Abstract
Objective
We highlight the past 25 years of cognitive neuroscience and neuropsychology, focusing on the impact to the field of the introduction in 1992 of functional magnetic resonance imaging (fMRI).
Methods
We reviewed the past 25 years of literature in cognitive neuroscience and neuropsychology, focusing on the relation and interplay of fMRI studies and studies utilizing the “lesion method” in human participants with focal brain damage.
Results
Our review highlights the state of localist/connectionist research debates in cognitive neuroscience and neuropsychology ca. 1992, and details how the introduction of fMRI into the field at that time catalyzed a new wave of efforts to map complex human behavior to specific brain regions. This, in turn, eventually evolved into many studies that focused on networks and connections between brain areas, culminating in recent years with large-scale investigations such as the Human Connectome Project.
Conclusions
We argue that throughout the past 25 years, neuropsychology – and more precisely, the “lesion method” in humans – has continued to play a critical role in arbitrating conclusions and theories derived from inferred patterns of local brain activity or wide-spread connectivity from functional imaging approaches. We conclude by highlighting the future for neuropsychology in the context of an increasingly complex methodological armamentarium.
Keywords: neuropsychology, lesion, fMRI, connectivity
Two fundamental properties of the nervous system are 1) the specificity of neurons and cortical areas to specific stimulus characteristics, and 2) the dense array of connections formed both at the neuronal and cortical level (e.g., Afif & Bergman, 2005). That the nervous system is both highly specific and densely interconnected has fascinated and driven intense debate regarding theories of brain-behavior relationships, as science has attempted to translate these fundamental properties into explanations for and formulations of the complex array of behaviors in animals and humans. This is particularly true when one considers the spectrum of higher cognitive functions that have been the interest of cognitive neuroscience and neuropsychology, such as language, memory, executive functions, and emotion. Histories of cognitive neuroscience point the to the early influence of Franz Joseph Gall on localization of brain functions to specific parts of the cortex, followed by the patient reports of Auburtin, Bouillaud, and Broca (Feinberg & Farah, 2003; Heilman & Valenstein, 2003). These “localizationists” (see Figure 1), as they were called, were followed by the rise in prominence of connectionist approaches, including the work of Wernicke, Liepmann, and Dejerine, who advocated that the connections between many cortical areas were key underpinnings of much complex behavior (Feinberg & Farah, 2003; Heilman & Valenstein, 2003). While the myeloarchitectonic mapping work of Oskar and Cecile Vogt (1903, see Nieuwenhuys, 2013 for a recent review) as well as the “new physiology” proposed by Brodmann’s cytoarchitetonic mapping (1909) kept a focus on the regional anatomical differences of the brain, both localizationist and connectionist approaches largely fell out of favor in the early 20th century (Heilman & Valenstein, 2003). In their place, holistic approaches such as those advocated by Lashley, Head and others came to dominate the field until the 1950s, when the callosal transection work of Myers and Sperry (1953, 1958) led to a renewed general interest in more closely linking specific areas of brain damage to specific deficits in behavior (Catani & Ffytche, 2005). This culminated in Geschwind’s seminal treatise on disconnection syndromes (1965a; 1965b), which reinvigorated the field with its focus on the connections between sensory and association areas as underpinning higher cognitive functions. The lasting influence of Geschwind’s work was such that between 1980 and 1985, “Disconnection Syndromes in Animals and Man” was cited once every 5 days (Absher & Benson, 1993).
Figure 1.
Prominent figures in local and global theories of brain function. Upper row: left, Pierre Paul Broca, pioneer of the localizationist tradition; right, Carl Wernicke, pioneer of connectionist theories. Bottom row: left, Norman Geschwind, novel elaboration of disconnection syndromes; right, Antonio and Hanna Damasio, leaders in lesion localization and lesion-deficit mapping using structural brain imaging (CT, MRI). (Broca and Wernicke photos courtesy of US National Library of Medicine; Geschwind and Damasio photos courtesy of Antonio Damasio).
