Xenopus laevis is a classic developmental model, but its allotetraploid genome has limited our ability to perform genetic manipulations. The advance of...
Keywords: CRISPR, kidney, targeted injection, Xenopus laevis, lhx1
Abstract
Studying genes involved in organogenesis is often difficult because many of these genes are also essential for early development. The allotetraploid frog, Xenopus laevis, is commonly used to study developmental processes, but because of the presence of two homeologs for many genes, it has been difficult to use as a genetic model. Few studies have successfully used CRISPR in amphibians, and currently there is no tissue-targeted knockout strategy described in Xenopus. The goal of this study is to determine whether CRISPR/Cas9-mediated gene knockout can be targeted to the Xenopus kidney without perturbing essential early gene function. We demonstrate that targeting CRISPR gene editing to the kidney and the eye of F0 embryos is feasible. Our study shows that knockout of both homeologs of lhx1 results in the disruption of kidney development and function but does not lead to early developmental defects. Therefore, targeting of CRISPR to the kidney may not be necessary to bypass the early developmental defects reported upon disruption of Lhx1 protein expression or function by morpholinos, antisense RNA, or dominant negative constructs. We also establish a control for CRISPR in Xenopus by editing a gene (slc45a2) that when knocked out results in albinism without altering kidney development. This study establishes the feasibility of tissue-specific gene knockout in Xenopus, providing a cost-effective and efficient method for assessing the roles of genes implicated in developmental abnormalities that is amenable to high-throughput gene or drug screening techniques.
XENOPUS laevis is a promising model for studying genes involved in human development and disease. Xenopus produce free-living, relatively transparent embryos, allowing for direct visualization of development and large-scale chemical screens (Tomlinson et al. 2005; Wheeler and Brändli 2009; Lienkamp et al. 2012). Large numbers of embryos are produced in a single clutch, allowing for hundreds of embryos to be manipulated by injection of antisense morpholino oligonucleotides or messenger RNAs (mRNAs). However, morpholino cost (∼$400 each) and toxicity concerns have hindered the ability to conduct large-scale genetic screens. CRISPR gene editing has made the use of Xenopus to study organogenesis and human disease genes more feasible (Blitz et al. 2013; Nakayama et al. 2013; Guo et al. 2014; Bhattacharya et al. 2015; Naert et al. 2016, 2017; Banach et al. 2017). Established Xenopus fate maps enable targeted microinjection into a selected blastomere that gives rise to an organ of interest (Moody 1987a,b; Moody and Kline 1990; Bauer et al. 1994; Karpinka et al. 2015; DeLay et al. 2016). Therefore, instead of generating conditional knockout mutant lines, Xenopus F0 embryos can be used to study gene function in a specific tissue, while avoiding early embryonic lethality associated with altering gene expression in the entire embryo.
Unlike mammalian metanephric kidneys, the Xenopus pronephros consists of a single functional nephron (Dressler 2006). The structure, as well as the spatial and developmental markers, is conserved between Xenopus pronephric and mammalian metanephric nephrons (Brändli 1999; Hensey et al. 2002). The Xenopus pronephros becomes functional within 2–3 days of fertilization, and visualization of the nephron through the epidermis makes Xenopus a simple model for studying genes affecting human kidney development and disease.
The LIM-class homeodomain transcription factor, lhx1 (lim1), is essential for mouse placenta formation, and rare surviving lhx1-null mice lack head structures, kidneys, and reproductive organs (Shawlot and Behringer 1995; Kobayashi et al. 2005). In Xenopus, lhx1 is expressed in the Spemann organizer and kidney (Taira et al. 1992, 1994). Injection of dominant negative lhx1 constructs leads to anterior somite defects, bent body axis, and diminished pronephric development, and morpholino injection causes bent body axis (Chan et al. 2000; Kodjabachian et al. 2001; Hukriede et al. 2003; Rankin et al. 2011). Due to the essential role of lhx1 in early development, targeted knockdown/out strategies have been employed to study its effects on mouse and Xenopus embryonic development. Therefore, lhx1 was selected to test the feasibility of kidney-targeted CRISPR knockout.
This study is the first to examine tissue-targeted knockout strategies in Xenopus. We demonstrate that CRISPR knockout can be targeted to a tissue of interest in F0 X. laevis embryos and establish a control single guide RNA (sgRNA) against slc45a2, a gene necessary for pigment production in zebrafish and humans (Ko et al. 2012; Irion et al. 2014). We also show that lhx1 knockout can be targeted to F0 embryonic kidneys, establishing the feasibility of using F0 X. laevis embryos for large-scale screens of genes implicated in human kidney disease. This targeting strategy can be easily applied to the study of other organ systems by selecting and injecting the appropriate blastomere in the early embryo (Moody 1987a,b; Moody and Kline 1990; Bauer et al. 1994; DeLay et al. 2016).
Materials and Methods
Embryos
Wild-type X. laevis adults were purchased from Nasco (LM00531MX) and embryos were obtained from these adults and reared as previously described (Sive et al. 2000). This protocol was approved by the University of Texas Health Science Center at Houston’s Center for Laboratory Animal Medicine Animal Welfare Committee, which serves as the Institutional Animal Care and Use Committee (protocol #: AWC-16-0111).
sgRNA design and production
slc45a2 genomic sequence was obtained from Xenbase (http://xenbase.org) and lhx1 sequences were obtained from the X. laevis genome browser at the Francis Crick Institute (http://genomes.crick.ac.uk/). sgRNAs against slc45a2 and lhx1 were designed using ZiFit (http://zifit.partners.org). Targets were blasted against the genome to ensure a unique hit and input into CRISPRscan to obtain predicted efficiency scores (http://www.crisprscan.org) (Moreno-Mateos et al. 2015). sgRNAs were designed to be complimentary to both the long and short chromosomes of lhx1. Slc45a2 transmembrane protein domains were determined and plotted using Protter (Omasits et al. 2014).
sgRNAs were produced using a PCR-based method (Bhattacharya et al. 2015). A modified universal reverse primer was used in conjunction with a gene-specific forward primer containing a T7 polymerase promoter (Table 1) to create a DNA template for subsequent sgRNA production (Bhattacharya et al. 2015).
Table 1. sgRNA production primers used in this study.
| Target gene | Sequence (5′ to 3′) | CRISPRscan score |
|---|---|---|
| Universal reverse | AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC | N/A |
| slc45a2 | CTAGCTAATACGACTCACTATAGGTTACATAGGCTGCCTCCAGTTTTAGAGCTAGAAATAGCAAG | Unscored |
| lhx1 exon 1 | CTAGCTAATACGACTCACTATAGGAGAAATGCTTCTCCAGGGGTTTTAGAGCTAGAAATAGCAAG | 64 |
| lhx1 exon 2 | CTAGCTAATACGACTCACTATAGGTGTGCGGGCTGTGCCCAGGTTTTAGAGCTAGAAATAGCAAG | 87 |
| lhx1 exon 3 | CTAGCTAATACGACTCACTATAGGCTCCCTTATGTGTCGGGTGTTTTAGAGCTAGAAATAGCAAG | 58 |
T7 polymerase was purified from Rosetta cells transformed with RP-pETHis6-T7 RNA polymerase expression plasmid (Ellinger and Ehricht 1998). Two units of T7 polymerase were combined with 8 µl PCR product, 2 µl each of 75 mM dNTPs, and 2 µl 10X T7 buffer (40 mM Tris-HCl, 6 mM MgCl2, 2 mM spermidine, 1 mM dithiothreitol, pH 7.9). The tube was incubated at 37° for 5 hr and 1 µl DNase was added, followed by a 15-min incubation at 37°. Fifteen microliters of ammonium acetate was added, and the reaction was brought up to 150 µl with RNase-free water. sgRNA was purified by acid phenol:chloroform extraction and ethanol precipitation.
Microinjection
Microinjections were performed as previously described (DeLay et al. 2016), with 10 nl of injection mix injected into individual blastomeres. For 8-cell injections, D1 blastomere was injected to target the eye, while the V2 blastomere was injected to target the kidney (Moody 1987a). sgRNA and Cas9 protein (CP01; PNA Bio) were incubated at room temperature for at least 5 min prior to co-injecting with either Alexa Fluor 488 fluorescent dextran or membrane-RFP RNA (Davidson et al. 2006; DeLay et al. 2016) tracers.
