duke University Medical Center (DUMC). The DUMC IACUC approved all of the vertebrate studies under protocol number A245-13-09. Mice studies were conducted in the Division of Laboratory Animal Resources facilities, which are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Data availability
Strains are available upon request. Table S1 lists all of the strains and their genotypes, and Table S2 lists all of the primer sequences used in this study.
Results
Calcineurin regulates unisexual reproduction independent of the transcription factor Crz1
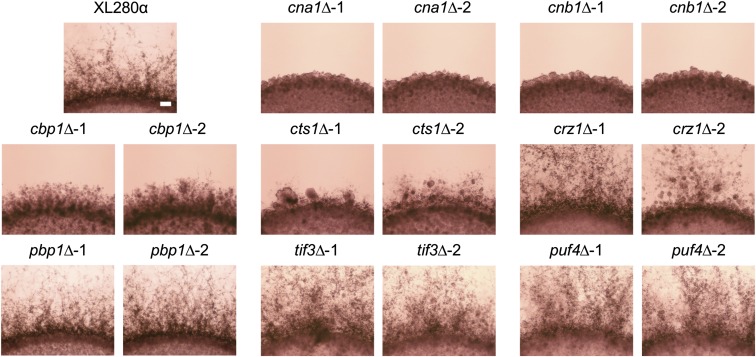
To test how the calcineurin pathway governs unisexual reproduction, we generated deletion mutants in the self-filamentous strain XL280α for eight genes (CNA1, CNB1, CBP1, CTS1, CRZ1, PBP1, TIF3, and PUF4) in the calcineurin pathway. These genes have been previously shown to regulate bisexual reproduction in C. neoformans and C. deneoformans (Cruz et al. 2001; Fox et al. 2003; Fox and Heitman 2005; Park et al. 2016; Chow et al. 2017). The calcineurin-dependent dephosphorylation sites in the three calcineurin targets Pbp1, Tif3, and Puf4 are conserved between C. neoformans and C. deneoformans (Figure S1). Deletion of the CNA1 or CNB1 gene completely abolished filamentation during early mating, and deletion of CBP1 or CTS1 delayed filamentation, while deletion of CRZ1, PBP1, TIF3, or PUF4 did not impact filamentation (Figure 1 and Figure S2). The unisexual mating phenotypes were consistent for the deletion mutants on both MS and V8 medium, with the exception of puf4∆ mutants, which exhibited a wild-type filamentation phenotype on MS medium but reduced filamentation on V8 medium (Figure 1 and Figure S3A). Under pheromone-independent unisexual mating-inducing conditions (Gyawali et al. 2017), cna1∆, cnb1∆, cbp1∆, cts1∆, and puf4∆ mutants displayed similar filamentation defects on V8 medium in the presence of 200 µM Cu2+ (Figure S3B). Although cna1∆ and cnb1∆ mutants eventually produced rare, short, blunted hyphae along the edges of the mating patch, these hyphae failed to form long mature hyphae and basidia (Figure S4). pbp1∆ and tif3∆ mutants produced basidia with four chains of spores similar to wild-type, while all other single mutants produced basidia lacking the characteristic four spore-chain structures (Figure S4).
Figure 1.
Unisexual mating phenotypes of calcineurin pathway deletion mutants. A wild-type (XL280α) strain and two independent cna1∆ (CF637 and CF645), cnb1∆ (CF1095 and CF1099), cbp1∆ (CF799 and CF802), cts1∆ (CF787 and CF794), crz1∆ (CF684 and CF688), pbp1∆ (CF1123 and CF1126), tif3∆ (CF1219 and CF1220), and puf4∆ (CF1194 and CF1195) deletion mutants were inoculated onto Murashige and Skoog medium, and incubated in the dark at room temperature for 14 days. Bar represents 200 μm.
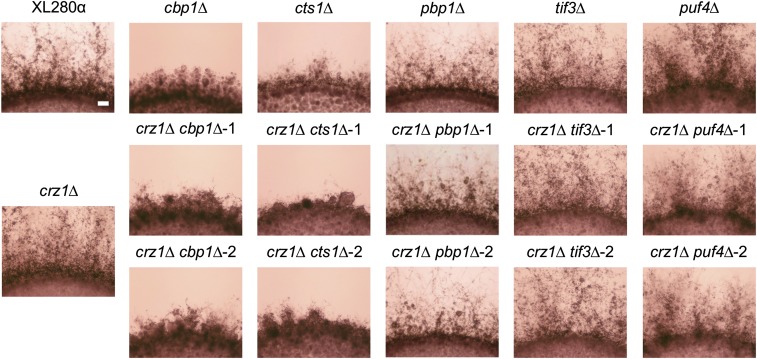
To test whether the transcription factor Crz1 is functionally redundant with other calcineurin target genes in governing unisexual reproduction, we generated double mutants of crz1 with each other calcineurin downstream gene by crossing the crz1∆ mutant with cbp1∆, cts1∆, pbp1∆, tif3∆, and puf4∆ mutants, individually. The double mutants exhibited the same mating phenotype during unisexual reproduction as the non-crz1 parental single mutants (Figure 2). All double mutants, including crz1∆ pbp1∆ and crz1∆ tif3∆ double mutants, produced bald basidia lacking spores, similar to the parental crz1∆ mutants (Figure S4). These results suggest that Crz1 is dispensable for filamentation but required for sporulation during unisexual reproduction.
Figure 2.
Unisexual mating phenotypes of double mutants of crz1 with calcineurin downstream target genes. A wild-type (XL280α) strain; crz1∆ (CF688), cbp1∆ (CF799), cts1∆ (CF787), pbp1∆ (CF1123), tif3∆ (CF1219), and puf4∆ (CF1194) single mutants; and crz1∆ cbp1∆ (CF1025 and CF1026), crz1∆ cts1∆ (CF998 and CF1001), crz1∆ pbp1∆ (CF1143 and CF1145), crz1∆ tif3∆ (CF1231 and CF1239), and crz1∆ puf4∆ (CF1200 and CF1203) double mutants were inoculated onto Murashige and Skoog medium, and incubated in the dark at room temperature for 14 days. Bar represents 200 μm.
