Abstract
Numerous clinical studies of vitamin D, its derivatives or analogs, have failed to clearly demonstrate sustained benefits when used for the treatment of human malignant diseases. However, given the strong preclinical evidence of anti-neoplastic activity and the epidemiological associations suggesting that vitamin D compounds may have a place in cancer therapy, attempts are continuing to devise new approaches to their therapeutic use. This laboratory has developed a strategy to enhance the effectiveness of the currently standard therapy of Acute Myeloid Leukemia (AML) by the immediate addition of the vitamin D2 analog Doxercalciferol combined with the plant polyphenol-derived Carnosic acid to AML cells previously treated with Cytarabine (AraC). Enhancement of AML cell death was noted to be dependent on VDR and BRAF kinase. Here we document that the stress-related kinase JNK is an important additional component of cell death enhancement in this protocol. Either the Knock-down or the inhibition of JNK activity reduced the enhancement of AraC-induced cell death, and we show that JNK signaling to the apoptosis regulator BIM and Caspase executioners of cell death are downstream of VDR and BRAF. A clear understanding of the molecular basis for the increased efficacy of AraC in the therapy of AML is expected to bring this regimen to a clinical trial.
Keywords: Vitamin D, JNK, BRAF, FOXO, Acute Myeloid Leukemia, Carnosic acid
Graphical Abstract
Potential signals downstream from VDR-BRAF pathway in the context of DNA damage.
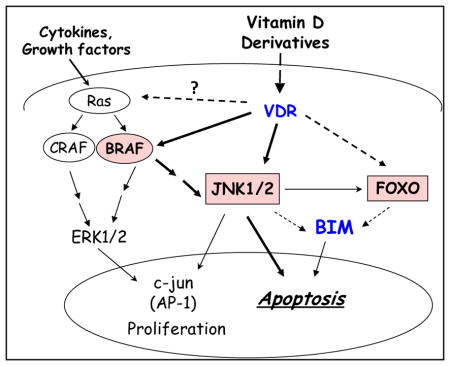
In this cell context, solid lines show proven signaling; bolded if shown here, and interrupted lines show signaling that needs further study.
INTRODUCTION
Standard therapy for Acute Myeloid Leukemia (AML), with cytotoxic agents such as Cytarabine (AraC) typically fails as curative therapy, especially in the elderly or relapsed patients (1–3). Not surprisingly, this has motivated numerous attempts to provide more than only generally cytotoxic therapeutic regimens to AML patients. Using molecularly targeted approaches, some initial clinical success has recently been reported in several subsets of patients, including patients with IDH1/2 mutations (4–6), and with the upregulation of HOXA9 transcription factor (7), or in AML with BCL2 dependence (8). Preclinical studies also suggest that chromatin regulators such as MLL1 and DOT1L can be therapeutic targets in NPM1-mutated AML (9–11).
The above studies generally depended on the use of small synthetic molecules which target cellular enzymes or transcription factors. In contrast, we have recently provided pre-clinical evidence that the small molecules related to natural compounds can increase the potentially therapeutic efficacy of AraC studied in AML blasts in ex vivo culture (12). These compounds, an analog of vitamin D2 Doxercalciferol (D2) and a plant-derived antioxidant carnosic acid (CA), have a powerful differentiating effect when combined, and this increases the cytotoxicity of AraC to AML blasts. Importantly, this action is selective for neoplastic cells, as the enhancement of cell death by this differentiation agent combination has no observable effect on the normal bone marrow cells (13, 14).
The initial investigation of the mechanism responsible for the enhancement of AraC-induced cell death by treating cells that have damaged DNA with a differentiation agent combination (D2/CA) has revealed a paradoxical tumor suppressor-like activity of BRAF. We found that reducing the level or inhibiting the kinase activity of BRAF diminishes the ability of D2/CA to enhance ALM cell death (13). Also, D2/CA increases BRAF expression in a VDR-dependent manner, which correlates with the increased levels of the DNA damage marker H2AX (14). We now provide data supporting the role of JNK as a transmitter of these signals to the cell death machinery, downstream from VDR-BRAF.