Genomic analysis
DNA was extracted from individual stage 10–12 embryos injected at the 1-cell stage (Bhattacharya et al. 2015). Regions surrounding the sgRNA target sites were amplified by PCR (Table 2). For lhx1, nested PCR was conducted to obtain the correct product. The lhx1 outer primers were used for the first PCR reaction, followed by a second reaction using lhx1 inner primers, with the resulting PCR product used for sequencing. PCR products were sequenced using the appropriate forward primer (Table 2). Insertion/deletion frequencies were calculated with TIDE (https://tide.nki.nl) (Brinkman et al. 2014).
Table 2. Genotyping primers used in this study.
| Target gene | Forward sequence (5′ to 3′) | Reverse sequence (5′ to 3′) |
|---|---|---|
| slc45a2.L | GTTCCCTTCGCTCATACAATGa | GCCAGAAAGGGGTTTATTGC |
| lhx1.L exon 1 (outer primer set) | CCGTAGCACTGGACGTGATGT | CAGCTTAGGCTACCACACTGCCGb |
| lhx1.L exon 1 (inner primer set) | TGCCTTCTATTCTCCTAATCCGCCCa,b | GAAGAGTTTGCTCCTTGCCCTC |
| lhx1.S exon 1 | CTGGACCGTTTCTTGTTGAATGa | GGTTTCACAAAGGGAAGTGCTG |
| lhx1.L exon 2 | CAGCAAGAGATGTAGCCAGCa | GTCCTACAACTATGGCGAAACG |
| lhx1.S exon 2 | CCTACAACAGTGGCGAAACa | GTCCTCCATTCTTCCTACGG |
| lhx1.L exon 3 | CATAGGGTGAAGAGGGCAAGa | CTCAAGTCTCCTTTGCAGCCAG |
| lhx1.S exon 3 | CCATTTGCAAGTTGATACCCa | GGTGAGACGGTTCATAGTGTG |
Forward primer used for genomic sequencing.
From Bachy et al. (2001).
Western blots
Embryos were staged (Nieuwkoop and Faber 1994) and collected to make protein lysates as previously described (Kim et al. 2002). One embryo equivalent of lysate was run in each well of an 8% SDS-PAGE gel. Protein was transblotted onto a 0.2-µm nitrocellulose membrane (GE Healthcare), and blocked for 3 hr (KPL block; SeraCare) at room temperature. Blots were incubated in 1:500 rabbit anti-Lhx1 primary antibody (Venegas-Ferrin et al. 2010) for 24 hr at 4° or 1:1000 rabbit anti-GAPDH (Santa Cruz FL-335) for 3 hr at room temperature. Blots were washed with TBST, incubated in goat anti-rabbit IgG horseradish peroxidase secondary antibody (1:5000; BioRad, Hercules, CA) for 2 hr at room temperature, and washed again with TBST prior to imaging (BioRad ChemiDoc XRS+) using enhanced chemiluminescence (Pierce Supersignal West Pico).
Immunostaining
Embryos were staged (Nieuwkoop and Faber 1994), fixed (DeLay et al. 2016), and immunostained using established protocols (Lyons et al. 2009). Proximal tubule lumens were labeled with 3G8 antibody (1:30) (Vize et al. 1995), and cell membranes of the intermediate, distal, and connecting tubules were labeled with 4A6 antibody (1:5) (Vize et al. 1995). Lhx1 was detected with Lhx1 antibody (1:250) (Venegas-Ferrin et al. 2010), and membrane-RFP tracer was labeled with RFP antibody (1:250; MBL International PM005). Goat anti-mouse IgG Alexa 488 (1:2000; Invitrogen, Carlsbad, CA), and goat anti-rabbit IgG Alexa 488 and Alexa 555 (1:2000; Invitrogen) secondary antibodies were used to visualize kidney and membrane-RFP tracer staining.
In situ hybridization
Digoxigenin-labeled RNA probes were prepared using a DIG RNA labeling kit (Roche). Constructs were linearized and synthesized using the listed enzyme and polymerase: lhx1-antisense XhoI/T7 (Taira et al. 1992; Carroll and Vize 1999), hnf1β-antisense SmaI/T7 (Demartis et al. 1994), atp1a1-antisense SmaI/T7b (Eid and Brändli 2001). Embryos were processed as described, eliminating the RNAse A/T1 step (Sive et al. 2000). 1:3000 dilution of Anti-DIG Fab fragments (Roche) and NBT/BCIP tablets (Roche) were used to detect probes.
Imaging
Embryos were scored and photographed using a Leica S8A80 stereomicroscope and Leica MC120 HD camera or an Olympus SZX16 fluorescent stereomicroscope and Olympus DP71 camera. 3G8/4A6 immunostained kidney images were taken using a Zeiss LSM800 confocal microscope. Images were processed with Adobe Photoshop and Illustrator CS6.
Data availability
Plasmids used in this study are available upon request. The authors declare that all data necessary for confirming the conclusions presented in the article are fully represented within the article.
Results
X. laevis CRISPR control
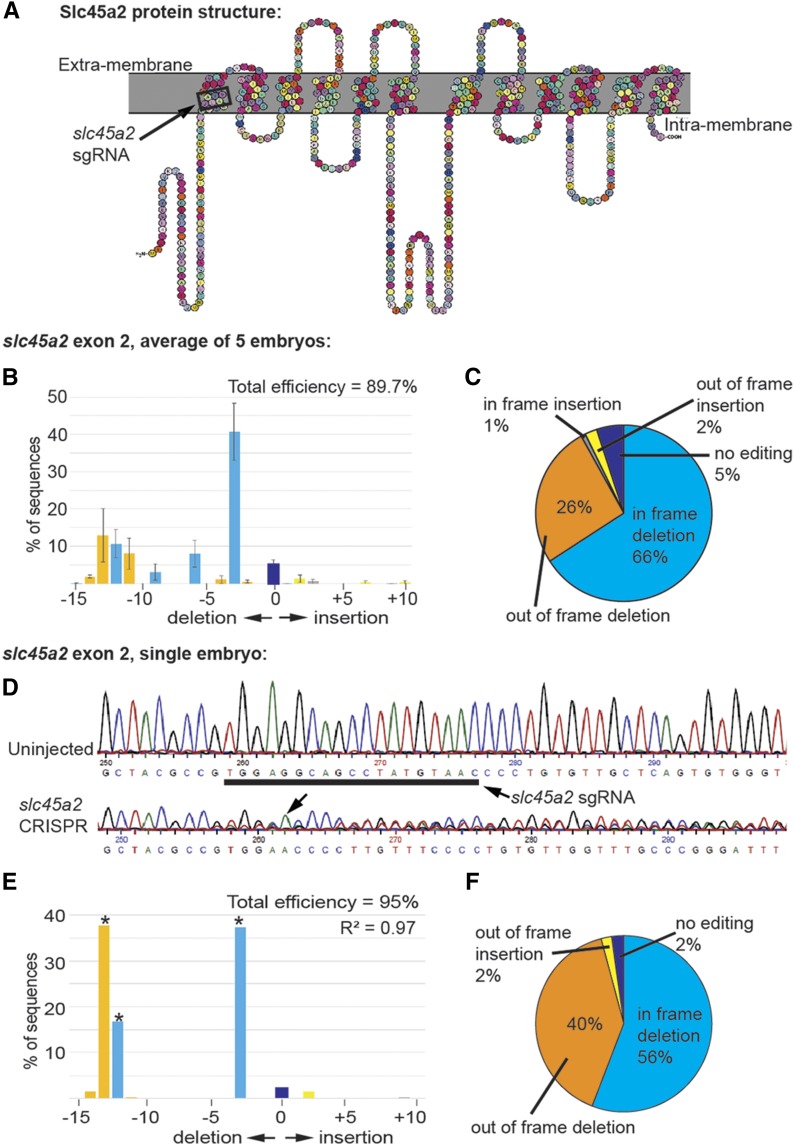
Knockout of slc45a2 (chromosome 1L) results in eye and skin pigment loss, providing a phenotypic readout of CRISPR editing without affecting kidney development (Irion et al. 2014; Shigeta et al. 2016). Although X. laevis is allotetraploid, there is only one homeolog of the slc45a2 gene: slc45a2.L. Therefore, the one sgRNA targeting the second exon of slc45a2.L (first transmembrane domain) hits the only copy of the gene (Figure 1A). Upon knockout, TIDE analysis indicated that slc45a2 was edited mosaically in all five embryos tested (Supplemental Material, Figure S1) (Brinkman et al. 2014). Overall, 89.7% of the DNA was edited, resulting most commonly in a three-base deletion (Figure 1, B and C). Sequence decomposition near the sgRNA binding region (Figure 1D) was confirmed in all embryos examined (Figure S1). Although the most common mutation was a three-base deletion (Figure 1, E and F), individual embryos showed varying mutation profiles (Figure S1).