State of the field-1992
These competing localizationist v. connectionist views had settled into an uneasy equilibrium as of 1992. Geschwind’s original framework that virtually all impairments in higher cognitive function could be viewed through a disconnectionist framework was refined and tempered by the subsequent work of students such as Antonio Damasio and Marsel Mesulam (Catani & Ffytche, 2005). In the case of Antonio and Hanna Damasio, their work using structural imaging methods such as CT and MR to localize and map lesion damage re-emphasized the role that specific focal damage can play in disorders of perception (e.g., Damasio, 1985) and language (e.g., A. Damasio & H. Damasio, 1983). Meanwhile Mesulam’s (e.g., 1990) work highlighted the fact that while complex behaviors are not localized to a singular anatomical site, they do arise from the complex interaction of distributed focal regions. Absher and Benson (1993) describe the lasting influence of Geschwind’s theory of disconnection syndromes as laying the foundation for “new and better conceptions of higher brain function” in the form of network theory. They describe this future paradigm of higher brain function as such:
Currently a network theory is used to explain many brain functions, each network consisting of several interconnected brain regions. Each separate region carries out specific functions, but complex behavior is based on combinations of these functions. Damage to a region or the disconnection of one or more regions will sometimes disrupt, but more often alter, the functions. While the site of damage influences clinical symptoms, damage at different sites may affect the network in similar ways. The network model is compatible with localizationism (functions are localized to some degree in the interconnected regions), holism (the network acts as a broad functional system), and disconnection (disconnection of different parts of the network creates different effects based on the regions isolated). Aspects of holism and localizationism are preserved and some of their weaknesses are circumvented. (Absher & Benson, 1993 p.866)
Meanwhile, the relatively recent introduction of CT and anatomical MRI imaging had already shifted the role of neuropsychological assessment, both for clinical and research purposes. Arthur Benton (1992), in his review of the field of clinical neuropsychology ca. 1960 to 1990, noted that the role of assessment had shifted from providing critical guidance on localizing the specific site of brain damage. Instead, Benton highlighted the role neuropsychological assessment plays in assessing the significance of focal damage, noting the frequent presence of incidental imaging findings without any measurable cognitive impact. Benton went on to emphasize the importance of connectivity and networks:
By now it is clear that, although it is of great value to neurologists and neurosurgeons in their diagnostic practice, the traditional concept of discrete areal localization, i.e., linking specific functions and cognitive abilities to specific regions of the brain, is dying (if it is not already dead). Neuroscientists now think in terms of extensive, highly complex neural networks, within which there is multiple simultaneous transmission of information, as the mediators of behavioral capacities. Far from being located in a discrete neural aggregate, these networks course through large parts of the brain and their functional properties are defined by dynamic relationships between neural aggregates. It is hard to specify what "localization" means in this context. It means the nature of the interrelations between these aggregates. If a function has to be "localized" somewhere, I suppose it would be in the several synapses of a network. (Now the really hard work begins. namely, to identify these synapses and to describe what happens at these sites). (Benton, 1992, p. 415)
In the midst of this equilibrium,Kwong et al. (1992) published the first study using functional magnetic resonance imaging (fMRI) to noninvasively examine the effects of sensory stimulation on the brain. fMRI quickly came to be a dominant force in cognitive neuroscience, and as we trace the last 25 years of the field, we see parallels in past efforts focusing on localizing discrete functions to discrete brain areas, along with a focus on distributed, interconnected brain networks for complex functions. Throughout, we hope to highlight the important role that neuropsychological studies of patients with focal brain damage have provided in regard to potential lessons from fMRI research.
Advances over the past 25 years
fMRI research in cognitive neuroscience over the past 25 years can be broadly described by two distinct, if somewhat overlapping periods. Early work in the field, starting from basic visual and motor processes and quickly evolving into higher cognitive functions, focused on fully utilizing the capabilities of the new technique to non-invasively map blood-oxygen-level-dependent (BOLD) derived brain activity (while participants were viewing stimuli or performing a task) to specific cortical regions. Among the most compelling examples of this work are the efforts by Kanwisher, McDermott, and Chun (1997) to define the role of the fusiform face area and its role in processing facial stimuli. Likewise, several careful studies of language (see Price, 2000 for a review) worked to isolate more narrowly the cortex active in the generation and perception of speech, beyond the relatively coarse anatomical localization that patient studies had provided.
However, the rush to map the hemodynamic-derived neural responses of healthy participants also led to more speculative (and in many cases, plainly dubious) studies on the neural correlates of love, politics, and other like topics. In his discussion of the challenges faced by the neuroimaging community’s efforts to map mental states onto the brain, Poldrack (2010, Table 1) elegantly illustrates how all of Gall’s 27 faculties of phrenology could be linked to modern fMRI equivalents. This type of imprecise reduction of abstract mental concepts to cortical areas generated broad criticism of fMRI as a research tool, such as William Uttal’s New Phrenology (2001). Meanwhile, other scholars pointed out valid critiques of experimental and statistical assumptions (and errors) underlying many neuroimaging analyses, such as Vul, Harris, Winkielman, and Pashler’s (2009) originally titled “Voodoo correlations in social neuroscience” (later changed to “Puzzling high correlations in social neuroscience”), as well as Bennett, Baird, Miller, and Wolford’s (2012) “Neural correlates of interspecies perspective taking in the post-mortem Atlantic salmon: An argument for proper multiple comparisons correction.”
In response to the renewed rush of localizationist research generated by fMRI studies, neuropsychological studies of brain-damaged patients over the past 25 years have provided much-needed tests on the necessity of fMRI-derived regions that are hemodynamically active during various experimental conditions. For example, early neuroimaging studies of language pointed to a specific role of greater hemodynamic left frontal operculum (FO) activity in reading pronounceable nonwords (e.g. “blist”) compared to most other word types, suggesting that FO maybe important for phonological, as opposed to semantic processing. This distinction was largely absent from the neuropsychological literature at the time, as most patients with left FO damage were broadly classified as having Broca’s aphasia and standard neuropsychological assessments did not probe nonword reading relative to word reading. Taking the imaging literature as a guide, Fiez, Tranel, Seager-Frerichs, and Damasio (2006) examined the ability of 11 patients with left FO damage to read nonwords, as well as high and low frequency words, comparing the left FO patients to matched brain-damaged and healthy comparison participants. The investigators demonstrated that, in alignment with the imaging literature, damage to the left FO led to a “phonological dyslexia” whereby patients were impaired at reading nonwords, but had intact word reading abilities. However, the left FO patients also showed greater difficulty reading low-frequency words with inconsistent spelling to sound pronunciations (e.g. “pint”) relative to low and high-frequency words with consistent spelling to sound pronunciations (e.g. “jade”). This second finding was unexpected from the neuropsychological literature, but entirely consistent with prior imaging work that indicated greater left FO activity for these inconsistent low-frequency words (Fiez, Balota, Raichle, & Petersen, 1999; Herbster, Mintun, Nebes, & Becker, 1997; Rumsey et al., 1997).