MATERIALS AND METHODS
Reagents
Arabinocytosine (AraC) and Doxercalciferol (1α-hydroxyvitamin D2; 1-D2) were purchased from Sigma-Aldrich (St. Louis, MO). Carnosic acid (CA) was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY). JNK inhibitor SP600125 was from Cell Signaling Inc (Danvers, MA). The antibodies that used for Western blotting as following: BRAF (#9433), CRAF (#9422), Phospho-JNK (Thr183/Tyr185, #9255), JNK (#9252), Phospho-p38 MAPK (Thr180/Tyr182, #4511), Phospho-C-JUN (Ser63, #2631), C-JUN (#9165), Phospho-BIM (Ser69, #4585), Phospho-H2AX (Ser139, #9718), FOXO3a (#2497), cleaved Caspase 9 (#9505), cleaved caspase 3 (#9661) and HRP-linked anti-rabbit (#7074) antibodies were purchased from Cell Signaling Technologies. BIM (sc-8625), VDR (sc-1008) and Crk-L (sc-319) were obtained from Santa Cruz Biotechnology (Dallas, TX).
Cell culture
HL60 is an AML cell line isolated from a patient with acute promyeloblastic leukemia (15). The search of the database in cBioPortal for Cancer Genomics shows no mutations of BRAF and JNKs in AML. However, it has been reported that BRAF mutations have been found in a small proportion of secondary AML (16), which is not the case in the specimen used in this study. The cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated bovine calf serum (BCS) at 37°C in a 5% CO2 atmosphere. Cells were passaged twice a week to maintain log phase growth. For experiments, the cells were seeded in 6-well plates at 1.5 ×105 cells/mL followed by the addition of the various agents for the indicated times. Cell viability was routinely determined by using the Trypan blue exclusion assay with a Neubauer hemocytometer.
Isolation of mononuclear cells from a patient blood sample
A blood sample was obtained during a diagnostic procedure from a patient volunteer diagnosed with FAB M5 subtype of AML, according to the Institutional Review Board protocol. Mononuclear cells were isolated from the specimen by using Histopaque-1077 (Sigma-Aldrich) gradient centrifugation as described previously (17).
Knockdown of proteins by siRNA oligonucleotides
HL60 cells were transfected with 100 nM of SignalSilence JNK siRNA, VDR siRNA, siBRAF, siCRAF or scrambled Control siRNA (Cell Signaling Inc), using Endo-Porter delivery reagent from Gene Tools Inc (Philomath, OR) for 48–72 hours before exposure to other agents for the indicated times. The cells were then examined to determine the extent of knockdown of JNK or VDR expression at both mRNA and protein levels.
Annexin V FITC assay
Following the indicated exposure of either HL60 cells or ex vivo AML blasts to AraC followed by D2/CA combination, the cells were collected, washed twice with 1xPBS, then re-suspended in the binding buffer, and stained using an Annexin V-FITC assay Kit (Fisher Scientific, Pittsburgh, PA). The cells were incubated with 50 μg/ml Annexin V and 10 μg/ml propidium iodide in 1x binding buffer at room temperature in the dark for 15 min, and immediately analyzed by flow cytometry (EPICS XL). Annexin V-positive/PI-negative cells were considered to be early apoptotic, cells with both Annexin V and PI positive to be late apoptotic, and Annexin V negative but PI positive to be “necrotic”(18).
Real time quantitative PCR
Total RNA was extracted by using Trizol (Invitrogen, CA) according to manufacturer’s protocol. Quantitative RT-PCR for JNK1/2 was carried out using an ABI 7500 Real-Time PCR System as described before (14). Fold changes of mRNA levels in target gene relative to the RNA polymerase II (RPII) control were calculated by using relative quantification analysis. Primers used for JNK1 were: forward 5′-CCCTGACAAGCAGTT AGATGAA-3′, reverse 5′-CTGTCTGTATCAGAGGCCAAAG-3′; JNK2, forward 5′-ATGGAGCTGGATCATGAAAGAA-3′, reverse 5′-GGTCAG TGCCTTGGAATATCA-3′, for RP II, forward 5′-GCACCACGTCCAATGA CAT-3′, reverse 5′-GTGCGG CTGCTTCCATAA-3′. The quality of all PCR products was monitored by using post-PCR melting curve analysis.