Calcineurin regulates unisexual reproduction independent of the pheromone response pathway
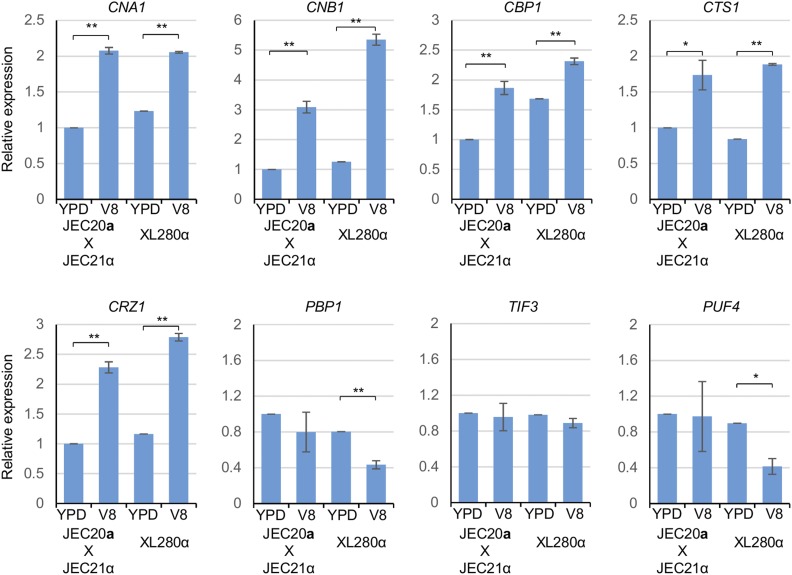
To further investigate the involvement of the calcineurin pathway in sexual reproduction, we performed real-time PCR to measure the expression levels of the genes encoding calcineurin and the calcineurin downstream components. During bisexual reproduction between JEC20a and JEC21α, expression levels of CNA1, CNB1, CBP1, CTS1, and CRZ1 increased significantly to approximately twice the expression level on nonmating-inducing YPD medium (2.1-fold for CNA1, P < 0.005; 3.1-fold for CNB1, P < 0.005; 1.9-fold for CBP1, P < 0.005; 1.7-fold for CTS1, P < 0.05; and 2.3-fold for CRZ1, P < 0.005) (Figure 3). PBP1, TIF3, and PUF4 were not upregulated during bisexual reproduction (Figure 3). During unisexual reproduction in XL280α, expression levels of CNA1, CNB1, CBP1, CTS1, and CRZ1 were also significantly increased, but at a range of 1.4–4.3-fold increase compared to the expression levels under nonmating-inducing conditions (1.7-fold for CNA1, P < 0.005; 4.3-fold change for CNB1, P < 0.005; 1.4-fold for CBP1, P < 0.005; 2.2-fold change for CTS1, P < 0.005; and 2.4-fold change for CRZ1, P < 0.005) (Figure 3). Interestingly, PBP1 and PUF4 showed no significant expression changes during bisexual reproduction, but were modestly downregulated during unisexual reproduction (1.9-fold decrease for PBP1 during unisex, P < 0.005, and 2.2-fold decrease for PUF4 during unisex, P < 0.05) (Figure 3). TIF3 was expressed at the same level during bisexual and unisexual reproduction compared to nonmating-inducing conditions (Figure 3).
Figure 3.
The calcineurin pathway is upregulated during sexual reproduction. Gene expression patterns for CNA1, CNB1, CBP1, CTS1, CRZ1, PBP1, TIF3, and PUF4 were examined by real-time PCR (* indicates P ;< 0.05 and ** indicates P < 0.005 for each pairwise comparison). A wild-type cross between JEC20a and JEC21α for bisexual reproduction and the wild-type strain XL280α for unisexual reproduction was grown on YPD and V8 agar medium for 36 hr. The expression levels of the cross between strains JEC20a and JEC21α on YPD medium were set to 1, and the remaining values were normalized to this. The error bars represent the SD of the mean for three biological replicates.
Because the pheromone response pathway regulates sexual reproduction in Cryptococcus, we examined whether the genes encoding pheromone (MFα), the pheromone receptor (STE3α), MAP kinase (CPK1), transcription factors (MAT2 and ZNF2), and a homeodomain protein (SXI1α) were misregulated in calcineurin pathway mutants. Under mating-inducing conditions, these genes were expressed at similar levels between wild-type and calcineurin pathway single mutants (cna1∆, cnb1∆, cbp1∆, and crz1∆), except in the cts1∆ mutants (Figure S5). MFα, STE3α, CPK1, and MAT2 were significantly upregulated in cts1∆ mutants compared to wild-type (2.5-fold for MFα, P < 0.05; twofold for STE3α, P < 0.05; twofold for CPK1, P < 0.05; and 3.6-fold for MAT2, P < 0.005) (Figure S5). ZNF2 and SXI1α were upregulated in cts1∆ compared to wild-type, although these were not statistically significantly different (4.3-fold for ZNF2, P = 0.0542, and 1.4-fold for SXI1α, P = 0.1229) (Figure S5). These expression patterns support the hypothesis that calcineurin does not regulate the pheromone response pathway during unisexual reproduction, with the exception of Cts1, which suppresses expression of the pheromone pathway genes.
Calcineurin downstream targets function synergistically in response to different environmental stresses
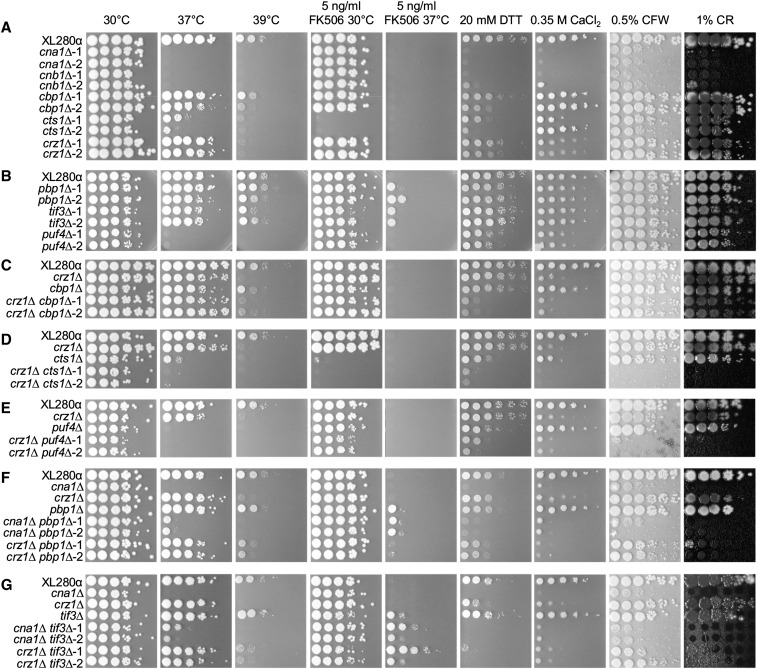
The calcineurin pathway governs Cryptococcus tolerance to various environmental stresses (Park et al. 2016; Chow et al. 2017). We tested calcineurin pathway mutants in the C. deneoformans XL280α strain background in the presence of high temperature (37 and 39°), a calcineurin inhibitor (5 ng/ml FK506 at 30 and 37°), an ER stress reagent (20 mM DTT), high calcium (0.35 M CaCl2), or cell wall stress reagents (0.5% CFW and 1% CR) (Figure 4).
Figure 4.
Stress response phenotypes of the calcineurin pathway single and double mutants. Stress response phenotypes were tested for: (A) XL280α and cna1∆ (CF637 and CF645), cnb1∆ (CF1095 and CF1099), cbp1∆ (CF799 and CF802), cts1∆ (CF787 and CF794), and crz1∆ (CF684 and CF688) mutants; (B) XL280α and pbp1∆ (CF1123 and CF1126), tif3∆ (CF1219 and CF1220), and puf4∆ (CF1194 and CF1195) mutants; (C) XL280α, crz1∆ (CF688) and cbp1∆ (CF799) single mutants, and crz1∆ cbp1∆ double mutants (CF1025 and CF1026); (D) XL280α, crz1∆ (CF688) and cts1∆ (CF787) single mutants, and crz1∆ cts1∆ double mutants (CF998 and CF1001); (E) XL280α, crz1∆ (CF688) and puf4∆ (CF1194) single mutants, and crz1∆ puf4∆ double mutants (CF1200 and CF1203); (F) XL280α, cna1∆ (CF637), crz1∆ (CF688) and pbp1∆ (CF1123) single mutants, and cna1∆ pbp1∆ (CF1288 and CF1289) and crz1∆ pbp1∆ (CF1143 and CF1145) double mutants; and (G) XL280α, cna1∆ (CF637), crz1∆ (CF688) and tif3∆ (CF1219) single mutants, and cna1∆ tif3∆ (CF1252 and CF1256) and crz1∆ tif3∆ (CF1231 and CF1239) double mutants. Strains were grown overnight at 30°, serially diluted 10-fold, and spot inoculated onto YPD and YPD agar medium supplemented with 5 ng/ml FK506, 20 mM DTT, 0.35 M CaCl2, 0.5% calcofluor white (CFW), and 1% Congo red (CR). Thermosensitivity of these strains was tested by comparing growth at 30, 37, and 39°.