Likewise, fMRI studies of neural areas active during moral judgment highlighted the medial prefrontal cortex (along with other regions) as an area that was particularly active during moral decisions requiring personal involvement (e.g., pushing one person from a footbridge onto the tracks to stop a runaway trolley from killing five people) compared to moral decisions requiring less personal engagement (e.g., pulling a switch to move the runaway trolley from a track where 5 people would be killed, to another track where one person would be killed, see Greene, Sommerville, Nystrom, Darley, & Cohen, 2001; Greene, Nystrom, Engell, Darley, & Cohen, 2004). Neuropsychological studies of patients with prefrontal damage demonstrated that damage to this area indeed impairs moral decision-making, but crucially, only in the context of personal moral decisions that have a high-degree of conflict (e.g., deciding to smother one’s baby to save a number of people, see Ciaramelli, Muccioli, Làdavas, & Di Pellegrino, 2007; Koenigs et al., 2007).
On the other hand, neuropsychological studies of patients have also provided compelling evidence refuting putative function-structure mapping suggested by imaging studies. For instance, a large body of imaging work has identified the anterior insula as important in processing bodily states (e.g., pain, temperature, pleasure), emotional feelings, and self-awareness (see reviews in Craig, 2002, 2009). However, detailed case studies of rare patients with bilateral insular damage resulting from infection with herpes simplex encephalitis have demonstrated normal experience and expression of many emotions and feelings (Feinstein et al., 2010; A. Damasio, H. Damasio, & Tranel 2013), as well as the experience of pain (Feinstein et al., 2016) and self-awareness (Phillipi et al., 2012; Damasio et al., 2013). These patient data make extreme interpretations of the fMRI data untenable, and prompt more nuanced, circumspect conclusions. Moreover, the patient data indicate that even if the particular brain area (the anterior insula in this example) may be normally involved in a function (emotions and feelings in this example), that brain area is not necessary for the function.
Similarly, a large body of neuroimaging work suggested overlapping neural architecture in the medial temporal lobe (MTL), especially in the hippocampus, was responsible for both Theory of Mind (ToM), or the ability to infer the mental states of others, as well as episodic memory (Buckner & Carroll, 2007), suggesting both behaviors would be affected by focal damage to the MTL. Instead, studying patients with focal MTL damage and severe retrograde episodic memory loss, Levine et al. (1998, 2009) demonstrated intact ToM functioning in these patients, suggesting the ability to infer another’s mental state did not require specific memories of one’s past mental state. These observations, in turn, drove other imaging studies that more discretely probed the interplay between episodic memory and ToM and demonstrated that familiarity of the ToM target seems to drive fMRI overlap with MTL areas active during episodic memory tasks (Rabin & Rosenbaum, 2012).
Finally, neuropsychological studies of patients with lesions have provided clarity regarding conflicting theories regarding the contributions that subregions of the prefrontal cortex make to various facets of working memory. In particular, one theory of prefrontal contributions to working memory focused on the “type of information,” whereby following the dorsal and ventral visual information streams from the posterior portions of the brain, the dorsolateral PFC handles spatial memory content whereas more ventral PFC regions are involved with memory for object information (Levy & Goldman-Rakic, 2000). Meanwhile, a competing theory focused on the “type of processing,” and emphasized the extent to which the content of memories has to be monitored or manipulated rather than spatial or object information (Miller, 1999). In this context, ventral PFC is involved with maintenance of memory information, while dorsolateral PFC is only recruited in the context of more active memory monitoring and manipulation. Both theories could point to electrophysiological and functional imaging studies as support. Muller, Machado and Knight (2002) used a lesion approach to tease apart these competing theories. Studying a series of patients with damage to the dorsolateral, ventromedial, or both aspects of the PFC,Muller et al. (2002) carefully manipulated both the degree of maintenance compared to manipulation (using one versus two-back tasks) as well as object (whether the test item was the same shape as the previously presented object) compared to spatial (whether the test item was in the same position as the previously presented object) information. They observed that patients with circumscribed damage localized to only the ventromedial PFC or only the dorsolateral PFC performed similarly to healthy comparison participants on all WM tests. Meanwhile, patients with damage that spanned both dorsal and ventral PFC regions showed significant impairments on the two-back task requiring manipulation, regardless if they were asked to remember object or position information. At the time, these results indicated that neither the dorsal nor ventral PFC are necessary for working memory, but are part of a broader network of frontal and more posterior regions, an observation that appears to be supported by subsequent task-based imaging studies (e.g., Dosenbach et al., 2006).