Western blotting
Western blotting was performed using 40 μg of whole cell extracts as described before (19). Briefly, membranes were incubated with primary antibodies for 1–2 hours according to the sensitivity of each antibody, and then blotted with a HRP-linked secondary antibody for 1 hour. The bands of protein were visualized using a chemiluminescence detection system (Pierce Biotechnology, Rockford, IL). Each membrane was stripped and reprobed for Crk-L, which was used as an internal loading control. The optical density of each band was quantified using ImageQuant 5.0 (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
Each experiment was repeated at least 3 times. The results are presented as the Mean ± Standard Deviation. Significance of the differences between mean values of two treatment groups was assessed by a Student’s T-test using Microsoft EXCEL program. Statistical significance of multiple treatment groups were assessed by one way ANOVA followed by the Tukey HSD test. p < 0.05 was considered statistically significant.
RESULTS
1. Phosphorylation of JNK1/2 is increased by D2/CA in AraC-treated cells and is required for the upregulation of BIM and the enhancement of cell death
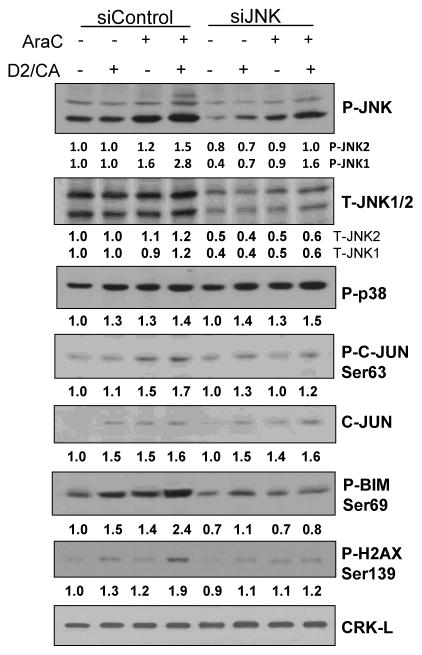
HL60 cells were treated with siRNA to JNK1/2 to reduce the expression of this stress-signaling kinase, and Table 1 shows that this treatment abrogated the D2/CA-induced increases in JNK1 mRNA, and reduced the increases in JNK2 mRNA after AraC or D2/CA exposure. Western blotting confirmed the marked knock-down (KD) of JNK protein isoforms, and resulted in a robust reduction of JNK activation by phosphorylation, as shown in Fig 1. While the expression of p38 MAPK was unaffected by the JNK KD, showing its specificity, the reduction of JNK activation was further documented by the reduced phosphorylation of C-JUN, a target of activated JNK (Fig 1). The decrease in P-JNK levels was also accompanied by the reduced expression of the pro-apoptotic protein BIM, and less evidence of DNA damage as shown by the P-H2AX levels (Fig 1). These signaling changes were associated with markedly reduced D2/CA-induced enhancement of the AraC-induced cell death in these cells (Table 2A). Similar treatment of a small blood sample of AML cells obtained from a patient with AML also resulted in increase in cell death induced by AraC and its enhancement by D2/CA, which was reduced in the cells with reduced levels of JNK that resulted from an exposure to siJNK (Table 2B).
Table 1.
