ABSTRACT
Yeast have one Atg8 protein; however, multiple Atg8 orthologs (LC3s and GABARAPs) are found in humans. We discovered that a population of the Atg8 ortholog GABARAP resides on the centrosome and the peri-centrosomal region. This centrosomal pool of GABARAP translocates to forming autophagosomes upon starvation to activate autophagosome formation in a non-hierarchical pathway. How this centrosome-to-phagophore delivery of GABARAP occurs was not understood. To address this, we have shown that the archetypal centriolar satellite protein PCM1 regulates recruitment of GABARAP to the centrosome. PCM1 recruits GABARAP, but not MAP1LC3B, directly to centriolar satellites through a LC3-interacting region (LIR) motif. Furthermore, PCM1, in concert with its interacting centriolar satellite E3 ligase MIB1, controls GABARAP stability, K48-linked ubiquitination and GABARAP-mediated autophagic flux.
KEYWORDS: ATG8, autophagy, centriolar satellites, centrosome, GABARAP, LIR, Mib1, PCM1, ubiquitination
A key family of Atg (autophagy-related) proteins in macroautophagy/autophagy and selective autophagy (such as mitophagy) are the Atg8 proteins. In mammals, these are the MAP1LC3 family (here LC3A, B, B2 and C) and the GABARAPs (GABARAP, GABARAPL1 and GABARAPL2). The functional and regulatory differences between these homologs is a subject of intense investigation. Our group recently identified a pool of GABARAP residing on the centrosome and peri-centrosomal region, which traffics to forming autophagosomes during amino acid starvation. Although we discovered the mechanism on the Golgi (specifically Golgi proteins WAC and GOLGA2/GM130) that controls this pool of GABARAP, and we could show that microtubules regulate the traffic from the Golgi to the centrosome, centrosomal proteins that regulate the centrosomal pool of GABARAP and its role in autophagy were completely unknown.
A clue to understanding the regulation of centrosomal GABARAP came from the Autophagy network published by Behrends et al. in Nature in 2010 where PCM1 was identified as an interactor of GABARAP and GABARAPL2. We confirmed the structural centriolar satellite protein PCM1 as a direct and endogenous interactor of GABARAP, and showed that PCM1 binds GABARAP through a canonical ULK-like LIR motif. Centriolar satellites are small (∼100 nm diameter) electron-dense granules that exist on and around the centrosome and are also peripherally distributed throughout the cell along microtubules. The centriolar satellites provide functions such as recruiting factors to the centrosome and participate in primary cilia formation. Centriolar satellites are not homogenous but exist as different subtypes containing different proteins; the functional importance of this is poorly understood.
We found GABARAP on a subset of PCM1-positive peripheral centriolar satellites and GABARAP localization to these structures requires the PCM1 LIR motif. Like many LIR motifs, the PCM1 LIR motif exhibits specificity and binds some Atg8-family proteins such as GABARAP but not others (e.g., MAP1LC3B). In addition, PCM1 promotes GABARAP's localization at the centrosome. Interestingly, we noticed PCM1-positive centriolar satellites colocalizing with an omegasome marker (GFP-ZFYVE1/DFCP1) and early autophagosomal markers such as ULK1 and WIPI2. In addition to GABARAP, PCM1 and its interacting centriolar satellite E3 ligase MIB1 is found on immuno-isolated omegasomes. These findings led us to wonder whether centriolar satellites are delivered to phagophores for their destruction; however, we found no evidence of PCM1 degradation by autophagy. Rather than being a cargo of the autophagosome, we found that PCM1 regulates the formation of GABARAP-positive (but not MAP1LC3B-positive) autophagosomes and the degradation of the autophagic cargo SQSTM1/p62.
After depletion of PCM1, GABARAP protein levels are reduced. This was due to enhanced proteasomal degradation of GABARAP. Conversely, ectopic expression of GFP-PCM1 stabilizes GABARAP protein levels, and this requires the PCM1 LIR motif. These findings suggest the LIR binding of PCM1 to GABARAP stabilizes the latter. Interestingly, the LIR motif in ATG4B is required to stabilize nonlipidated GABARAP against proteasomal degradation. LIR motifs function to bind to cargo receptors such as SQSTM1/p62, and cargo adaptors such as PLEKHM1 to Atg8-family proteins. It will be interesting to discover whether LIR motif stabilization of Atg8-family proteins from proteasomal degradation is an additional general mechanism across the Atg8 family.