There is one other area where localizationist functional imaging approaches in the past 25 years have strongly impacted neuropsychology in computational approaches to analyzing datasets. When studying groups of patients with focal damage, accurate registration of the site of focal damage to a common reference space is critical for accurate assessments of brain-behavior relationships. While this was originally carried out by manual hand-registration techniques (H. Damasio & A.R. Damasio, 1989) the rise of computer-based registration algorithms by neuroimaging analysis packages have improved the speed and effort required to register large samples of patients to a common space (Andersson, Jenkinson, & Smith, 2007; Brett, Leff, Rorden, & Ashburner, 2001). In addition, mass-univariate statistical techniques first developed for analyzing voxelwise group differences in fMRI conditions have been adapted for use in the comparing patients with brain damage in relation to performance on cognitive and behavioral measures of interest (Rorden & Karnath, 2004; Rorden, Karnath, & Bonilha, 2007). Such approaches have been useful in the study of intelligence (Gläscher et al., 2009, 2010), executive functions (Gläscher et al., 2012), as well as other measures of interest (but see Mah, Husain, Rees, & Nachev 2014 for a recent critique of this approach).
In summary, the introduction of fMRI into cognitive neuroscience precipitated a veritable “gold rush” of studies attempting to noninvasively map various cognitive states to specific brain areas, mirroring the early dominance of localizationist approaches in the late 1800s and early 1900s. These early studies provided some significant advances in our understanding of brain function, especially in healthy populations, but these advances were tempered with critiques of the correlative nature of the approach, as well as overly zealous interpretations of (often) poorly-defined cognitive constructs. Neuropsychological studies of various claims from this work have provided significant clarity to the neuroimaging literature, confirming or denying the necessity of areas implicated by fMRI studies, or arbitrating between conflicting theories in the imaging literature.
While the initial rush of fMRI research favored localizationist approaches to mapping discrete functions to specific cortical areas (as much as possible, given the method’s effective resolution), these early studies immediately captured the curiosity of those more interested in understanding how these regions interacted in the context of complex behavior. Biswal, Yetkin, Haughton, and Hyde (1995) made the early observation that low frequency fluctuations of the BOLD signal were highly correlated between the same areas of left and right primary motor cortex, in the same regions that were evoked during finger-tapping in a task-based fMRI paradigm. This remarkable observation went relatively unnoticed in the cognitive neuroscience domain until 2005, when Fox et al. (2005) used the technique to examine correlated brain areas during task performance as compared to rest. This “task-negative” or “default mode network” catalyzed a new rush of studies attempting to explore the interconnected nature of the brain, and how those networks interface in the context of complex human behavior. Functional connectivity approaches have subsequently identified networks implicated in attention (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006), executive control (Dosenbach et al., 2006), salience (Seeley et al., 2007), and other functions (see Yeo et al., 2015 for a recent attempt to assign connectivity network parcels to cognitive functions). This effort to understand the brain through its connectivity has even resulted in large-scale research investments in the form of the Human Connectome Project, as well as similar projects in rodent models such as the Allen Mouse Brain Connectivity Atlas.
Despite the wealth of studies indicating differences between healthy and patient groups in their functional connectomes (see Fornito, Zalesky & Breakspear, 2015 for a review on the connectomics of brain disorders), tying differences in connectivity networks to observed differences in behavior has remained elusive. Rarer still are efforts to understand the effects of focal brain damage on these networks, and whether network damage is related to observed cognitive or behavioral deficits in patient samples. However, as connectome-driven studies of the brain have flourished in the past 12 years, neuropsychological approaches have begun to weigh in on the necessities of these networks in the context of behavior. For example, in a study of patients with hemispatial neglect, resting-state fMRI showed acute decreases in connectivity of the undamaged dorsal attention network that returned to normal chronically, while connectivity in the damaged ventral attention network remained poor at both acute and chronic time points (He et al., 2007). Another study (Carter et al., 2010) highlighted acute (defined as an average of 18 days post-stroke) interhemispheric disruptions in the dorsal attention and somatomotor networks in patients with focal damage due to stroke. These disruptions were correlated with the degree of behavioral impairments on visual and motor tasks. The default mode network has been linked to several functions including mind-wandering, self-referential processing, as well as autobiographical memory (Buckner, Andrews-Hanna, & Schacter, 2008). Philippi, Tranel, Duff, and Rudrauf (2015) used voxel-lesion-symptom mapping techniques to examine whether focal damage to the nodes of the default mode network resulted in deficits to autobiographical memory in a sample of 92 patients. They observed significant impairments in self-reported autobiographical memory associated with focal damage in known default mode network regions including the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), inferior parietal lobule (IPL) and medial temporal lobe (MTL). Furthermore, hemispheric distinctions were seen between damage that affected semantic autobiographical memory (left mPFC and MTL) compared to episodic autobiographical memory (right mPFC and MTL).
More recent connectivity approaches have borrowed from the domain of graph theory in an attempt to identify critical hubs or nodes within and between these large-scale brain networks (see Power, Schlaggar, & Petersen, 2014, for a recent review of these techniques). There has been disagreement on the “best” way to identify critical nodes in brain networks, at both the local and regional level. Here too, neuropsychology approaches have provided much-needed insight. In a striking example of synergy between imaging and neuropsychological approaches,Warren et al. (2014) built off previous studies by Power and colleagues (2011, 2013) to identify nodes in the brain which exhibited high measures of interacting with multiple functional brain networks (so called “hub regions”), as determined by functional connectivity data in healthy participants. Warren et al. (2014) then identified patients with focal brain damage that included these “target” hub areas, as well as a comparison sample of “control” cases with damage to areas with a large number of connections that mainly were within a single network (so-called high-degree nodes). Analyzing patients’ neuropsychological functions across nine classic cognitive and behavioral domains (Lezak, Howieson, Bigler, & Tranel, 2012), the investigators found that patients with damage to target regions showed impaired cognitive and behavioral functions across multiple domains. By contrast, patients with damage to control areas showed impaired functioning in only one or two domains. Subsequent work showed that the scope and severity of impairment in target cases greatly exceeded that predicted by expert clinician ratings based only on knowledge of the lesion anatomy (Warren et al., 2017). This line of work shows promise for utilizing normative data from functional connectivity analyses to inform projected behavioral and cognitive morbidity in clinical populations.