Knockdown of JNK1 and JNK2 with siJNK diminishes the AraC-D2/CA-induced increases in JNK1/2 mRNA
| Table 1A | mRNA | vs siCTL | vs AraC | vs CA+D2 | P value |
|---|---|---|---|---|---|
|
| |||||
| HL60 | JNK1 | p value | p value | p value | vs corresp group |
| siControl-Control | 0.98 | ||||
| siControl-D2/CA | 1.26 | 0.180 | |||
| siControl-AraC | 0.82 | 0.038 | |||
| siControl-AraC-D2/CA | 1.46 | 0.179 | 0.040 | 0.261 | |
|
| |||||
| siJNK-Control | 0.89 | 0.157 | |||
| siJNK-D2/CA | 0.87 | 0.788 | 0.147 | ||
| siJNK-AraC | 0.85 | 0.462 | 0.656 | ||
| siJNK-AraC-D2/CA | 0.81 | 0.326 | 0.126 | 0.327 | 0.105 |
| Table 1B | mRNA | vs siCTL | vs AraC | vs CA+D2 | P value |
|---|---|---|---|---|---|
|
| |||||
| HL60 | JNK2 | p value | p value | p value | vs corresp group |
| siControl-Control | 1.13 | ||||
| siControl-D2/CA | 1.75 | 0.011 | |||
| siControl-AraC | 2.48 | 0.020 | |||
| siControl-AraC-D2/CA | 2.96 | 0.007 | 0.018 | 0.050 | |
|
| |||||
| siJNK-Control | 0.80 | 0.003 | |||
| siJNK-D2/CA | 1.09 | 0.069 | 0.010 | ||
| siJNK-AraC | 1.33 | 0.024 | 0.061 | ||
| siJNK-AraC-D2/CA | 1.92 | 0.006 | 0.042 | 0.047 | 0.033 |
HL60 cells were transfected with 100 nM of JNK siRNA or scrambled Control siRNA for 48 hr, then the transfected cells were pretreated with 100 nM AraC for 72 hr, followed by the addition of 100 nM D2 and 10 uM CA for 96 hr. Cells were harvested to check the effects of siJNK inhibition of JNK1 and JNK2 mRNA by RT-qPCR.
Figure 1.
Western blots showing that phosphorylation of JNK1/2 and their downstream target C-JUN is increased by D2/CA in AraC-treated cells, and is required for the upregulation of BIM, and the DNA damage marker P-H2AX. The lack of change in P-p38 levels indicates the specificity of siJNK. CRK-L was used as an internal control for loading.
Table 2.
Reduction of AraC-induced cell death by D2/CA when JNK expression is knocked down by a siJNK construct.
|
|
||||
|---|---|---|---|---|
| HL60 | AML ex vivo blasts | |||
|
|
|
|||
| TB Permeable | Annexin V | TB Permeable | Annexin V | |
|
|
|
|||
| Cell death % | Cell death % | Cell death % | Cell death % | |
| siControl-Control | 8.3 ± 1.8 | 10.4 ± 1.8 | 13.3 ± 3.6 | 15.6 ± 2.8 |
| siControl-D2/CA | 11.2 ± 2.5 | 15.5 ± 2.6 | 15.7 ± 2.9 | 16.7 ± 3.5 |
| siControl-AraC | 33.0 ± 4.1 | 40.5 ± 3.6 | 42.3 ± 4.4 | 40.2 ± 3.9 |
| siControl-AraC-D2/CA | 49.2 ± 5.9 | 56.9 ± 4.6 | 55.3 ± 6.2 | 58.9 ± 6.1 |
|
|
|
|||
| siJNK-Control | 9.7 ± 2.8 | 11.7 ± 3.0 | 12.0 ± 3.1 | 14.4 ± 3.5 |
| siJNK-D2/CA | 12.3 ± 2.9 | 16.3 ± 2.9 | 14.7 ± 2.6 | 16.4 ± 3.2 |
| siJNK-AraC | 31.0 ± 4.5 | 33.6 ± 4.8 | 38.0 ± 4.8 | 35.7 ± 4.2 |
| siJNK-AraC-D2/CA | 39.0 ± 4.7 | 42.6 ± 4.4 | 44.3 ± 4.9 | 45.5 ± 4.9 |
|
|
|
|||
There was statistically significant enhancement of cell death (p < 0.05, n=3) in siControl- treated cells (both HL60 and AML ex vivo), but not in siJNK-treated cells.
Thus, the reduction in JNK levels results in a decrease in signals for and the execution of cell death in AML cells with DNA damage.