Figure 1.
sgRNA targeting slc45a2 efficiently edits Xenopus embryo DNA. All data shown are from stage 10–12 embryos injected with slc45a2 sgRNA and Cas9 protein at the 1-cell stage. (A) Diagram of Slc45a2 protein showing the 12 transmembrane domains, with the region where the slc45a2 sgRNA binds outlined in black. (B) Percent of sequenced slc45a2 DNA containing different insertions and deletions. Bars shown are the mean of the percent of indel sequences from five individual embryos, error bars represent SEM. (C) Percent of indels that lead to in-frame or out-of-frame mutations. Data shown are the mean of the sequencing data from five individual embryos. (D–F) Results from a single representative embryo shown. (D) Sequencing chromatogram shows DNA editing after injection of slc45a2 sgRNA and Cas9 protein. Underlined region indicates the sgRNA binding sequence. Arrow indicates the start of degradation of the sequence due to CRISPR editing. (E) TIDE analysis shows degradation of the sequence trace in the slc45a2 CRISPR embryo after the expected Cas9 editing site. * P < 0.001 as identified by TIDE. (F) TIDE analysis prediction of the indels present in the single embryo, indicating that 3-, 12-, and 13-bp deletions are the most common indels in this embryo.
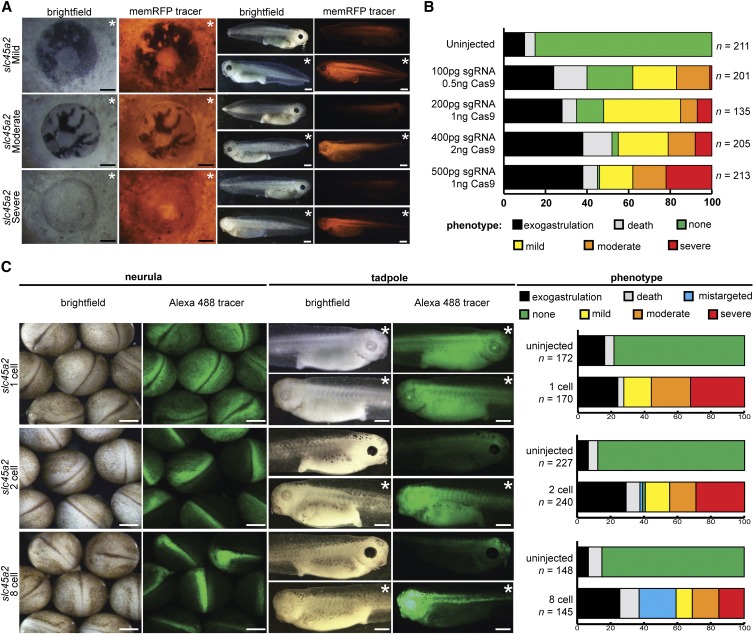
Since body pigment loss is difficult to assess, eye pigment loss was used to evaluate slc45a2 gene editing efficiency (Figure 2A). Less than 50% loss was scored as “mild,” >50% loss was scored as “moderate,” and nearly complete loss was scored as “severe.” Varying amounts of slc45a2 sgRNA and Cas9 protein were injected into one cell of 2-cell embryos, and mortality and eye phenotype severity were assessed (Figure 2B). Of the conditions tested, 500 pg sgRNA and 1 ng Cas9 protein provided the best balance between phenotype severity and mortality (Figure 2B).
Figure 2.
slc45a2 knockout results in loss of eye and body pigmentation and can efficiently be targeted to the eye. (A) Phenotype severity scoring system based on pigment loss in the eye. Mild phenotype: >50% of eye pigment present; moderate phenotype: <50% of eye pigment present; severe phenotype: almost no eye pigment present. Embryos injected with 500 pg slc45a2 sgRNA, 1 ng Cas9 protein, and memRFP RNA tracer in one cell of a 2-cell embryo. (B) Survival and phenotype severity of embryos injected with varying amounts of slc45a2 sgRNA and Cas9 protein. Exogastrulation: embryo death prior to gastrulation; death: embryo death after gastrulation and before stage 39–41; none: no eye pigment loss. Surviving stage 39–41 embryos were scored based on phenotype severity. (C) Phenotype severity eye-targeted CRISPR gene editing. Embryos injected with 500 pg slc45a2 sgRNA, 1 ng Cas9 protein, and Alexa 488 dextran tracer at 1-, 2-, and 8-cell stages (blastomere D1) show slc45a2 editing in the targeted eye tissue. Embryos shown at neurula (NF stage 19–20) and tadpole (NF stage 39–41) stages. Mistargeted: tracer not present in the eye. (A and C) White bar, 500 µm, black bar, 100 µm. * Injected side of embryo.
Tissue-targeted slc45a2 knockout
slc45a2 knockout was targeted to the eye by injecting embryos at the 1-, 2-, and 8-cell (left D1 blastomere) stages (Moody 1987a). Each of these injection stages showed eye pigment loss (Figure 2C). One-cell injections resulted in embryos with pigment loss in both eyes, while 2- and 8-cell injections caused pigment loss only on the injected side. Although embryos injected at the 2-cell stage exhibited both eye and trunk pigment loss, embryos properly injected at the 8-cell stage lost eye pigmentation but not pigmentation on the head or trunk (Figure 2C). Alexa 488:dextran tracer showed that 8-cell targeted injections yielded greater restriction to the eye than 1- or 2-cell injections. These data suggest that tissue-targeted knockout is possible and targeting slc45a2 knockout to the eye produces embryos lacking eye pigmentation.
lhx1 knockout
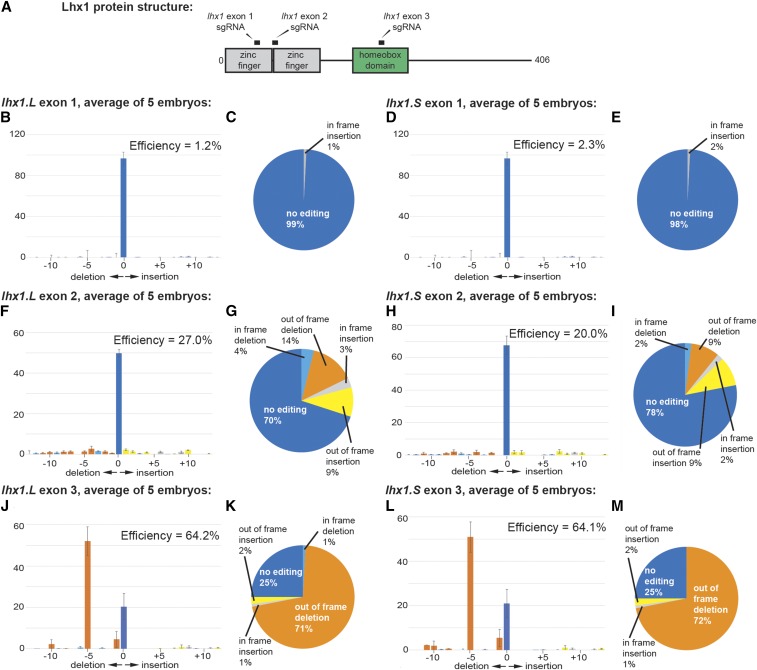
lhx1 has two homeologs, located on chromosomes 2L and 2S. Three sgRNAs, each complimentary to both the long and short homeologs of lhx1, were designed to target exons 1, 2, and 3, corresponding to the first and second zinc finger and the homeobox domain of the Lhx1 protein, respectively (Figure 3A) (Table 1) (Sander et al. 2007, 2010; Moreno-Mateos et al. 2015). All three of the sgRNAs were designed so that there were no mismatches between the sgRNA target site and the sgRNA sequence on either of the lhx1 homeologs.
Figure 3.
Comparison of the editing efficiency of three different sgRNAs designed to edit lhx1. (A) Diagram of the domains present in Lhx1 protein. Regions corresponding to the three lhx1 sgRNAs used in this study are represented by the black bars above the protein domains. Each sgRNA was complementary to both lhx1.L and lhx1.S. (B, C, F, G, J, and K) TIDE analysis results from the long chromosome, with pooled results from five embryos reported. (D, E, H, I, L, and M) TIDE analysis results from the short chromosome, with pooled results from five embryos reported. (B, D, F, H, J, and L) Percentage of DNA editing contributed to insertions and deletions of different sizes. Error bars represent the SEM. (C, E, G, I, K, and M) Percent of in-frame and out-of-frame insertions and deletions.