Altogether, the impact of fMRI over the past 25 years of cognitive neuroscience research has been marked by a striking recapitulation of past debates and shifts in research from localizationist efforts to assign discrete functions to parts of the cortex, to connectionist emphasis on widely distributed networks as the critical drivers of cognition and behavior. Throughout these swings in the imaging literature, neuropsychological approaches to studying cognition and behavior using patient samples have provided critical contributions as to the necessity of areas that are “active” in response to fMRI tasks, as well as key tests of predictions derived from network-level connectivity studies. Likewise, developments in analysis techniques and statistical approaches (and caveats) driven by neuroimaging research in the past 25 years have opened new doors in the study of patient samples and linking cognitive deficits to observed neurological damage or disease.
Predictions about further advances
Neuroscience generally, and cognitive neuroscience in particular, is increasingly a multi-disciplinary team effort. The focus and emphasis by governments and funding agencies for translational approaches to understanding health and disease will require a sustained investment in understanding brain-behavior relationships at the human level. Increasing collaboration and interaction between neuroimaging and neuropsychological approaches in cognitive neuroscience will provide confidence in the conclusions derived from imaging studies, as well as increasing the power and impact of patient observations. In the current era of “crisis” with regards to replication and validity of scientific findings, the need for converging lines of evidence using multiple methods is needed more urgently than ever. In the final section of our essay, we discuss some encouraging trends combining imaging and neuropsychological approaches, as well as predictions about where this work is headed.
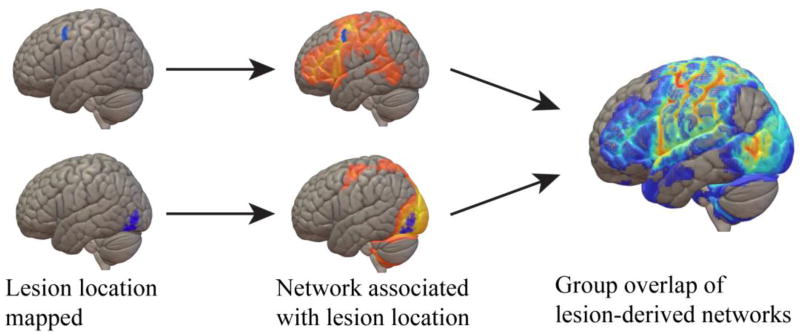
One development in the last couple of years has been initial studies combining traditional lesion analysis with normative imaging datasets. One method, termed “lesion-network mapping” (Figure 2), uses publically available healthy subject resting-state functional connectivity data, combined with traditional lesion mapping approaches, to infer the areas that would be connected with each patient's lesion area in healthy adults. Initial studies using this approach (Boes et al., 2015; Laganiere, Boes, & Fox, 2016) have shown some promise in explaining the neural bases for rare patient syndromes that defy parsimonious lesion localization, such as peduncular hallucinosis (characterized by vivid, dynamic, well-formed visual hallucinations following a lesion to the pons, midbrain, or thalamus), or hemichorea-hemiballismus (a post-stroke syndrome characterized by the presence on one side of the body of both chorea-jerky, aggressive, twisting movements of the arm or trunk or face, and ballism-violent throwing motions of the entire limb, Postuma & Lang, 2003). Applied to neuropsychology, this technique may be also useful in understanding cognitive deficits in patients who do not display predictable lesion characteristics or clear lesion overlap. One example of this involved utilizing data from the Iowa Gambling Task (IGT), a measure of complex decision-making under risk and ambiguity, and examining patients who either performed well or poorly on the test, but had focal damage outside of the prefrontal or limbic areas where damage has clearly been shown to affect performance (Sutterer et al., 2016). A traditional lesion overlap analysis showed these patients had largely non-overlapping damage; however, lesion-network mapping demonstrated that the patients who were impaired on the IGT showed overlap in their lesion connectivity maps with somatosensory, motor and insula cortices, to a greater extent than patients who had unimpaired performance on the IGT. An analogous approach utilizing diffusion tractography imaging (DTI) data has also shown promise in understanding outcome from stroke (Kuceyeski et al., 2016). Future refinements to these network-derived lesion-mapping approaches (for example, combining DTI and resting-state data) hold further promise for better appreciating the remote effects of damage in the context of neuropsychological studies of brain and behavior.
Figure 2.
Lesion network mapping. Lesion masks in standard space are used as seed regions-of-interest for resting-state fMRI data from healthy participants. The resulting connectivity maps are then binarized and summed together similar to the tradition lesion overlap approach, creating overlap maps of lesion-derived connectivity for a group of patients.