The calcineurin phosphatase catalytic subunit cna1∆ mutants and the regulatory subunit cnb1∆ mutants were sensitive to high-temperature (37 and 39°), ER, and high-calcium stress, and exhibited intermediate sensitivity to cell wall stress (Figure 4A and Table 1). Other calcineurin pathway mutants were less sensitive to one or more stress conditions compared to the cna1∆ and cnb1∆ mutants. The cbp1∆ mutants were only mildly sensitive to high temperature at 39° (Figure 4A). The cts1∆ mutants were less sensitive to all stress conditions except the calcineurin inhibitor FK506 compared to the cna1∆ and cnb1∆ mutants, and the cts1∆ mutants exhibited hypersensitivity to FK506 at 30° (Figure 4A). The crz1∆ mutants were only mildly sensitive to high temperature at 39° and high calcium (Figure 4A). The pbp1∆ mutants were not sensitive to any stress tested except FK506 (Figure 4B). The tif3∆ mutants were only mildly sensitive to high temperature at 39° and cell wall stresses. Both the pbp1∆ and the tif3∆ mutants exhibited an enhanced high-temperature tolerance phenotype in the presence of FK506 compared to wild-type (Figure 4B). The puf4∆ mutants were sensitive to high temperature at both 37 and 39° at similar levels, and less sensitive to ER stress and high calcium compared to the cna1∆ and cnb1∆ mutants (Figure 4B).
Table 1. Summary of stress phenotypes for calcineurin pathway mutants.
| 37° | 39° | 5 ng/ml FK506 30° | 5 ng/ml FK506 37° | 20 mM DTT | 0.35 M CaCl2 | 5 mg/ml CFW | 1% Congo red | |
|---|---|---|---|---|---|---|---|---|
| Wild type | NS | S | NS | SSS | NS | NS | NS | NS |
| cna1∆ | SSS | SSS | WT | WT | SSS | SSS | SS | SS |
| cnb1∆ | SSS | SSS | WT | WT | SSS | SSS | SS | SS |
| cbp1∆ | WT | S | WT | WT | WT | WT | WT | WT |
| cts1∆ | SS | SS | SSS | WT | S | S | S | S |
| crz1∆ | WT | SS | WT | WT | WT | S | WT | WT |
| pbp1∆ | WT | WT | WT | R | WT | WT | WT | WT |
| tif3∆ | WT | S | WT | R | WT | WT | S | S |
| puf4∆ | SSS | SSS | WT | WT | S | S | WT | WT |
| crz1∆ cbp1∆ | WT | S | WT | WT | SS | SS | WT | WT |
| crz1∆ cts1∆ | SSS | SSS | SSS | WT | SS | SSS | SSS | SSS |
| crz1∆ puf4∆ | SSS | SSS | WT | WT | SS | SS | SSS | SSS |
| cna1∆ pbp1∆ | SS | SSS | WT | R | SSS | SSS | SS | SS |
| crz1∆ pbp1∆ | WT | S | WT | WT | S | SSS | S | S |
| cna1∆ tif3∆ | SS | SSS | WT | R | SSS | SSS | SS | SS |
| crz1∆ tif3∆ | WT | S | WT | R | SS | SS | S | S |
| cbp1∆ pbp1∆ | SS | SS | WT | WT | S | S | WT | WT |
NS, the strain exhibited no sensitive phenotype under the stress; S, the strain exhibited a mild sensitivity phenotype under the stress; SS, the strain exhibited an intermediate sensitivity phenotype under the stress; SSS, the strain failed to grow under the stress; WT, deletion mutant exhibited a similar stress tolerance phenotype as the wild type; R, deletion mutant exhibited an enhanced stress tolerance phenotype compared to the wild type.
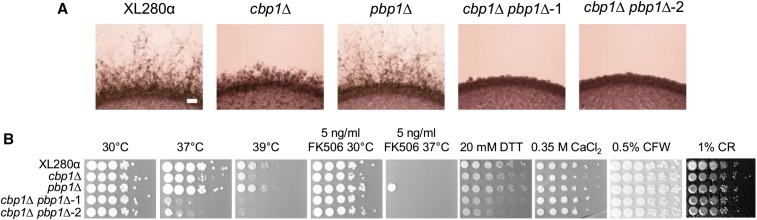
Due to the nearly wild-type stress tolerance phenotypes of the cbp1∆ and pbp1∆ mutants under most stress conditions tested, we sought to determine whether or not CBP1 and PBP1 are functionally redundant. Interestingly, the cbp1∆ pbp1∆ double mutants displayed reduced filamentation during unisexual reproduction compared to the cbp1∆ and pbp1∆ single mutants (Figure 5A). The cbp1∆ pbp1∆ double mutants were also more sensitive than the single mutants to high-temperature (37 and 39°), ER, and high-calcium stress, but were not sensitive to cell wall stress (CR and CFW) (Figure 5B and Figure S6A). The cbp1∆ single and cbp1∆ pbp1∆ double mutants exhibited mild sensitivity to 1% CFW compared to the wild type and the pbp1∆ single mutant, confirming that PBP1 does not synergistically interact with CBP1 in response to cell wall stresses (Figure S6A). These phenotypic analyses suggest that CBP1 and PBP1 function redundantly downstream of calcineurin in regulating high-temperature and ER stress tolerance, and filamentation, during unisexual reproduction.
Figure 5.
CBP1 and PBP1 synergistically regulate unisexual reproduction and stress responses in C. deneoformans. (A) Wild-type XL280α, cbp1∆ (CF799) and pbp1∆ (CF1123) single mutants, and two cbp1∆ pbp1∆ double mutants (CF1260 and CF1268) were grown on Murashige and Skoog agar medium for 14 days. Bar represents 200 μm. (B) Stress response phenotypes were tested for wild-type XL280α, cbp1∆ (CF799), pbp1∆ (CF1123), and two cbp1∆ pbp1∆ double mutants (CF1260 and CF1268). Strains were grown overnight at 30°, serially diluted 10-fold, and spot inoculated onto YPD and YPD agar medium supplemented with 5 ng/ml FK506, 20 mM DTT, 0.35 M CaCl2, 0.5% calcofluor white (CFW), and 1% Congo red (CR). Thermosensitivity of these strains was tested at 30, 37, and 39°.