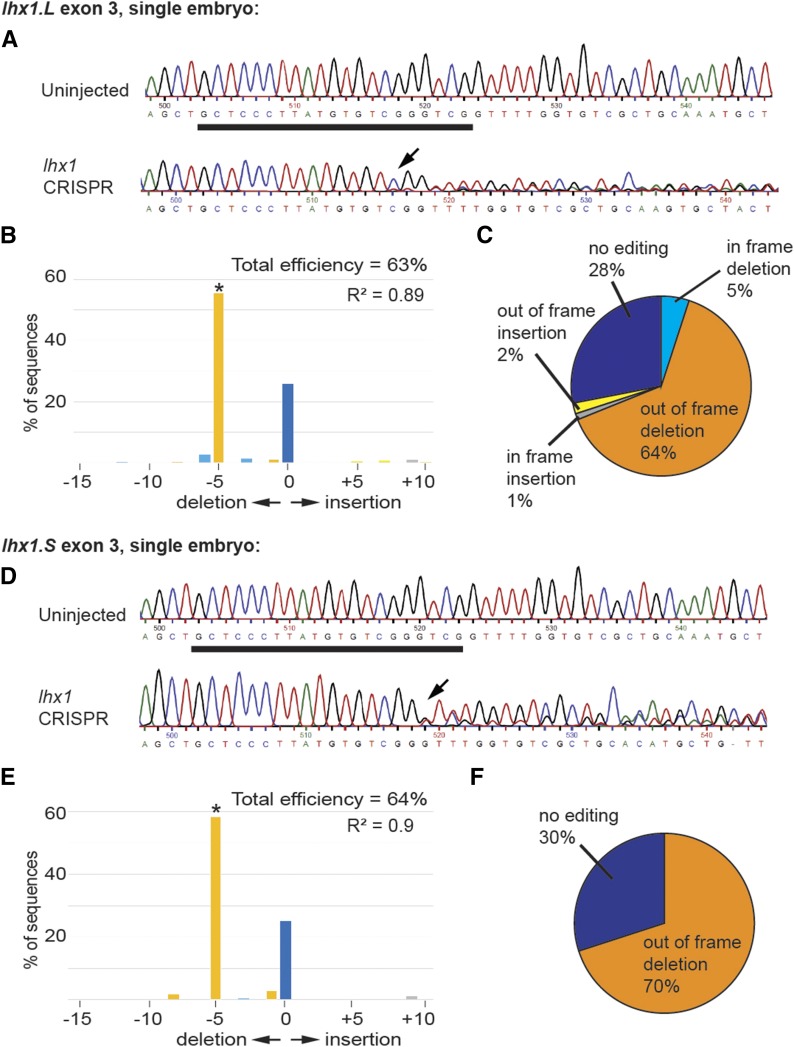
Sequencing and TIDE analysis of DNA from stage 10–12 embryos indicates that the sgRNA targeting exon 1 was least efficient (Figure 3, B and D), resulting in mostly in-frame insertions (Figure 3, C and E), while editing of exon 2 was more efficient (Figure 3, F and H), resulting in mostly out-of-frame deletions and insertions (Figure 3, G and I). The sgRNA targeting exon 3 was most efficient, resulting in mostly a 5 bp out-of-frame deletion and a premature stop codon (Figure 3, J–M and Figure 4). The exon 3 sgRNA edited 64% of the DNA for both short and long homeologs (Figure 3, J and L). Additionally, the percent of aberrant sequences, defined as the difference in nucleotide chromatogram peak height between the unedited and knockout sequences, was significant downstream of the Cas9 cut site and greatly reduced upstream for the exon 3 sgRNA only (Figure S2). Individual embryos had similar editing efficiency of both the long and short homeologs (Figure 4), suggesting that the exon 3 sgRNA is able to efficiently edit both homeologs in the same embryo. Due to the greater editing efficiency of the exon 3 sgRNA, we chose to use this sgRNA for subsequent experiments.
Figure 4.
Comparison of the editing efficiency of lhx1 exon 3 sgRNA in a single embryo. Results shown in A–C and D–F are from the same embryo. (A–C) Editing efficiency in lhx1.L. (D–F) Editing efficiency in lhx1.S. (A and D) Sequencing chromatogram showing degradation of the sequence in the region of the sgRNA binding site. Underlined region corresponds to the sgRNA binding site, and the black arrow marks the point of sequence degradation in the lhx1 knockout embryo. (B and E) TIDE analysis results from a single embryo showing percentage of DNA editing due to insertions and deletions of different sizes. * P < 0.001 as identified by TIDE. (C and F) Percent of in-frame and out-of-frame insertions and deletions.
lhx1 knockout disrupts pronephric development
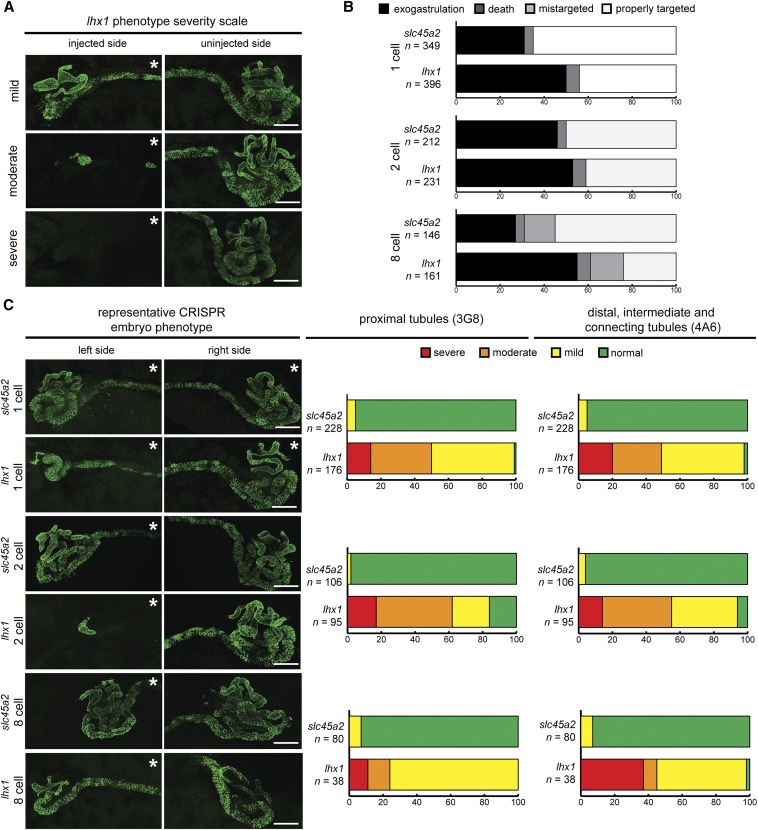
lhx1 was knocked out at the 1-, 2-, or 8-cell stage (V2 blastomere) to target knockout to the kidney (Moody 1987a). As a negative control, slc45a2 was knocked out. The kidney phenotype was rated as “mild” for slight impairments in kidney development, “moderate” if tubule regions were missing, and “severe” if kidney tubules were missing entirely. Kidneys were scored as “normal” if no differences existed between the injected and uninjected sides (Figure 5A). For embryos injected at the 1-cell stage, phenotype severity was assessed relative to slc45a2 knockout embryos at the same stage.
Figure 5.
Knockout of lhx1 leads to kidney developmental defects. (A) Scoring system used to assess the phenotypic severity of lhx1 knockout embryos. Mild: decrease in kidney tubulogenesis in comparison to the uninjected side of the embryo; moderate: portions of the kidney tubules missing; severe: kidney tubules absent. Embryos stained with antibodies 3G8 (to label proximal tubule lumen) and 4A6 (to label the cell membranes of the intermediate, distal, and connecting tubules). (B) Targeting CRISPR knockout of lhx1 to the kidney does not decrease embryo mortality. Embryos injected at the 1-, 2- and 8-cell (blastomere V2) stages. Exogastrulation: embryos die during gastrulation; death: embryos survive gastrulation but die prior to stage 40; mistargeted: tracer not present in the kidney. (C) lhx1 targeted knockout reduces late markers of kidney development. Embryos assessed at stage 39–41 using immunostaining with 3G8 and 4A6 antibodies. (A and C) * denotes injected side of embryo. White bar, 100 μm.