While structural imaging of brain-damaged populations has been an important and long-standing effort in neuropsychological studies, functional imaging studies of such populations have often been constrained in their scope and interpretation, due to “feasibility and cooperation” factors such as the difficulty of some populations to complete tasks in the scanning environment (e.g., motor responses in patients with hemiparesis, language comprehension and responding in patients with aphasia). One clear advantage of connectivity-based imaging techniques, such as resting-state fMRI and DTI, is that even participants with relatively severe impairments can participate and provide useful data (although experimenters must still be mindful of motion and sleep confounds in the scanner in the case of resting-state fMRI studies). These network-focused approaches have begun to allow researchers to explore the question of how brain networks reorganize after damage, and how the reorganization affects observed cognitive and behavioral outcomes. For example,Ramsey et al. (2016) studied how functional connectivity changed from 2 weeks to 6 months after stroke in patients recovering from hemispatial neglect. They found that improvement of attention deficits was correlated with improvements of initially reduced interhemispheric connectivity across sensory, motor, and attention networks, and a recovery of the normal negative correlation between dorsal attention/motor regions and default-mode/frontoparietal regions, particularly in the damaged hemisphere. In another example of a study utilizing connectivity-based imaging in patient studies, Eldaief, McMains, Hutchison, Halko, and Pascual-Leone (2017) examined the effect of circumscribed damage to the medial prefrontal cortex, a critical hub of the default-mode network, on functional connectivity within the DMN as well as with other networks. They observed largely preserved connectivity between the other undamaged nodes of the DMN, but weaker negative correlations between the DMN and attentional and sensory networks. Unfortunately, they did not report on any relationships between these network-level changes and behavioral or cognitive measures in the patients. We expect the next 5 to 10 years to hold great promise in utilizing neuropsychological approaches in concert with imaging to better understand the importance of connectivity networks in relation to higher cognitive functions, and how these networks change and reorganize in the context of damage. Enhancing our knowledge in this area will be beneficial not only in the context of diagnosis and treatment of brain-damaged individuals, but also in informing the promise and limits of plasticity-based interventions such as cognitive training.
Recent years have seen an increased emphasis on reproducibility and rigor across biomedical and psychological fields, and neuropsychology is no exception (Gelman & Geurts, 2017). We anticipate the next 5 to 10 years will see a shift in neuropsychological research to meet this emphasis, for example, moving from traditionally small samples of patients with focal damage, to larger, multi-site patient samples. This shift will coincide with an increase in the use of multivariate or classifier-based approaches to examining brain-behavior interactions (Adolphs, 2016). To meet the need for larger samples, we also anticipate a shift from traditional manual lesion-mapping approaches to an increased reliance on automated or semi-automated mapping techniques. While there have been persistent efforts in this domain over the years (e.g. Seghier, Ramlackhansingh, Crinion, Leff & Price, 2008), comparisons of existing methods have traditionally fallen short of “gold-standard” manual lesion mapping (for a review of these methods in stroke and multiple sclerosis populations, see Wilke, de Haan, Juenger, & Karnath, 2011 and García-Lorenzo, Francis, Narayanan, Arnold & Collins, 2013, respectively). However, ongoing work such as the Ischemic Stroke Lesion Segmentation (ISLES, Maier et al., 2015) challenge (www.isles-challenge.org) provides a publically available benchmark dataset to refine and improve these methods, as well as a defined challenge for the research community to tackle. We anticipate that such efforts will lead to improvement in these tools over the coming decade.
In parallel with the changes in methodological approaches in neuropsychology, we anticipate that the next 5 to 10 years will re-emphasize the need for carefully defining and measuring cognitive and behavioral constructs, perhaps leveraging tools from fields such as bioinformatics to help group studies and concepts together to develop a formal cognitive ontology (cf. Krakauer, Ghazanfar, Gomez-Marin, Maciver, & Poeppel, 2017; Poldrack & Yarkoni, 2016). We feel researchers in the field of neuropsychology are well-positioned to meet this need with their historical focus on careful observations of cognition and behavior, and considering the “whole patient” rather than specific test scores. In addition, a major challenge facing both neuropsychological and neuroimaging research in the coming years is translating group results into predictions that are useful at the level of an individual, specific person, particularly with regards to predicting outcome and recovery of individual patients following neurological injury. There have been some early attempts in this domain, especially with regards to stroke (Hope, Seghier, Leff & Price, 2013; Price, Hope & Seghier, 2017; Rehme et al., 2015). However, significant challenges remain. In particular, the results of data-driven analyses (e.g. using machine learning algorithms on large lesion datasets to identify variables that are most predictive of outcomes) need to be integrated wth a priori models of brain damage and recovery. These new models will function best when cross-validated with imaging studies in healthy and brain-injured participants, and further refined by evaluating the predictive accuracy on new sets of data from patients with brain damage (c.f. Price, Hope & Seghier 2017 for a more in-depth discussion). We believe that significant strides will be made in the next decade, as computational algorithms improve and large consortium-based projects provide sufficiently large samples with multi-modal imaging and behavioral measures for both data-driven analyses and model-based predictive testing.
Concluding Remarks
This essay summarizes remarkable changes in cognitive neuroscience and neuropsychology over the past 25 years, and how the introduction of fMRI in 1992 catalyzed a wave of studies in both the localizationist tradition, as well as the more recent (dis)connectionist focus in the era of “connectomics.” We believe the future holds promise in unlocking the secrets of how these networks work, how they relate to brain and behavior, and using this knowledge to inform predictions at the individual patient level. The neuropsychological approach holds a key role in that future, as it has across the entire history of our field.