To test whether the transcription factor Crz1 synergistically interacts with other calcineurin downstream targets in regulating stress tolerance, we examined stress response phenotypes for crz1 double mutants. The crz1∆ cbp1∆ double mutants were more sensitive to ER stress and high calcium compared to crz1∆ and cbp1∆ single mutants (Figure 4C). At lower concentrations of CFW and CR, the double mutants and the single mutants exhibited a wild-type phenotype. However, at higher concentrations of cell wall stressors, the crz1∆ cbp1∆ double mutants displayed sensitive phenotypes similar to that of the crz1∆ single mutant (Figure 4C and Figure S6A). The crz1∆ cts1∆ double mutants were more sensitive to all stresses tested (except FK506 at 30°) compared to the crz1∆ and cts1∆ single mutants (Figure 4D). At lower temperatures of 17 and 25°, the crz1∆ cts1∆ double mutants were more sensitive to FK506 compared to the single mutants (Figure S6B). The crz1∆ puf4∆ double mutants were more sensitive to ER, high-calcium, and cell wall stresses compared to crz1∆ and puf4∆ single mutants (Figure 4E). The crz1∆ pbp1∆ and crz1∆ tif3∆ double mutants were more sensitive to ER, high-calcium, and cell wall stresses compared to the single mutants, but less sensitive to high temperature at 39° than crz1∆ mutants (Figure 4, F and G). The crz1∆ tif3∆ and crz1∆ pbp1∆ double mutants displayed a more resistant phenotype at high temperature in the presence of FK506 (Figure 4, F and G and Figure S6C). Taken together, these results support a model in which Crz1 functions together with Cbp1, Cts1, Pbp1, Tif3, and Puf4 in branched pathways downstream of calcineurin to control stress responses.
PBP1 and TIF3 are required for virulence in C. deneoformans
Growth at the host temperature of 37° is a key virulence factor for Cryptococcus infection (Odom et al. 1997). Inhibition of the calcineurin pathway by FK506, or deletion of CNA1 or CNB1, prevents C. deneoformans growth at 37°; however, cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants were partially suppressed for the high-temperature-sensitive phenotype compared to the cna1∆ mutants (Figure 4, F and G). To analyze the thermotolerant phenotype in further detail, pbp1∆ and tif3∆ single mutants, cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants, and crz1∆ pbp1∆ and crz1∆ tif3∆ double mutants were tested at high temperatures ranging from 35 to 37° in the presence of the calcineurin inhibitor FK506. Growth of the cna1∆ and crz1∆ mutants was completely inhibited at 37°, and the mutants were sensitive to high temperature at 35 and 36° Compared to wild-type (Figure 4 and Figure S6C). In comparison, the pbp1∆ and tif3∆ mutants, the crz1∆ pbp1∆ and crz1∆ tif3∆ double mutants, and the cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants were resistant to high temperature at 35, 36, and 37° in the presence of FK506 compared to the wild type or the cna1∆ and crz1∆ single mutants (Figure 4 and Figure S6C).
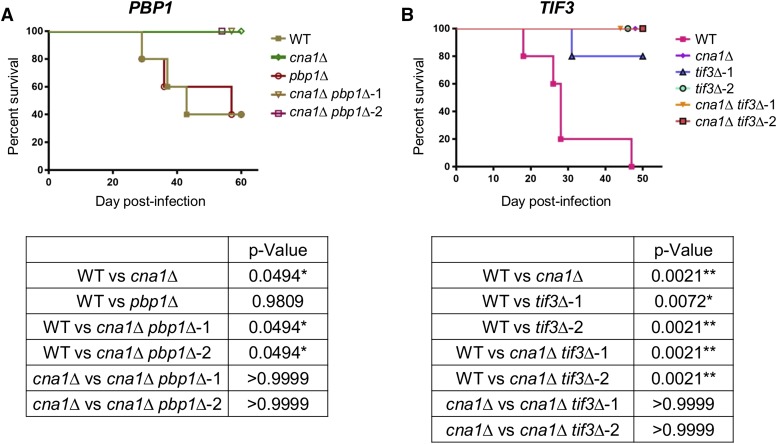
To determine whether high-temperature tolerance impacts Cryptococcus virulence, pbp1∆ and tif3∆ single mutants, and cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants, were tested in the murine inhalation infection model. The pbp1∆ and tif3∆ virulence experiments were performed with different anesthetic methods (isoflurane for the pbp1∆ mutant group and pentobarbital for the tif3∆ group). The wild-type strain, XL280α, produced a 60% mortality rate using the inhalation anesthetic isoflurane, and a 100% mortality rate was observed using subcutaneous injection of the anesthetic sodium pentobarbital (Figure 6, A and B). The different anesthetic methods may vary in the depths of sedation in mice, which may influence lodging of fungal cells in the host lungs and ultimately impact fungal virulence outcome. Infection with the pbp1∆ mutant gave a similar survival curve to infection with the wild type (Figure 6A). The cna1∆ pbp1∆ double mutants were avirulent, similar to a cna1∆ mutant (Figure 6A). Fungal burden in the lung on day 20 postinfection was significantly lower in the pbp1∆ mutant compared to wild-type, suggesting that the virulence of the pbp1∆ mutant is attenuated in mice; however, fungal burden in the brain was only detected in fewer animals (three for wild-type, none for cna1∆, two for pbp1∆, one for cna1∆ pbp1∆-1, and one for cna1∆ pbp1∆-2, out of five tested for each group), therefore the data were not statistically significant between different groups due to the sample size (Figure S7A). At the end of the virulence experiment, the fungal burden in both the lungs and brain were detected in all mice that survived cryptococcal infection with the pbp1∆ mutant (two out of two), but not the cna1∆ mutant or the cna1∆ pbp1∆-2 double mutant (Figure S8). Only one out of five mice infected with the cna1∆ pbp1∆-1 double mutant was detected with fungal burden in the lung. The virulence of the tif3∆ mutants was attenuated compared to wild-type, and the cna1∆ tif3∆ double mutants were as avirulent as the cna1∆ mutant (Figure 6B). The fungal burden in the lung on day 20 postinfection was significantly lower in the cna1∆ and tif3∆ mutants compared to wild-type, and the fungal burden in the cna1∆ tif3∆ double mutants was higher than the cna1∆ mutant, but only statistically significant for one double mutant (Figure S7B). The fungal burden in the brain was detectable in mice infected with wild-type (five out of five mice), tif3∆ mutants (four out of four mice for one mutant and three out five for the other), and one cna1∆ tif3∆ double mutant (two out of five mice), but not detectable in mice infected with the cna1∆ mutant and the other cna1∆ tif3∆ double mutant. The fungal burden for the tif3∆ mutants and the cna1∆ tif3∆ double mutant was lower than wild-type, but not statistically significant due to the small sample size (Figure 7B). Despite the fact that this finding did not meet statistical significance (P > 0.05), the fungal burdens in mice brains infected with the pbp1∆ and tif3∆ single mutants did exhibit a trend and were consistently lower than with the wild-type strains. This may indicate a potential dissemination defect of the pbp1∆ and tif3∆ mutants into the central nervous system during cryptococcal infection (Figure S7). The virulence experiments showed that the virulence of the thermotolerant pbp1∆ and tif3∆ mutants was attenuated, while the cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants were avirulent in mice. In summary, these results revealed that both Pbp1 and Tif3 play significant roles in C. deneoformans pathogenesis.
Figure 6.
Deletion of PBP1 and TIF3 attenuates C. deneoformans virulence in a murine infection model. (A) Virulence was tested for the wild-type (WT) XL280α, cna1∆ (CF637) and pbp1∆ (CF1123) single mutants, and cna1∆ pbp1∆ double mutants (CF1288 and CF1289). (B) Virulence was tested for the WT XL280α, cna1∆ (CF637) and tif3∆ (CF1219 and CF1220) single mutants, and cna1∆ tif3∆ double mutants (CF1252 and CF1256). Strains were grown overnight in YPD liquid medium at 30° and washed with PBS. For each strain, five female A/J mice were inoculated with 5 × 106 cells via intranasal infection, and animal survival was monitored up to 60 days postinfection. P-values for the virulence experiments are provided in the bottom panels.