Because lhx1 knockout is embryonically lethal in mice, embryonic mortality was assessed upon targeting lhx1 knockout to the kidney. We anticipated that 8-cell targeting would reduce embryo death compared to knockout in 1- or 2-cell embryos, but no difference in exogastrulation was observed for the three lhx1 injection conditions (P = 0.74, Figure 5B).
The effect of lhx1 knockout on kidney development was assessed in stage 39–41 embryos using antibodies to stain differentiated kidney tubules (3G8 and 4A6) (Vize et al. 1995). Embryos were injected at the 1-, 2-, or 8-cell (V2 blastomere) stages to assess the efficiency of kidney-targeted lhx1 knockout. lhx1 knockout disrupted proximal, distal, intermediate, and connecting tubule development for all three injection stages, while slc45a2 knockout did not (Figure 5C). Embryos injected at the 2-cell stage exhibited the highest proportion of kidneys with moderate or severe phenotypes, while embryos injected at the 8-cell stage showed the lowest proportion of moderate or severe kidney phenotypes. Therefore, 2-cell injections were the most efficient at producing moderate and severe kidney phenotypic effects.
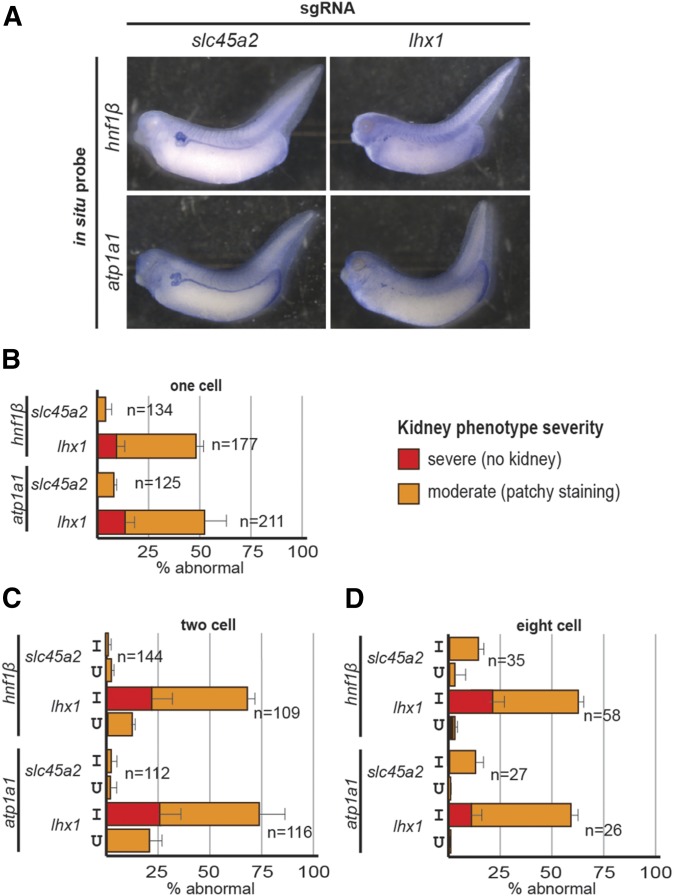
in situ staining with probes that label the majority of the pronephros (hnf1β and atp1a1) revealed decreased kidney tubulogenesis upon lhx1 knockout (Figure 6A). Over 75% of the embryos injected at the 2- and 8-cell stages showed reduced hnf1β and atp1a1 expression, with the majority displaying moderate or severe kidney phenotypes (Figure 6, C and D). In contrast, fewer embryos injected at the 1-cell stage showed moderate or severe phenotypes (Figure 6B), suggesting that 2- or 8-cell injections result in increased lhx1 knockout phenotype severity in comparison to 1-cell injections.
Figure 6.
Knockout of lhx1 leads to a decrease in kidney developmental markers as seen by in situ hybridization. (A) Representative embryos showing that knockout of lhx1 leads to a decrease in hnf1β and atp1a1 expression in the pronephros, while slc45a2 knockout does not cause a decrease in these markers of kidney development. (B) lhx1 knockout in 1-cell embryos leads to a decrease in hnf1β and atp1a1 in comparison to slc45a2 knockout control embryos. (C) lhx1 knockout in 2-cell embryos leads to a decrease in hnf1β and atp1a1 in comparison to slc45a2 knockout control embryos. (D) lhx1 knockout in 8-cell embryos leads to a decrease in hnf1β and atp1a1 in comparison to slc45a2 knockout control embryos. I, injected side of embryo; U, uninjected side of embryo.
lhx1 knockout does not lead to head or axis defects
Because lhx1 knockout did not lead to developmental head or axis defects seen using other techniques (Taira et al. 1992; Carroll and Vize 1999; Chan et al. 2000; Rankin et al. 2011), a Xenopus Lhx1 antibody was utilized to assess Lhx1 levels by immunoblot after CRISPR knockout (Venegas-Ferrin et al. 2010). Lhx1 levels were decreased in lhx1 knockout embryos in comparison to slc45a2 knockout embryos starting at stage 10–12 (Figure 7A and Figure S4), suggesting that lhx1 knockout results in decreased Lhx1 protein production prior to axis establishment and kidney specification. Importantly, knockout did not result in complete loss of Lhx1, which may explain why the knockout embryos did not display head or axis defects.
Figure 7.
Lhx1 protein and lhx1 RNA levels are decreased upon CRISPR knockout. (A) Immunoblot (IB) showing that knockout of lhx1 leads to a decrease in Lhx1 protein in comparison to slc45a2 knockout controls as early as embryonic stage 10–12. One-cell embryos were injected with 1 ng Cas9 protein and 500 pg of either slc45a2 or lhx1 sgRNA. (B) Immunoblot of embryo lysates from different stages of Xenopus development, ranging from 1-cell embryo (stage 1) to tadpole (stage 43). Levels of Lhx1 and GAPDH (loading control) protein show that Lhx1 is present in embryos throughout pronephric development (stages 12.5–43). (C) lhx1 in situ of stage 20 embryos shows loss of kidney staining on the injected side (white arrowheads) of lhx1 CRISPR embryos, but not in slc45a2 CRISPR embryos. No decrease in neural staining in the lhx1 knockout embryos was observed in comparison to the slc45a2 controls (black arrowheads). CRISPR knockout done in one cell of 2-cell embryos. White bar, 200 µm. Graph depicts severity of lhx1 loss on the injected side of the embryo. None: no loss of lhx1 staining; mild: decrease in lhx1 staining; moderate: patchy loss of lhx1 staining; severe: complete loss of lhx1 staining. (D) lhx1 immunostaining of stage 32 embryos shows loss of kidney staining on the injected side of lhx1 CRISPR embryos, but not in slc45a2 CRISPR embryos. CRISPR knockout done in one cell of 2-cell embryos. The epidermis of the embryo was removed prior to imaging. White bar, 100 µm. Graph depicts severity of lhx1 immunostaining loss on the injected side of the embryo. None: no loss of lhx1 staining; mild: decrease in lhx1 staining; moderate: patchy loss of lhx1 staining; severe: complete loss of lhx1 staining.
Immunoblot analysis was performed to determine whether Lhx1 protein was present prior to zygotic transcription initiation. Lhx1 protein was first detected in 1-cell embryos, with increased expression around gastrulation (stage 10–12) after the midblastula transition (Figure 7B). Lhx1 levels decreased between stages 25 and 30 and increased during later tadpole stages (35–40), corresponding to previously published mRNA expression data (Taira et al. 1992; Session et al. 2016; Watanabe et al. 2017) and the results seen in slc45a2 knockout embryos (Figure 7A). Taken together, these data indicate that low levels of maternally loaded lhx1 RNA and/or Lhx1 protein may compensate for the genomic knockout during head formation, and that knockout of slc45a2 does not alter the embryo stage-specific Lhx1 expression pattern.
in situ analysis of stage 20 embryos was conducted to determine whether knockout affects early kidney development. lhx1 knockout in 2-cell embryos resulted in decreased lhx1 expression in the kidney anlagen, indicating a loss of lhx1 transcript (Figure 7C). No decrease in lhx1 staining in the neural structures was observed in the lhx1 knockout embryos (Figure 7C).