Public Significance Statement.
This review covers the last 25 years of research in cognitive neuroscience and neuropsychology. The introduction of functional magnetic resonance imaging (fMRI) in 1992 generated a number of studies that echo historical debates on the importance of localizing behavior to specific brain areas, compared to focusing on the importance of connections between brain areas. Neuropsychology has played a critical role in arbitrating theories derived from fMRI, and we predict it will continue to do so in the future.
Acknowledgments
This work was supported in part by grant P50MH094258-04A1 from the National Institute of Mental Health, grant 220020387 from the James S. McDonnell Foundation, and a grant from the Spastic Paralysis Research Foundation of Kiwanis International.
Contributor Information
Matthew J. Sutterer, Department of Neurology, University of Iowa
Daniel Tranel, Department of Neurology, Department of Psychological and Brain Sciences, University of Iowa.
References
- Absher JR, Benson DF. Disconnection syndromes: An overview of Geschwind’s contributions. Neurology. 1993;43(5):862–7. doi: 10.1212/wnl.43.5.862. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Human lesion studies in the 21st century. Neuron. 2016;90(6):1151–1153. doi: 10.1016/j.neuron.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi AK, Bergman RA. Functional Neuroanatomy. 2. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. 2007 (Technical Report TR07JA2). Retrieved from Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Department of Clinical Neurology, Oxford University, Oxford, UK website: http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf.
- Bennett CM, Baird AA, Miller MB, Wolford GL. Neural correlates of interspecies perspective taking in the post-mortem Atlantic salmon: An argument for proper multiple comparisons correction. Journal of Serendipitous and Unexpected Results. 2012;1:1–5. [Google Scholar]
- Benton A. Clinical neuropsychology: 1960–1990. Journal of Clinical and Experimental Neuropsychology. 1992;14(3):407–417. doi: 10.1080/01688639208407616. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boes AD, Prasad S, Liu Q, Fox MD. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(10):3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Leipzig:J.A. Barth (Translated and edited Laurence J. Garey 1999 London: Imperial College Press) 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of Neurology. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128(10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Muccioli M, Làdavas E, Di Pellegrino G. Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Social Cognitive and Affective Neuroscience. 2007;2(2):84–92. doi: 10.1093/scan/nsm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33(12):1573–83. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Disorders of complex visual processing: agnosias, achromatopsia, Balint's syndrome, and related difficulties of orientation and construction. In: Mesulam MM, editor. Principles of behavioral neurology. Vol. 1. Philadelphia, PA: Davis; 1985. pp. 259–88. [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cerebral Cortex. 2013;23(4):833–846. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Damasio A. Lesion analysis in neuropsychology. New York, NY: Oxford University Press; 1989. [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, McMains S, Hutchison RM, Halko MA, Pascual-Leone A. Reconfiguration of intrinsic functional coupling patterns following circumscribed network lesions. Cerebral Cortex. 2017;27(5):2894–2910. doi: 10.1093/cercor/bhw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg TE, Farah JJ. The development of modern behavioral neurology and neuropsychology. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology, 2nd Edition (pp. 3–21) New York: McGraw-Hill; 2003. pp. 3–21. [Google Scholar]
- Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D. Bilateral limbic system destruction in man. Journal of Clinical and Experimental Neuropsychology. 2010;32(1):88–106. doi: 10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, Lee JE, Rudrauf D. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Structure and Function. 2016;221(3):1499–1511. doi: 10.1007/s00429-014-0986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Tranel D, Seager-Frerichs D, Damasio H. Specific reading and phonological processing deficits are associated with damage to the left frontal operculum. Cortex. 2006;42(4):624–643. doi: 10.1016/s0010-9452(08)70399-x. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nature Reviews Neuroscience. 2015;16(3):159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lorenzo D, Francis S, Narayanan S, Arnold DL, Collins DL. Review of automatic segmentation methods of multiple sclerosis white matter lesions on conventional magnetic resonance imaging. Medical Image Analysis. 2013;17(1):1–18. doi: 10.1016/j.media.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Gelman A, Geurts HM. The statistical crisis in science: How is it relevant to clinical neuropsychology? The Clinical Neuropsychologist. 2017 Jun;4046:1–15. doi: 10.1080/13854046.2016.1277557. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965a;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965b;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Rudrauf D, Paul LK, Colom R, Tranel D, Damasio H, Adolphs R. Distributed neural system for general intelligence revealed by lesion mapping. Proceedings of the National Academy of Sciences. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision-making in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44(2):389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293(5537):2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Introduction. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. 4. New York: Oxford University Press; 2003. pp. 1–13. [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Human Brain Mapping. 1997;5(2):84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hope TMH, Seghier ML, Leff AP, Price CJ. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage: Clinical. 2013;2(1):424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: Evidence from the ultimatum game. Journal of Neuroscience. 2007;27(4):951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, Maciver MA, Poeppel D. Neuroscience needs behavior: Correcting a reductionist bias. Neuron. 