2. Inhibition of JNK activation by SP600125 reduces the expression of FOXO3a and inhibits the activation of the apoptotic cascade
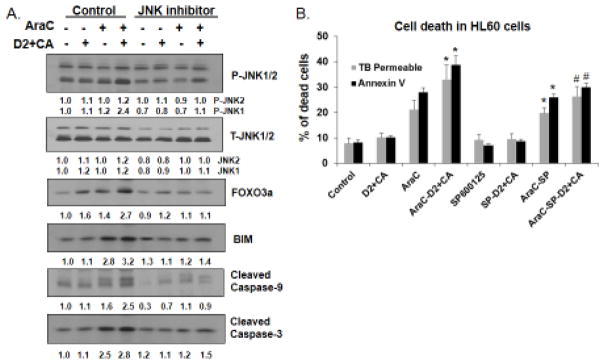
We also sought to confirm the role of JNK as a component of cell death signaling in this system by inhibiting the kinase activity of JNK with the selective pharmacological inhibitor SP600125. Indeed, the Westerns illustrated in Fig 2A show that in cells treated with AraC the enhancement of JNK phosphorylation by D2/CA was abrogated by this compound. This series of experiments also demonstrated that in this system the FOXO3a transcription factor is upregulated in a JNK-dependent manner by D2/CA. Since it is known that FOXO3a can control apoptosis by transcriptional regulation of BIM (20), the parallel effects of D2/CA and SP600125, observed here on FOXO3a, BIM, and on the activation by cleavage of Caspase 9 and Caspase 3, suggest that JNK is an upstream activator of a cascade of events that lead to apoptosis. Indeed, the effect of SP6000125 on cell death correlated well with its inhibition of JNK1 (Fig 2B).
Figure 2.
A. Inhibition of JNK activity by SP600125 reduces protein levels of FOXO3a and BIM in cells with AraC-induced cell damage treated with D2/CA. The levels of cleaved Caspases 9 and 3 are also reduced in these cells. B. The reduction of the enhancement of the cell death by D2/CA by inhibition of JNK phosphorylation parallels the molecular changes shown in panel A. *= p < 0.05, when compared with AraC treated group. # = p < 0.05, when compared with corresponding control group. N=3
3. VDR is required for the optimal JNK upregulation
Given that the cell death enhancement by D2/CA combination involves the vitamin D2 analog Doxercalciferol, we tested whether vitamin D receptor (VDR) is required JNK activation. Indeed, the KD of VDR with a silencing construct previously shown to be effective (13) reduced JNK activating phosphorylation in parallel with cell death reduction, but did not reduce JNK protein levels (Fig 3). This shows that the activation of JNK by phosphorylation does require optimal levels of VDR.
Figure 3.
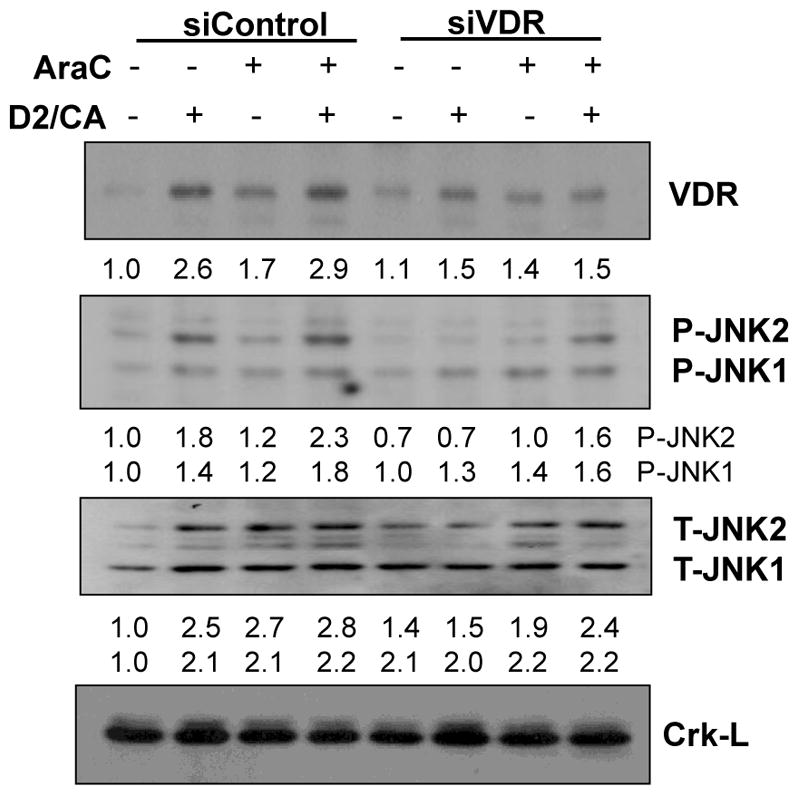
Knockdown of VDR with siVDR reduces JNK phosphorylation, but does not reduce JNK protein levels. CRK-L was used as a loading control.