To confirm that knockout decreases Lhx1 protein levels within the kidney, embryos were injected in one cell at the 2-cell stage and immunostained at stage 30 using an Lhx1 antibody that stains the nucleus of kidney cells and neural structures (Irion et al. 2014). Lhx1 knockout embryos showed reduced Lhx1 in the kidney on the injected side in comparison to the uninjected side of the embryo (Figure 7D). Similar to the range of kidney loss seen in later stage embryos visualized with 3G8 and 4A6 antibodies, we observed a range of lhx1 expression loss in lhx1 knockout embryos (Figure S3).
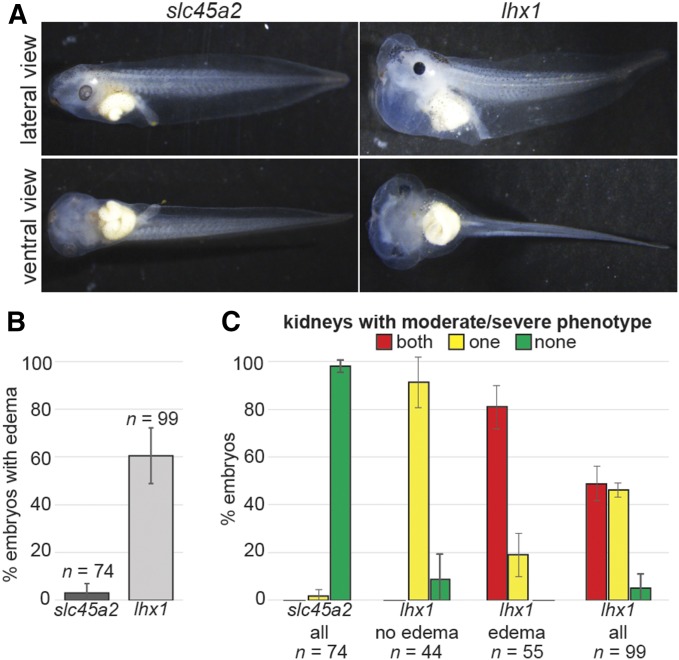
Knockout of lhx1 leads to edema
Assessment of edema was used as a readout of kidney function. Injections into both cells of 2-cell embryos resulted in edema in the head and thorax (Figure 8A) (Nieuwkoop and Faber 1994). Sixty percent of lhx1 knockout embryos displayed edema, compared to 3% of slc45a2 knockout controls (Figure 8B). Prior to edema development (stage 43–46), the embryos looked phenotypically normal and did not display head or axis defects.
Figure 8.
Knockout of lhx1 leads to edema formation. Embryos were injected into both cells of 2-cell embryos with 1 ng Cas9 protein and 500 pg of either slc45a2 or lhx1 sgRNA, and reared to stage 43–46 for immunostaining and scoring. Error bars represent the SEM. (A) Knockout of lhx1 leads to edema formation in Xenopus embryos. (B) The percent of lhx1 knockout embryos displaying edema at stage 43–46 is higher than slc45a2 control embryos. (C) Percent of embryos with moderate (missing parts) and severe (absent) kidney phenotypes. Most lhx1 knockout embryos with edema display moderate/severe kidney phenotypes in both kidneys, while most embryos without edema have at least one normal kidney.
Immunostaining showed that none of the lhx1 knockout embryos displaying edema had normal kidneys (Figure 8C), while all of the lhx1 knockout embryos without edema had at least one normal kidney. Likewise, the majority of the slc45a2 knockout embryos had two normal kidneys. These data suggest that lhx1 knockout prevents functional kidney formation.
Discussion
CRISPR gene editing is new in the allotetraploid X. laevis (Lane et al. 2015; Wang et al. 2015; Jaffe et al. 2016; Banach et al. 2017), and tissue-targeted CRISPR knockouts have not been reported. Our results indicate that CRISPR can efficiently target a tissue of interest. Although X. laevis has two homeologs for many of its genes, our results demonstrate that a single sgRNA can efficiently target both homeologs in F0 embryos. In fact, the sgRNA tested gave similar DNA editing efficiencies for both homeologs of lhx1.
We observed a range of pigment loss phenotypes upon knockout of slc45a2 ranging from mild to complete pigment loss. Approximately 40% of the total DNA sequenced from five individual embryos had a 3-bp deletion, while ∼26% had an out-of-frame deletion. Each embryo is mosaic, with individual cells (and alleles within those cells) potentially having different slc45a2 mutations. This was evidenced by our sequencing results, which showed that individual embryos displayed a range of mutations (Figure 1, D–F). It is unlikely that the in-frame deletions had no effect on slc45a2 function, as these mutations account for the vast majority of the sequenced mutations, and the majority of the surviving embryos were missing more than half the pigment in their eye (moderate and severe phenotypes). Instead, it is possible that the variation in pigmentation that was observed in our slc45a2 knockout embryos is due to functional maternal RNA or protein, or incomplete penetrance. We used wild-type, pigmented Xenopus for this study. The adult frogs and resulting embryos display varying degrees of pigmentation. Therefore, if the parent is highly pigmented, this may have an effect on the embryo’s pigment as well. In fact, slc45a2 mRNA levels are highest in unfertilized oocytes, suggesting that maternal effects may play a role in an embryo’s pigmentation (Session et al. 2016).
lhx1 knockout led to abnormally developed kidney tubules in embryos injected at the 1-, 2-, and 8-cell stages. Injecting embryos at the 2-cell stage results in embryos with one lhx1 knockout kidney and one unedited kidney, serving as an internal control. No advantage to targeting at the 8-cell stage in comparison to injecting one cell of a 2-cell embryo was observed. Eight-cell microinjections are challenging because the embryo must be turned prior to injection, and identification of the correct cell can be difficult, while at the 2-cell stage either blastomere can be injected. As early developmental defects are not seen with 2-cell injections, CRISPR editing of genes implicated in kidney development can be carried out at the 2-cell stage, which is not a viable strategy for morpholino-mediated knockdown of essential genes.
Of the three lhx1 sgRNAs tested, the sgRNA targeting exon 3 was most efficient, although its predicted efficiency was the least (Table 1). Additionally, the slc45a2 sgRNA resulted in editing of over 89% of the DNA, but CRISPRscan did not score this sgRNA. Although CRISPRscan was designed against a library of Xenopus tropicalis sgRNAs, our results suggest that guide quality predicted by this software does not necessarily apply to X. laevis.
lhx1 plays an important role in early embryonic development of head structures in both mice and Xenopus (Shawlot and Behringer 1995; Yasuoka et al. 2014). In mouse, lhx1 knockout is embryonically lethal (Shawlot and Behringer 1995). For this reason, previous lhx1 loss-of-function studies in Xenopus were accomplished by targeted injection to bypass early developmental defects (Chan et al. 2000; Hukriede et al. 2003; Cirio et al. 2011; Rankin et al. 2011). Unexpectedly, we found no difference in lethality between embryos injected at the 1-, 2-, or 8-cell stages. We did not observe early developmental defects (missing head structures, bent spines) in lhx1 knockout embryos, suggesting that targeted knockout is not necessary. However, disrupted kidney development was observed as reported in prior knockdown studies (Chan et al. 2000; Cirio et al. 2011). Similar to mouse, we found that lhx1 knockout led to kidneys with regions of the tubules missing, suggesting that there are multiple points of kidney induction (Kobayashi et al. 2005).
Although the majority of lhx1 knockout embryos displayed kidney defects, we did not observe head or axis defects. There are multiple potential explanations for this observation. Unlike morpholinos or dominant negative constructs, CRISPR should not affect maternal protein or RNA (Watanabe et al. 2017). Prior studies suggest that CRISPR may not influence early Xenopus embryonic development due to unaffected maternal mRNA and protein deposits (Bhattacharya et al. 2015). Alternatively, the low levels of Lhx1 protein in knockout animals may be enough for the head organizer to develop normally, whereas pronephric development may be more sensitive to lower Lhx1 levels. The mosaic nature of lhx1 knockout may allow the embryos to develop without early developmental defects, because unlike the organizer, the pronephros may be affected cell-autonomously by lhx1 knockout. Similar to our results, morpholino knock down of β-catenin in X. tropicalis results in ventralization, but CRISPR knockout does not (Bhattacharya et al. 2015). These data suggest that CRISPR knockout in F0 embryos of Xenopus and other models, such as zebrafish, may be useful to study genes that are embryonically lethal in null mutants, especially for studying later developmental processes such as organ formation. To disrupt early developmental processes, such as dorsal organizer function, it may be necessary to perform “leap-frogging” to knock out the gene only in the germ cells or oocyte transfer to create F0 mutations (Blitz et al. 2016; Aslan et al. 2017).