2017;93(3):480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- Kuceyeski A, Navi BB, Kamel H, Raj A, Relkin N, Toglia J, O’Dell M. Structural connectome disruption at baseline predicts 6-months post-stroke outcome. Human Brain Mapping. 2016;37(7):2587–2601. doi: 10.1002/hbm.23198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology. 2016;86(23):2187–2195. doi: 10.1212/WNL.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, Stuss DT. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121(10):1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Turner GR, Mandic M, Mackey A. Behavioral and functional neuroanatomical correlates of anterograde autobiographical memory in isolated retrograde amnesic patient M.L. Neuropsychologia. 2009;47(11):2188–2196. doi: 10.1016/j.neuropsychologia.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133(1):23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Mah Y-H, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137(9):2522–2531. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier O, Menze BH, von der Gablentz J, Häni L, Heinrich MP, Liebrand M, Reyes M. ISLES 2015 - A public evaluation benchmark for ischemic stroke lesion segmentation from multispectral MRI. Medical Image Analysis. 2017;35:250–269. doi: 10.1016/j.media.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: Complex neural properties for complex behavior. Neuron. 1999;22(1):15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: Evidence from brain lesions in humans. Journal of Cognitive Neuroscience. 2002;14(5):673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Myers RE, Sperry RW. Interocular transfer of a visual from discrimination habit in cats after section of the optic chiasm and corpus callosum. Anatomical Record. 1953;115(2):351–352. [Google Scholar]
- Myers RE, Sperry RW. Interhemispheric communication through the corpus callosum: Mnemonic carry-over between the hemispheres. A.M.A. Archives of Neurology & Psychiatry. 1958;80(3):298–303. doi: 10.1001/archneurpsyc.1958.02340090034004. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The myeloarchitectonic studies on the human cerebral cortex of the Vogt–Vogt school, and their significance for the interpretation of functional neuroimaging data. Brain Structure and Function. 2013;218(2):303–352. doi: 10.1007/s00429-012-0460-z. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Tranel D, Duff M, Rudrauf D. Damage to the default mode network disrupts autobiographical memory retrieval. Social Cognitive and Affective Neuroscience. 2014;10:318–326. doi: 10.1093/scan/nsu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Feinstein JS, Khalsa SS, Damasio A, Tranel D, Landini G, Rudrauf D. Preserved self-awareness following extensive bilateral brain damage to the insula, anterior cingulate, and medial prefrontal cortices. PLoS ONE. 2012;7(8):e38413. doi: 10.1371/journal.pone.0038413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Mapping mental function to brain structure: How can cognitive neuroimaging succeed? Perspectives on Psychological Science. 2010;5(6):753–761. doi: 10.1177/1745691610388777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Yarkoni T. From brain maps to cognitive ontologies: Informatics and the search for mental structure. Annual Review of Psychology. 2016;67(1):587–612. doi: 10.1146/annurev-psych-122414-033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Lang AE. Hemiballism: Revisiting a classic disorder. The Lancet Neurology. 2003;2(11):661–668. doi: 10.1016/s1474-4422(03)00554-4. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Studying brain organization via spontaneous fmri signal. Neuron. 2014;84(4):681–696. doi: 10.1016/j.neuron.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Hope TM, Seghier ML. Ten problems and solutions when predicting individual outcome from lesion site after stroke. NeuroImage. 2017;145:200–208. doi: 10.1016/j.neuroimage.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin JS, Rosenbaum RS. Familiarity modulates the functional relationship between theory of mind and autobiographical memory. NeuroImage. 2012;62(1):520–529. doi: 10.1016/j.neuroimage.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey LE, Siegel JS, Baldassarre A, Metcalf NV, Zinn K, Shulman GL, Corbetta M. Normalization of network connectivity in hemispatial neglect recovery. Annals of Neurology. 2016;80(1) doi: 10.1002/ana.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Volz LJ, Feis DL, Eickhoff SB, Fink GR, Grefkes C. Individual prediction of chronic motor outcome in the acute post-stroke stage: Behavioral parameters versus functional imaging. Human Brain Mapping. 2015;36(11):4553–4565. doi: 10.1002/hbm.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain. 1997;120(5):739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Ramlackhansingh A, Crinion J, Leff AP, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41(4):1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterer MJ, Bruss J, Boes AD, Voss MW, Bechara A, Tranel D. Canceled connections: Lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex. 2016;78:31–43. doi: 10.1016/j.cortex.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttal WR. The new phrenology: The limits of localizing cognitive processes in the brain. Cambridge, MA: The MIT press; 2001. [Google Scholar]
- Vogt O. Zur anatomischen Gliederung des Cortex cerebri. Journal fur Psychologie und Neurologie. 1903;2:160–180. [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, Sun H, Tranel D. Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences. 2014;111(39):14247–14252. doi: 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Denburg NL, Power JD, Bruss J, Waldron EJ, Sun H, Tranel D. Brain network theory can predict whether neuropsychological outcomes will differ from clinical expectations. Archives of Clinical Neuropsychology. 2017;32(2017):40–52. doi: 10.1093/arclin/acw091. [DOI] [PubMed] [Google Scholar]
- Wilke M, de Haan B, Juenger H, Karnath H-O. Manual, semi-automated, and automated delineation of chronic brain lesions: a comparison of methods. NeuroImage. 2011;56(4):2038–46. doi: 10.1016/j.neuroimage.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Chee MWL. Functional specialization and flexibility in human association cortex. Cerebral Cortex. 2015;25(10):3654–3672. doi: 10.1093/cercor/bhu217. [DOI] [PMC free article] [PubMed] [Google Scholar]