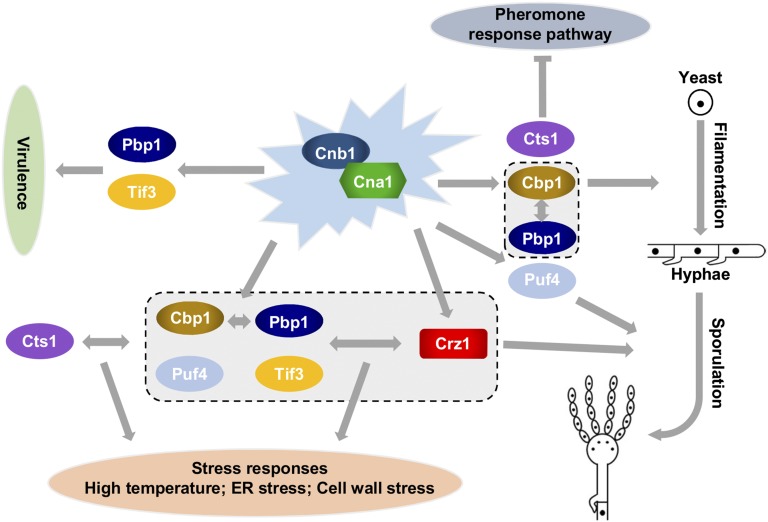
Figure 7.
The calcineurin pathway controls unisexual reproduction, stress responses, and virulence in C. deneoformans. Calcineurin Cna1/Cnb1 functions as the central hub in the calcineurin pathway. During unisexual reproduction, calcineurin regulates the yeast–hyphal morphological transition through Cbp1 and Cts1, and via a pathway in which Cbp1 synergistically interacts with Pbp1, whereas Cts1 inhibits the pheromone response pathway. Crz1 and Puf4 are required for sporulation. In response to different environmental stresses, Crz1 synergistically interacts with other calcineurin downstream targets via complex branched pathways. Finally, Pbp1 and Tif3 are key virulence factors downstream of calcineurin in C. neoformans.
Discussion
Calcineurin orchestrates mating, stress responses, and virulence in Cryptococcus via distinct pathways (Cruz et al. 2001; Park et al. 2016; Chow et al. 2017). To elucidate the mechanisms via which calcineurin elicits unisexual reproduction, we deleted individual genes involved in the calcineurin pathway in the self-filamentous strain XL280α (Lin et al. 2005, 2006) and analyzed the mating phenotypes during unisexual reproduction. The calcineurin phosphatase catalytic subunit Cna1, regulatory subunit Cnb1, calcineurin-binding protein Cbp1, and calcineurin temperature suppressor Cts1 are each required for filamentation during both bisexual and unisexual reproduction (Cruz et al. 2001; Fox et al. 2003; Fox and Heitman 2005). In the wild-type strains, the expression levels for the CNA1, CNB1, CBP1, and CTS1 genes were upregulated during both unisexual and bisexual reproduction, confirming that the calcineurin pathway is involved in mating of C. deneoformans. Deletion of these genes caused defects in hyphal production to varying degrees during unisexual reproduction. Compared to other calcineurin pathway mutants, the cna1∆ and cnb1∆ mutants produced few blunted hyphae after a prolonged incubation period, suggesting that Cna1 and Cnb1 play central roles in the calcineurin pathway promoting unisexual reproduction.
The three calcineurin downstream targets Pbp1, Tif3, and Puf4, identified by a phospho-proteomic study, are involved in C. neoformans bisexual reproduction (Park et al. 2016). In S. cerevisiae, Pbp1, Tif3, and Puf4 are involved in controlling the extent of mRNA polyadenylation, translation, and stability, respectively (Altmann et al. 1995; Gerber et al. 2004; Mangus et al. 2004). The poly(A)-binding protein-binding protein Pbp1 has also been shown to be required for mating type switch in S. cerevisiae and for normal sexual development in A. nidulans (Tadauchi et al. 2004; Soukup et al. 2017). In C. neoformans, deletion of PBP1 or TIF3 leads to a reduction in dikaryotic hyphal production, and deletion of PUF4 leads to a hyper-filamentous phenotype (Park et al. 2016). In contrast to bisexual reproduction in C. neoformans, deletion of PBP1, TIF3, and PUF4 did not impact unisexual reproduction in C. deneoformans, and these genes were not upregulated during unisexual reproduction, indicating that the calcineurin pathway may govern unisexual and bisexual reproduction in these related Cryptococcus species via different downstream targets. Interestingly, C. deneoformans puf4∆ mutants exhibited different filamentation phenotypes on MS and V8 medium, suggesting that Puf4 plays a role in driving unisexual reproduction on V8 medium. pbp1∆ mutants did not display any defect in filamentation during unisexual reproduction, and the cbp1∆ pbp1∆ double mutants exhibited a severe filamentation defect phenotype compared to the cbp1∆ and pbp1∆ single mutants, implying that Pbp1 plays a role in promoting unisexual reproduction that is functionally redundant with Cbp1. Similarly, Tif3 may also play a role in unisexual reproduction, but have redundant functions with other calcineurin downstream factors, as both proteins have been shown to regulate C. neoformans bisexual reproduction (Park et al. 2016).
The calcineurin-responsive zinc finger transcription factor Crz1 is not required for bisexual reproduction in C. neoformans (Park et al. 2016; Chow et al. 2017). Similarly, deletion of CRZ1 did not cause any filamentation defect during unisexual reproduction in XL280α, and all double mutants of crz1 with other calcineurin downstream target genes exhibited the non-crz1 parental deletion mutant unisexual mating phenotype, suggesting that hyphal production during unisexual reproduction is independent of the transcription factor Crz1. During pheromone-independent unisexual reproduction in the presence of high copper, Cna1 and Cnb1 are required for filamentation, but the transcription factor Crz1 is not (Gyawali et al. 2017), further demonstrating that Crz1 is dispensable for hyphal production during this process. In our study, the expression of the pheromone response pathway genes, including MFα (pheromone), STE3 (pheromone receptor), CPK1 (MAP kinase), and MAT2 and ZNF2 (transcription factors downstream of the pheromone pathway) (Feretzaki and Heitman 2013), was not affected by the deletion of CNA1, CNB1, CBP1, or CRZ1 during unisexual reproduction, supporting the hypothesis that the calcineurin pathway regulates filamentation independently of the pheromone response pathway. Interestingly, deletion of CTS1 upregulated the pheromone response pathway; however, the elevated pheromone signaling failed to rescue the filamentation defect of the cts1∆ mutants. This indicates that Cts1 suppresses the pheromone response pathway and that Cts1 may serve as a conduit between these two pathways during unisexual reproduction.
Although the calcineurin pathway mutants produced different amounts of hyphae, the cna1∆, cnb1∆, cts1∆, and cbp1∆ mutants were defective in sporulation. Additionally, crz1∆ and puf4∆ mutants, which produced wild-type levels of hyphae, formed basidia without spore chains, suggesting that the calcineurin pathway may play a role during the meiotic process or in spore chain formation, and that this function is dependent on Crz1 and Puf4. Taken together, calcineurin regulates unisexual reproduction at different stages: the Crz1-independent stage during initial filamentation and hyphal production, and the Crz1- and Puf4-dependent sporulation stage (Figure 7).