Successful knockout of lhx1 in F0 embryos requires editing of all four lhx1 alleles to produce a complete loss-of-function phenotype. Additionally, all four alleles must be edited in a way that produces a non-functional protein, such as with out-of-frame insertions or deletions. The lhx1-knockout embryos produced in this study showed a range of phenotypes, from a decrease in tubule development to a complete loss of tubules. One possible explanation for the range of observed phenotypes is that embryos with the most severe phenotype have all four lhx1 alleles edited, while embryos with moderate and mild phenotypes have one or more unedited lhx1 alleles. Sequencing of the two lhx1 homeologs showed that ∼64% of the lhx1.L and lhx1.S DNA was edited. Therefore, the probability that all four alleles were edited in a single cell was ∼41%. This frequency is similar to the range of phenotypes observed in knockout embryos, with the phenotype severity likely corresponding to the percentage of cells that have all four alleles knocked out. Therefore, it is possible that embryos with a severe phenotype have all four alleles edited in most of their kidney cells, while embryos that have a moderate or mild phenotype have one or more unedited lhx1 alleles in the majority of their kidney cells.
The two genes knocked out in this study, slc45a2 and lhx1, have known functions in human, mouse, and Xenopus (Shawlot and Behringer 1995; Chan et al. 2000; Kodjabachian et al. 2001; Hukriede et al. 2003; Kobayashi et al. 2005; Rankin et al. 2011; Ko et al. 2012). Although the F0 embryos generated in this study displayed varying phenotype severities, the resulting pigment loss and kidney defect phenotypes were as expected from previous X. tropicalis and Danio rerio CRISPR knockout (slc45a2) and X. laevis knockdown (lhx1) studies (Cirio et al. 2011; Irion et al. 2014; Shigeta et al. 2016). We saw a range of phenotype severities in the slc45a2 and lhx1 knockout embryos, similar to what has been reported in other reports of slc45a2 CRISPR knockout in X. tropicalis and antisense knockdown of lhx1 (Cirio et al. 2011; Shigeta et al. 2016). Therefore, our results show that CRISPR is a reliable way of producing large numbers of F0 embryos with known genetic knockout phenotypes. However, the range of phenotypes observed in our knockout embryos may make the characterization of the function of unknown genes more difficult. In this case, CRISPR may be a good option for quickly and cheaply screening large numbers of genes for potential phenotypic effects. When a gene is found to produce a phenotype in F0 embryos, follow-up experiments can be performed to verify gene function. Alternatively, mutant lines may be bred to create homozygous F1 individuals with known genetic mutations. Although it may be easy to create lines with knockout of nonessential genes like slc45a2, a knockout line with a gene such as lhx1 may not be feasible. In this case, phenotypic analysis of F0 embryos may be a more effective strategy for studying the function of essential genes.
X. laevis is a unique model for studying genes implicated in human diseases using CRISPR genome editing (Tandon et al. 2017). Approximately 79% of genes implicated in human diseases have Xenopus orthologs, including genes that are involved in human kidney disorders (Hellsten et al. 2010). In addition, Xenopus embryos develop functional kidneys within 2–3 days of fertilization, allowing for the assessment of large numbers of F0 mutant kidneys in a short time. Therefore, CRISPR genome editing allows for high-throughput screening of candidate human disease genes with the benefit that each embryo has an uninjected control side. Although we showed that targeted CRISPR knockout is highly efficient in the eye and kidney, the technique described here would be applicable to the study of other organs in the developing Xenopus embryo. The quick embryonic development time, large clutch size, and ability to target a tissue of interest on one side of the embryo make CRISPR genome editing in X. laevis an innovative model for the study of human gene function and genetic disorders.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300468/-/DC1.
Acknowledgments
We thank the instructors and teaching assistants of the 2015 and 2016 Cold Spring Harbor Laboratory Xenopus Course, in particular K.J. Liu, M.K. Khokha, and E.K. Mis, for training in Xenopus CRISPR gene editing and in situ hybridization techniques. The instructors of the 2017 National Xenopus Resource Xenopus Genome Editing Workshop also provided advice on genome editing techniques. We also thank N.R. De Lay for the gift of the T7 polymerase expression plasmid and Rosetta cells, as well as for advice on sgRNA production and purification. We are grateful to the members of the laboratories of R.K.M. and P.D. McCrea, as well as to M. Kloc, for their helpful suggestions and advice throughout this project. In particular, we thank V. Krneta-Stankic for early help with injections. Additionally, we thank S.-T. Yen for assessing the lhx1 CRISPR sgRNA sequences, as well as S. McNamara and N. Aryal for guidance on CRISPR sgRNA design. We especially thank R.R. Behringer for advice on CRISPR knockout of lhx1 and for critically reading this article and providing guidance throughout this project. We are grateful to the University of Texas Health Science Center Office of the Executive Vice President and Chief Academic Officer and the Department of Pediatrics Microscopy Core for funding and maintaining the Zeiss LSM800 confocal microscope used in this work. These studies were supported by a National Institutes of Health (NIH) KO1 grant (K01DK092320 to R.K.M.), startup funding from the Department of Pediatrics Pediatric Research Center at the University of Texas McGovern Medical School (to R.K.M.), an NIH National Xenopus Resource Center grant (P40OD010997 to M.E.H.), and an NIH R01 grant (R01HD084409 to M.E.H.).
Author contributions: B.D.D. performed microinjections, sequencing and TIDE analysis, analyzed embryo phenotypes, and wrote the article. M.E.C. performed microinjections, in situ hybridization, and edema experiments. H.L.H. performed microinjections. M.S. and M.E.H. designed the slc45a2 sgRNA. J.M.D. helped to design and generate the lhx1 sgRNAs. N.S. and M.T. generated the Lhx1 antibody. R.K.M. conceived of the project, helped to design the lhx1 sgRNAs, and oversaw the experiments and article preparation. All authors critically read and edited the article.
Footnotes
Communicating editor: O. Hobert
Literature Cited
- Aslan Y., Tadjuidje E., Zorn A. M., Cha S. W., 2017. High-efficiency non-mosaic CRISPR-mediated knock-in and indel mutation in F0 Xenopus. Development 144: 2852–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachy I., Vernier P., Retaux S., 2001. The LIM-homeodomain gene family in the developing Xenopus brain: conservation and divergences with the mouse related to the evolution of the forebrain. J. Neurosci. 21: 7620–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach M., Edholm E. S., Robert J., 2017. Exploring the functions of nonclassical MHC class Ib genes in Xenopus laevis by the CRISPR/Cas9 system. Dev. Biol. 426: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D. V., Huang S., Moody S. A., 1994. The cleavage stage origin of Spemann’s organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development 120: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D., Marfo C. A., Li D., Lane M., Khokha M. K., 2015. CRISPR/Cas9: an inexpensive, efficient loss of function tool to screen human disease genes in Xenopus. Dev. Biol. 408: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz I. L., Biesinger J., Xie X., Cho K. W., 2013. Biallelic genome modification in F0 Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis 51: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz I. L., Fish M. B., Cho K. W., 2016. Leapfrogging: primordial germ cell transplantation permits recovery of CRISPR/Cas9-induced mutations in essential genes. Development 143: 2868–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändli A. W., 1999. Towards a molecular anatomy of the Xenopus pronephric kidney. Int. J. Dev. Biol. 43: 381–395. [PubMed] [Google Scholar]
- Brinkman E. K., Chen T., Amendola M., van Steensel B., 2014. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42: e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll T., Vize P. D., 1999. Synergism between Pax-8 and lim-1 in embryonic kidney development. Dev. Biol. 214: 46–59. [DOI] [PubMed] [Google Scholar]
- Chan T. C., Takahashi S., Asashima M., 2000. A role for Xlim-1 in pronephros development in Xenopus laevis. Dev. Biol. 228: 256–269. [DOI] [PubMed] [Google Scholar]
- Cirio M. C., Hui Z., Haldin C. E., Cosentino C. C., Stuckenholz C., et al. , 2011. Lhx1 is required for specification of the renal progenitor cell field. PLoS One 6: e18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. A., Marsden M., Keller R., Desimone D. W., 2006. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr. Biol. 16: 833–844. [DOI] [PubMed] [Google Scholar]