GABARAP is degraded by the proteasome, and multiple ubiquitination sites have been found on GABARAP. Surprisingly, we found that GABARAP is equally degraded by the proteasomal and lysosomal pathways, under basal conditions of low autophagic flux. This highlights the idea that proteasomal stability of GABARAP is a mechanism of GABARAP regulation. We found that the MIB1 E3 ligase, a component of centriolar satellites, which binds to and is regulated by PCM1, promotes the K48-linked ubiquitination of GABARAP. MIB1-mediated ubiquitination of GABARAP occurs at K13 and K23, within an N-terminal helix that is less conserved between Atg8-family proteins. Interestingly, K13 and K23 are absent in the LC3 subfamily of Atg8-family proteins and these findings open up the idea that ubiquitination of Atg8-family proteins allows a specific mode of regulation between the family members. In addition, ubiquitination may regulate the function of Atg8-family proteins in other ways than stability. Finally, we observe multiple species (mono, di, tri, poly) of ubiquitinated GABARAP. Any functional differences between these forms requires further investigation.
We propose a model whereby centriolar satellites can carry GABARAP to phagophores to perform its function. In addition, centriolar satellites provide the link and control the balance of GABARAP between the centrosome and autophagosome. This is likely to be separate from the role of centriolar satellites in primary cilium formation. It will be interesting to see how centriolar satellites position themselves next to forming autophagosomes, using ultrastructural methods, especially electron microscopy. For example, if centriolar satellites are not ‘eaten’ are they therefore found only on the convex side of the phagophore? When do these centriolar satellites dissociate from the forming autophagosome? How GABARAP that is bound to centriolar satellites, likely in its unlipidated form, is mobilized to perform its autophagic function is unknown. In the cell there are many LIR-containing proteins, and how GABARAP selects and binds to the PCM1 LIR, for example, on the centriolar satellite, or to another LIR on a different structure is poorly understood (Fig. 1). Likewise, it will be fascinating to understand the signaling mechanisms that control the function of centriolar satellites in autophagy and the purpose of PCM1 selectivity for GABARAP over MAP1LC3B. Finally, it is worth considering that GABARAP may have a nonautophagosomal function at centriolar satellites and on the centrosome itself.
Figure 1.
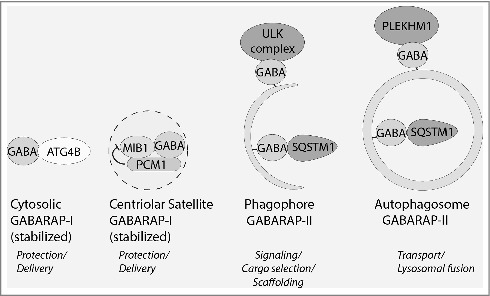
GABARAP function is controlled by LIR motif interactions with different binding partners. Examples of GABARAP (GABA) protein-protein interactions mediated through LIR motifs. Nonlipidated GABARAP (GABARAP-I) in the cytosol and on centriolar satellites is stabilized from proteasomal degradation by LIR motifs on ATG4B and PCM1. This likely maintains a reservoir of GABARAP, which is delivered to forming autophagosomes when required. The E3 ligase MIB1 on centriolar satellites binds GABARAP, but it is unknown if this is through a LIR motif. Lipidated GABARAP (GABARAP-II) on the phagophore acts as a scaffold and activates the ULK complex through multiple LIR motifs, to sustain ULK signaling during autophagosome biogenesis. In addition, GABARAP binds multiple cargo receptors such as SQSTM1/p62 for sequestration of cargoes. GABARAP on fully formed autophagosomes may bind adaptors that facilitate autophagosome transport, and LIR motif interaction with PLEKHM1 facilitates autophagosome-lysosome fusion.
FUNDING
The Francis Crick Institute ID: FC001187