Besides the regulation of mating, calcineurin functions as a hub for the calcineurin signaling network in regulating various stress responses, and Crz1 and other calcineurin downstream targets function in branched pathways (Park et al. 2016; Chow et al. 2017). Supporting this model, the cna1∆ and cnb1∆ mutants in the XL280α background exhibited the most sensitive phenotypes against ER, high-temperature, high-calcium, and cell wall stresses, while mutants of calcineurin downstream targets, including Cbp1, Cts1, Crz1, Pbp1, Tif3, and Puf4, displayed intermediate to nonsensitive phenotypes toward these stresses. These results suggest that Cna1 and Cnb1 function early on in the calcineurin pathway to control stress responses, and that the downstream targets share redundant functions in stress tolerance (Figure 7).
In S. cerevisiae, inactivation of calcineurin promotes the activity of the vacuolar H+/Ca2+ exchanger (Vcx1), which in turn enables yeast cells to tolerate high concentrations of calcium (Cunningham and Fink 1996). Moreover, calcineurin regulates calcium homeostasis through the transcription factor Crz1 in yeast (Matheos et al. 1997; Stathopoulos and Cyert 1997). In C. neoformans, calcineurin and Crz1 positively regulate expression of the VCX1 gene and the PMC1 gene encoding the vacuolar calcium transporter Pmc1 to confer higher tolerance to calcium (Kmetzsch et al. 2010, 2013; Chow et al. 2017; Squizani et al. 2017). Several studies have shown that the calcineurin/Crz1 regulatory network has been extensively rewired in different fungal species (Yoshimoto et al. 2002; Kim et al. 2010; Soriani et al. 2010; Chatfield-Reed et al. 2016; Chow et al. 2017), which may account for the high calcium sensitivity observed in cna1∆ and cnb1∆ mutants in Cryptococcus.
Based on the ER and high-calcium stress tolerance phenotypes, Crz1 synergistically interacts with all of the other calcineurin downstream targets. However, all of the crz1 double mutants were less sensitive to these stresses than the cna1∆ and cnb1∆ mutants, suggesting that Crz1 and these downstream targets function in multiple parallel pathways for ER stress and high-calcium tolerance. In addition, CBP1 and PBP1 synergistically regulate ER and high-calcium stress responses, but the double mutants were only mildly sensitive to these stresses, supporting the hypothesis that complex multiple branched pathways function downstream of calcineurin to regulate responses to ER and high-calcium stresses (Figure 7).
In response to the cell wall stressors CR, known to interact with β-glucans (Teather and Wood 1982), and CFW, which binds to chitin (Elorza et al. 1983), Crz1 synergistically interacts with Cts1, Puf4, Tif3, and Pbp1, but not Cbp1. Interestingly, cbp1∆ single mutants and cbp1∆ pbp1∆ double mutants exhibited largely wild-type tolerance to CR and CFW, except for a mild sensitivity to 2% CFW, indicating that Cbp1 does not play a significant role in response to these cell wall stresses. Double mutants of crz1 with tif3 or pbp1 were less sensitive to cell wall stresses than the cna1∆ and cnb1∆ mutants, indicating that these three genes are functionally redundant and that additional factors may contribute to cell wall stress tolerance. Double mutants of crz1 with cts1 or puf4 were more sensitive to cell wall stresses compared to the cna1∆ and cnb1∆ mutants, suggesting that Cts1 and Puf4 may function independently of the calcineurin pathway in regulating cell wall stress tolerance.
Calcineurin is essential for survival at high temperature at 37°. Deletion of CNA1 or CNB1, or inhibition of the calcineurin pathway by the inhibitor FK506, leads to high-temperature sensitivity to 37° (Odom et al. 1997). Among the calcineurin downstream targets analyzed in this study, only the puf4 mutants exhibited the same high-temperature-sensitive phenotype as the cna1∆ and cnb1∆ mutants, and the crz1∆ puf4∆ double mutants had similar thermosensitivity to the puf4∆ single mutants, indicating that Puf4 plays a key role downstream of calcineurin in governing high-temperature stress tolerance in C. deneoformans. Interestingly, Puf4 was previously shown to synergistically interact with Crz1 in C. neoformans in orchestrating high-temperature stress responses (Park et al. 2016), suggesting that the calcineurin pathway has been rewired in the Cryptococcus sister species. Furthermore, deletion of PUF4 caused a defect in hyphal production in C. neoformans during bisexual reproduction (Park et al. 2016), but not in C. deneoformans during unisexual reproduction. Puf4 belongs to the pumilio-FBF (fem-3 mRNA binding factor) family of mRNA-binding proteins and it controls the turnover of the unfolded protein response pathway transcription factor Hxl1 in C. neoformans (Glazier et al. 2015). Puf4 may impact thermosensitivity in C. deneoformans via similar mechanisms by promoting the stability of calcineurin pathway mRNAs.
Overexpression of CTS1 was previously shown to suppress the thermosensitive phenotype of cna1∆ mutants (Fox et al. 2003). Cts1 colocalizes with Cna1 in mRNA-processing P-body sites, and dephosphorylation of Cts1 is dependent on calcineurin, suggesting that Cts1 functions as an effector downstream of Cna1 in the calcineurin pathway (Aboobakar et al. 2011). However, in this study, deletion of CTS1 caused hypersensitivity to 30° in the presence of FK506, and crz1∆ cts1∆ double mutants were more sensitive to cell wall stresses compared to the cna1∆, cnb1∆, and crz1∆ single mutants, suggesting that Cts1 may function in a pathway parallel, with calcineurin enabling responses to various stress conditions.
In contrast to the cna1∆ and cnb1∆ mutants, the pbp1∆ and tif3∆ single mutants, and the cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants, were resistant to 37°, both in the absence and in the presence of the calcineurin inhibitor FK506, suggesting that Pbp1 and Tif3 function as suppressors in the calcineurin pathway regulating the stress responses to high temperature. Thermosensitivity at 37° is considered a key virulence factor that contributes to the avirulent phenotype in mice for the cna1∆ mutants (Odom et al. 1997; Cruz et al. 2000). However, the thermosensitive puf4∆ mutants were reported to be as virulent as the wild type in mice (Glazier et al. 2015), and the virulence for the high-temperature-tolerant pbp1∆ mutants in C. neoformans was attenuated (Park et al. 2016), indicating that thermosensitivity may not be entirely predictive of virulence potential. Supporting this hypothesis, the high-temperature-resistant phenotype for pbp1∆ and tif3∆ mutants in C. deneoformans did not confer hypervirulence in mice, as both the survival curve and fungal burden in the lung and brain for the single mutants were attenuated compared to wild-type. The modestly thermotolerant cna1∆ pbp1∆ and cna1∆ tif3∆ double mutants were avirulent like the cna1∆ mutants, and fungal burdens were comparable between the double mutants and the cna1∆ single mutants, further suggesting that host temperature sensitivity at 37° alone does not explain the avirulent phenotype observed for the cna1∆ mutants. Interestingly, the thermosensitive C. neoformans puf4∆ mutants exhibited lower fungal burden than the wild-type, suggesting that growth at host temperature contributes to fungal survival in the host, but may not predict the overall virulence outcome (Glazier et al. 2015). C. deneoformans puf4∆ mutants are more sensitive to 37° than C. neoformans puf4∆ mutants, which is likely due to species-specific responses, in that the C. deneoformans calcineurin mutant is more sensitive to higher temperature than the C. neoformans cna1∆ mutant (Cruz et al. 2000; Glazier et al. 2015; Park et al. 2016). The severe thermosensitive phenotype may further influence growth of the C. deneoformans puf4∆ mutant in the host. Our studies indicate that Pbp1 and Tif3 are key virulence factors, which regulate virulence downstream of calcineurin in a temperature-independent manner in C. deneoformans. Pbp1 and Tif3 synergistically interact with Crz1 in responses to ER, high-calcium,and cell wall stresses, indicating that Pbp1 and Tif3 play additional roles in regulating multiple stress responses downstream of calcineurin, thereby contributing to virulence in mice.