- DeLay B. D., Krneta-Stankic V., Miller R. K., 2016. Technique to target microinjection to the developing Xenopus kidney. J. Vis. Exp. 111: 10.3791/53799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demartis A., Maffei M., Vignali R., Barsacchi G., 1994. Cloning and developmental expression of LFB3/HNFIβ transcription factor in Xenopus laevis. Mech. Dev. 47: 19–28. [DOI] [PubMed] [Google Scholar]
- Dressler G. R., 2006. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 22: 509–529. [DOI] [PubMed] [Google Scholar]
- Eid S. R., Brändli A. W., 2001. Xenopus Na,K-ATPase: primary sequence of the beta2 subunit and in situ localization of alpha1, beta1, and gamma expression during pronephric kidney development. Differentiation 68: 115–125. [DOI] [PubMed] [Google Scholar]
- Ellinger T., Ehricht R., 1998. Single-step purification of T7 RNA polymerase with a 6-histidine tag. Biotechniques 24: 718–720. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhang T., Hu Z., Zhang Y., Shi Z., et al. , 2014. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development 141: 707–714. [DOI] [PubMed] [Google Scholar]
- Hellsten U., Harland R. M., Gilchrist M. J., Hendrix D., Jurka J., et al. , 2010. The genome of the Western clawed frog Xenopus tropicalis. Science 328: 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensey C., Dolan V., Brady H. R., 2002. The Xenopus pronephros as a model system for the study of kidney development and pathophysiology. Nephrol. Dial. Transplant. 17: 73–74. [DOI] [PubMed] [Google Scholar]
- Hukriede N. A., Tsang T. E., Habas R., Khoo P. L., Steiner K., et al. , 2003. Conserved requirement of Lim1 function for cell movements during gastrulation. Dev. Cell 4: 83–94. [DOI] [PubMed] [Google Scholar]
- Irion U., Krauss J., Nüsslein-Volhard C., 2014. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 141: 4827–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe K. M., Grimes D. T., Schottenfeld-Roames J., Werner M. E., Ku T. S., et al. , 2016. c21orf59/kurly controls both cilia motility and polarization. Cell Rep. 14: 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinka J. B., Fortriede J. D., Burns K. A., James-Zorn C., Ponferrada V. G., et al. , 2015. Xenbase, the Xenopus model organism database; new virtualized system, data types and genomes. Nucleic Acids Res. 43: D756–D763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W., Fang X., Ji H., Paulson A. F., Daniel J. M., et al. , 2002. Isolation and characterization of XKaiso, a transcriptional repressor that associates with the catenin Xp120(ctn) in Xenopus laevis. J. Biol. Chem. 277: 8202–8208. [DOI] [PubMed] [Google Scholar]
- Ko J. M., Yang J. A., Jeong S. Y., Kim H. J., 2012. Mutation spectrum of the TYR and SLC45A2 genes in patients with oculocutaneous albinism. Mol. Med. Rep. 5: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Kwan K. M., Carroll T. J., McMahon A. P., Mendelsohn C. L., et al. , 2005. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: 2809–2823. [DOI] [PubMed] [Google Scholar]
- Kodjabachian L., Karavanov A. A., Hikasa H., Hukriede N. A., Aoki T., et al. , 2001. A study of Xlim1 function in the Spemann-Mangold organizer. Int. J. Dev. Biol. 45: 209–218. [PubMed] [Google Scholar]
- Lane A. B., Strzelecka M., Ettinger A., Grenfell A. W., Wittmann T., et al. , 2015. Enzymatically generated CRISPR libraries for genome labeling and screening. Dev. Cell 34: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienkamp S. S., Liu K., Karner C. M., Carroll T. J., Ronneberger O., et al. , 2012. Vertebrate kidney tubules elongate using a planar cell polarity-dependent rosette-based mechanism of convergent extension. Nat. Genet. 44: 1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. P., Miller R. K., Zhou X., Weidinger G., Deroo T., et al. , 2009. Requirement of Wnt/β-catenin signaling in pronephric kidney development. Mech. Dev. 126: 142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S. A., 1987a Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev. Biol. 119: 560–578. [DOI] [PubMed] [Google Scholar]
- Moody S. A., 1987b Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev. Biol. 122: 300–319. [DOI] [PubMed] [Google Scholar]
- Moody S. A., Kline M. J., 1990. Segregation of fate during cleavage of frog (Xenopus laevis) blastomeres. Anat. Embryol. (Berl.) 182: 347–362. [DOI] [PubMed] [Google Scholar]
- Moreno-Mateos M. A., Vejnar C. E., Beaudoin J., Fernandez J. P., Mis E. K., et al. , 2015. CRISPRscan: designing highly efficient sgRNAs for CRISPR/Cas9 targeting in vivo. Nat. Methods 12: 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert T., Colpaert R., Van Nieuwenhuysen T., Dimitrakopoulou D., Leoen J., et al. , 2016. CRISPR/Cas9 mediated knockout of rb1 and rbl1 leads to rapid and penetrant retinoblastoma development in Xenopus tropicalis. Sci. Rep. 6: 35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert T., Van Nieuwenhuysen T., Vleminckx K., 2017. TALENs and CRISPR/Cas9 fuel genetically engineered clinically relevant Xenopus tropicalis tumor models. Genesis 55: 1–2. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Fish M. B., Fisher M., Oomen-Hajagos J., Thomsen G. H., et al. , 2013. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis 51: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J., 1994. Normal Table of Xenopus laevis (Daudin): A Systematical & Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. Garland Publishing, Inc., New York. [Google Scholar]
- Omasits U., Ahrens C. H., Muller S., Wollscheid B., 2014. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30: 884–886. [DOI] [PubMed] [Google Scholar]
- Rankin S. A., Kormish J., Kofron M., Jegga A., Zorn A. M., 2011. A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev. Biol. 351: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J. D., Zaback P., Joung J. K., Voytas D. F., Dobbs D., 2007. Zinc Finger Targeter (ZiFiT): an engineered zinc finger/target site design tool. Nucleic Acids Res. 35: W599–W605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J. D., Maeder M. L., Reyon D., Voytas D. F., Joung J. K., et al. , 2010. ZiFiT (Zinc Finger Targeter): an updated zinc finger engineering tool. Nucleic Acids Res. 38: W462–W468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Session A. M., Uno Y., Kwon T., Chapman J. A., Toyoda A., et al. , 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W., Behringer R. R., 1995. Requirement for Lim1 in head-organizer function. Nature 374: 425–430. [DOI] [PubMed] [Google Scholar]
- Shigeta M., Sakane Y., Iida M., Suzuki M., Kashiwagi K., et al. , 2016. Rapid and efficient analysis of gene function using CRISPR-Cas9 in Xenopus tropicalis founders. Genes Cells 21: 755–771. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M., Harland R. M., 2000. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B., 1992. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 6: 356–366. [DOI] [PubMed] [Google Scholar]
- Taira M., Otani H., Jamrich M., Dawid I. B., 1994. Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 120: 1525–1536. [DOI] [PubMed] [Google Scholar]
- Tandon P., Conlon F., Furlow J. D., Horb M. E., 2017. Expanding the genetic toolkit in Xenopus: approaches and opportunities for human disease modeling. Dev. Biol. 426: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M. L., Field R. A., Wheeler G. N., 2005. Xenopus as a model organism in developmental chemical genetic screens. Mol. Biosyst. 1: 223–228. [DOI] [PubMed] [Google Scholar]
- Venegas-Ferrin M., Sudou N., Taira M., del Pino E. M., 2010. Comparison of Lim1 expression in embryos of frogs with different modes of reproduction. Int. J. Dev. Biol. 54: 195–202. [DOI] [PubMed] [Google Scholar]
- Vize P. D., Jones E. A., Pfister R., 1995. Development of the Xenopus pronephric system. Dev. Biol. 171: 531–540. [DOI] [PubMed] [Google Scholar]
- Wang F., Shi Z., Cui Y., Guo X., Shi Y. B., et al. , 2015. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Yasuoka Y., Mawaribuchi S., Kuretani A., Ito M., et al. , 2017. Conservatism and variability of gene expression profiles among homeologous transcription factors in Xenopus laevis. Dev. Biol. 426: 301–324. [DOI] [PubMed] [Google Scholar]
- Wheeler G. N., Brändli A. W., 2009. Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Dev. Dyn. 238: 1287–1308. [DOI] [PubMed] [Google Scholar]
- Yasuoka Y., Suzuki Y., Takahashi S., Someya H., Sudou N., et al. , 2014. Occupancy of tissue-specific cis-regulatory modules by Otx2 and TLE/Groucho for embryonic head specification. Nat. Commun. 5: 4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plasmids used in this study are available upon request. The authors declare that all data necessary for confirming the conclusions presented in the article are fully represented within the article.