In summary, by utilizing the self-filamentous C. deneoformans strain XL280α, we elucidated the functions of the calcineurin pathway in the control of unisexual reproduction, stress responses, and virulence (Figure 7). The calcineurin-responsive transcription factor Crz1 is not required for the yeast–hyphal morphological transition, but it is important for spore chain formation. Crz1 synergistically interacts with other calcineurin downstream targets in coordinating responses to high-temperature, ER, high-calcium, and cell wall stresses. The widespread synergy among calcineurin downstream targets suggests that the calcineurin pathway is highly plastic, which allows for rapid signaling network rewiring in adaptation to changing environments during evolution.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300422/-/DC1.
Acknowledgments
We thank Giuseppe Ianiri, Kaila Pianalto, Praveen Rao Juvvadi, and Shelby Priest for critical reading of the manuscript, and Anna Floyd-Averette for assistance with the virulence studies. This work was supported by National Institutes of Health (NIH)/National Cancer Institute grant R01 CA-154499 to M.E.C., and NIH/National Institute of Allergy and Infectious Diseases grants R37 AI-39115-20 and R01 AI-50113-13 to J.H.
Footnotes
Communicating editor: A. Mitchell
Literature Cited
- Aboobakar E. F., Wang X., Heitman J., Kozubowski L., 2011. The C2 domain protein Cts1 functions in the calcineurin signaling circuit during high-temperature stress responses in Cryptococcus neoformans. Eukaryot. Cell 10: 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shanti N., Stewart C. E., 2009. Ca2+/calmodulin-dependent transcriptional pathways: potential mediators of skeletal muscle growth and development. Biol. Rev. Camb. Philos. Soc. 84: 637–652. [DOI] [PubMed] [Google Scholar]
- Altmann M., Wittmer B., Methot N., Sonenberg N., Trachsel H., 1995. The Saccharomyces cerevisiae translation initiation factor Tif3 and its mammalian homologue, eIF-4B, have RNA annealing activity. EMBO J. 14: 3820–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J., Heitman J., Crabtree G. R., 2004. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 5: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader T., Bodendorfer B., Schroppel K., Morschhäuser J., 2003. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 71: 5344–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals C. R., Clipstone N. A., Ho S. N., Crabtree G. R., 1997. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 11: 824–834. [DOI] [PubMed] [Google Scholar]
- Blankenship J. R., Heitman J., 2005. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect. Immun. 73: 5767–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. R., Steinbach W. J., Perfect J. R., Heitman J., 2003a Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4: 192–199. [PubMed] [Google Scholar]
- Blankenship J. R., Wormley F. L., Boyce M. K., Schell W. A., Filler S. G., et al. , 2003b Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield-Reed K., Vachon L., Kwon E. J., Chua G., 2016. Conserved and diverged functions of the calcineurin-activated Prz1 transcription factor in fission yeast. Genetics 202: 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. L., Brand A., Morrison E. L., Silao F. G., Bigol U. G., et al. , 2011. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot. Cell 10: 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. L., Konieczka J. H., Springer D. J., Bowen S. E., Zhang J., et al. , 2012. Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3 (Bethesda) 2: 675–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. L., Yu S. J., Huang H. Y., Chang Y. L., Lehman V. N., et al. , 2014. Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryot. Cell 13: 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E. W., Clancey S. A., Billmyre R. B., Averette A. F., Granek J. A., et al. , 2017. Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans. PLoS Genet. 13: e1006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R., 1992. Identification of calcineurin as a key signaling enzyme in lymphocyte-T activation. Nature 357: 695–697. [DOI] [PubMed] [Google Scholar]
- Coelho C., Casadevall A., 2016. Cryptococcal therapies and drug targets: the old, the new and the promising. Cell. Microbiol. 18: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen L. E., Lindquist S., 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309: 2185–2189. [DOI] [PubMed] [Google Scholar]
- Cramer R. A. Jr., Perfect B. Z., Pinchai N., Park S., Perlin D. S., et al. , 2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 7: 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M. C., Sia R. A., Olson M., Cox G. M., Heitman J., 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 68: 982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M. C., Fox D. S., Heitman J., 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20: 1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M. C., Goldstein A. L., Blankenship J. R., Del Poeta M., Davis D., et al. , 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21: 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R., 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Kunisawa R., Kaim D., Thorner J., 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 88: 7376–7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. C., Blankenship J. R., Kraus P. R., de Jesus Berrios M., Hull C. M., et al. , 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148: 2607–2615. [DOI] [PubMed] [Google Scholar]
- Dewenter M., von der Lieth A., Katus H. A., Backs J., 2017. Calcium signaling and transcriptional regulation in cardiomyocytes. Circ. Res. 121: 1000–1020. [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Rico H., Sentandreu R., 1983. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 129: 1577–1582. [DOI] [PubMed] [Google Scholar]
- Feretzaki M., Heitman J., 2013. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genet. 9: e1003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan W. M., Corthesy B., Bram R. J., Crabtree G. R., 1991. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352: 803–807. [DOI] [PubMed] [Google Scholar]
- Fox D. S., Heitman J., 2002. Good fungi gone bad: the corruption of calcineurin. BioEssays 24: 894–903. [DOI] [PubMed] [Google Scholar]
- Fox D. S., Heitman J., 2005. Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans. Eukaryot. Cell 4: 1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. S., Cruz M. C., Sia R. A., Ke H., Cox G. M., et al. , 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12–FK506 in Cryptococcus neoformans. Mol. Microbiol. 39: 835–849. [DOI] [PubMed] [Google Scholar]
- Fox D. S., Cox G. M., Heitman J., 2003. Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans. Eukaryot. Cell 2: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J. L., Norris C. M., 2014. Calcineurin and glial signaling: neuroinflammation and beyond. J. Neuroinflammation 11: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. P., Herschlag D., Brown P. O., 2004. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2: E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier V. E., Kaur J. N., Brown N. T., Rivera A. A., Panepinto J. C., 2015. Puf4 regulates both splicing and decay of HXL1 mRNA encoding the unfolded protein response transcription factor in Cryptococcus neoformans. Eukaryot. Cell 14: 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach J., Fox D. S., Cutler N. S., Cox G. M., Perfect J. R., et al. , 2000. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. EMBO J. 19: 3618–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali R., Zhao Y., Lin J., Fan Y., Xu X., et al. , 2017. Pheromone independent unisexual development in Cryptococcus neoformans. PLoS Genet. 13: e1006772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A., 2010. A tetrad analysis of the basidiomycete fungus Cryptococcus neoformans. Genetics 185: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi P. R., Fortwendel J. R., Rogg L. E., Burns K. A., Randell S. H., et al. , 2011. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 82: 1235–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi P. R., Lamoth F., Steinbach W. J., 2014. Calcineurin-mediated regulation of hyphal growth, septation, and virulence in Aspergillus fumigatus. Mycopathologia 178: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karababa M., Valentino E., Pardini G., Coste A. T., Bille J., et al. , 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59: 1429–1451. [DOI] [PubMed] [Google Scholar]
- Kim S., Hu J., Oh Y., Park J., Choi J., et al. , 2010. Combining ChIP-chip and expression profiling to model the MoCRZ1 mediated circuit for Ca2+/calcineurin signaling in the rice blast fungus. PLoS Pathog. 6: e1000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipanyula M. J., Kimaro W. H., Seke Etet P. F., 2016. The emerging roles of the calcineurin-nuclear factor of activated T-lymphocytes pathway in nervous system functions and diseases. J. Aging Res. 2016: 5081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H., 1979. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl. Acad. Sci. USA 76: 6270–6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L., Staats C. C., Simon E., Fonseca F. L., de Oliveira D. L., et al. , 2010. The vacuolar Ca2+ exchanger Vcx1 is involved in calcineurin-dependent Ca2+ tolerance and virulence in Cryptococcus neoformans. Eukaryot. Cell 9: 1798–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L., Staats C. C., Cupertino J. B., Fonseca F. L., Rodrigues M. L., et al. , 2013. The calcium transporter Pmc1 provides Ca2+ tolerance and influences the progression of murine cryptococcal infection. FEBS J. 280: 4853–4864. [DOI] [PubMed] [Google Scholar]
- Kozubowski L., Lee S. C., Heitman J., 2009. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 11: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68: 821–833. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Edman J. C., Wickes B. L., 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60: 602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Li A., Calo S., Heitman J., 2013. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 9: e1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Li A., Calo S., Inoue M., Tonthat N. K., et al. , 2015. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 97: 844–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S., Desmarini D., Chayakulkeeree M., Sorrell T. C., Djordjevic J. T., 2012. The Crz1/Sp1 transcription factor of Cryptococcus neoformans is activated by calcineurin and regulates cell wall integrity. PLoS One 7: e51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Hull C. M., Heitman J., 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434: 1017–1021. [DOI] [PubMed] [Google Scholar]
- Lin X., Huang J. C., Mitchell T. G., Heitman J., 2006. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATα allele enhances filamentation. PLoS Genet. 2: e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Lane W. S., Friedman J., Weissman I., et al. , 1991a Calcineurin is a common target of cyclophilin-cyclosporine A and FKBP-FK506 complexes. Cell 66: 807–815. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ishii S., Tokai M., Tsutsumi H., Ohki O., et al. , 1991b The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol. Gen. Genet. 227: 52–59. [DOI] [PubMed] [Google Scholar]
- Mangus D. A., Smith M. M., McSweeney J. M., Jacobson A., 2004. Identification of factors regulating poly(A) tail synthesis and maturation. Mol. Cell. Biol. 24: 4196–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti O., Entenza J. M., Sanglard D., Bille J., Glauser M. P., et al. , 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44: 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos D. P., Kingsbury T. J., Ahsan U. S., Cunningham K. W., 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11: 3445–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranova Z., Virtudazo E., Hricova K., Ohkusu M., Kawamoto S., et al. , 2014. The CRZ1/SP1-like gene links survival under limited aeration, cell integrity and biofilm formation in the pathogenic yeast Cryptococcus neoformans. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 158: 212–220. [DOI] [PubMed] [Google Scholar]
- Ni M., Feretzaki M., Li W., Floyd-Averette A., Mieczkowski P., et al. , 2013. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol. 11: e1001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K., Cox G. M., Wang P., Toffaletti D. L., Perfect J. R., et al. , 2003. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect. Immun. 71: 4831–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J. P., Ho S. N., Chen L., Thomas D. J., Timmerman L. A., et al. , 1994. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature 369: 497–502. [DOI] [PubMed] [Google Scholar]
- Odom A., Muir S., Lim E., Toffaletti D. L., Perfect J., et al. , 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16: 2576–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyewu C., Wormley F. L., Jr, Perfect J. R., Heitman J., 2004. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 72: 7330–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., et al. , 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530. [DOI] [PubMed] [Google Scholar]
- Park H. S., Chow E. W., Fu C., Soderblom E. J., Moseley M. A., et al. , 2016. Calcineurin targets involved in stress survival and fungal virulence. PLoS Pathog. 12: e1005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingham R., Smith R. M., Park B. J., Jarvis J. N., Govender N. P., et al. , 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D., Ischer F., Marchetti O., Entenza J., Bille J., 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48: 959–976. [DOI] [PubMed] [Google Scholar]
- Santos M., de Larrinoa I. F., 2005. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 48: 88–100. [DOI] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., et al. , 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriani F. M., Malavazi I., Savoldi M., Espeso E., Dinamarco T. M., et al. , 2010. Identification of possible targets of the Aspergillus fumigatus CRZ1 homologue, CrzA. BMC Microbiol. 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup A. A., Fischer G. J., Luo J., Keller N. P., 2017. The Aspergillus nidulans Pbp1 homolog is required for normal sexual development and secondary metabolism. Fungal Genet. Biol. 100: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squizani E. D., Oliveira N. K., Reuwsaat J. C. V., Marques B. M., Lopes W., et al. , 2017. Cryptococcal dissemination to the central nervous system requires the vacuolar calcium transporter Pmc1. Cell. Microbiol. DOI.10.1111/cmi.12803 [DOI] [PubMed] [Google Scholar]
- Stajich J. E., Harris T., Brunk B. P., Brestelli J., Fischer S., et al. , 2012. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 40: D675–D681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A. M., Cyert M. S., 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11: 3432–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos-Gerontides A., Guo J. J., Cyert M. S., 1999. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13: 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach W. J., Schell W. A., Blankenship J. R., Onyewu C., Heitman J., et al. , 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48: 1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach W. J., Cramer R. A., Jr, Perfect B. Z., Asfaw Y. G., Sauer T. C., et al. , 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach W. J., Reedy J. L., Cramer R. A., Jr, Perfect J. R., Heitman J., 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5: 418–430. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P., 1982. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 137: 80–84. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Sio S. O., Shuntoh H., Kuno T., 2002. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7: 619–627. [DOI] [PubMed] [Google Scholar]
- Tadauchi T., Inada T., Matsumoto K., Irie K., 2004. Posttranscriptional regulation of HO expression by the Mkt1-Pbp1 complex. Mol. Cell. Biol. 24: 3670–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J., 1982. Use of Congo red polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 43: 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffaletti D. L., Rude T. H., Johnston S. A., Durack D. T., Perfect J. R., 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175: 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhai B., Lin X., 2012. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 8: e1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto H., Saltsman K., Gasch A. P., Li H. X., Ogawa N., et al. , 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277: 31079–31088. [DOI] [PubMed] [Google Scholar]
- Zhai B., Zhu P., Foyle D., Upadhyay S., Idnurm A., et al. , 2013. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect. Immun. 81: 2626–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Silao F. G., Bigol U. G., Bungay A. A., Nicolas M. G., et al. , 2012. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS One 7: e44192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. Table S1 lists all of the strains and their genotypes, and Table S2 lists all of the primer sequences used in this study.