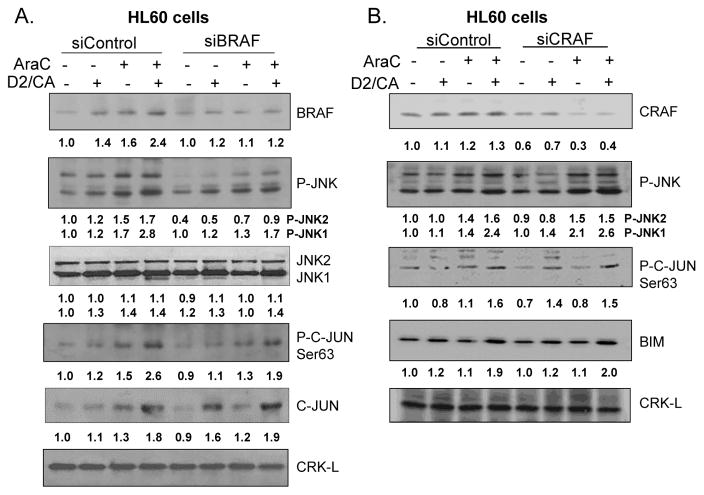
4. JNK is downstream of BRAF, but not of CRAF
We have previously shown that the enhancement of cell death by D2/CA requires BRAF (14), but the possibility of an involvement of JNKs was not examined in that study. Using a pool of siBRAF construct oligonucleotides we now show that BRAF is required for JNK phosphorylation in this system, and JNK activation is also demonstrated by the dependence on BRAF of C-JUN phosphorylation, which is a target of JNK (Fig 4A). In contrast, KD of CRAF had no such effect, consistent with its apparent lack of effect on the levels of BIM (Fig 4B), and on cell death (data not shown).
Figure 4.
Downstream targets of RAF signaling. A: Knockdown of BRAF with siBRAF shows that BRAF is required for optimal phosphorylation of JNK and its target C-JUN. B. Knockdown of CRAF with siCRAF showed little if any effect on the phosphorylation of JNK or C-JUN, and the levels of its downstream target BIM.
DISCUSSION
The JNK pathway is a well-recognized transmitter of stress and genotoxicity-related cell death in many cell types (21, 22). We, and others, have shown that JNK is activated during VDD-induced differentiation of AML cells (23–26), but whether this is a biomarker of this process, or has a causative role is not clear. JNK can have a protective role in some cell types (27) and some specific cell context in AML blasts (28). However, it has been reported that anthracycline resistance of AML patients can be due to the failure of JNK activation and apoptosis (29), and our evidence suggests that in HL60 cells and in ex vivo AML blasts JNK depletion reduces both AraC cell death and its enhancement (Table 2).
In our system, cell death signals from JNK appear to be transmitted by FOXO3a, at least in part. As pointed out above, FOXO3a controls apoptosis by transcriptional regulation of BIM in some cells (20, 30, 31), and JNK is one of FOXO3a upstream regulators.
On one hand, JNK can trigger apoptosis by the upregulation of genes which promote cell death such as BIM, PUMA and BNIP3, at least in part by modulating the nuclear localization, and thus the levels of FOXO3a protein in a number of different cell types (32–34). Specifically, phosphorylation of FOXO3a by JNK diminishes the interaction of FOXO3a with 14-3-3 protein, which protects FOXO3a from proteosomal degradation in the cytoplasm (35, 36). Nuclear localization of FOXO3a allows this transcription factor to upregulate a variety of genes, including BIM.
On the other hand, AKT promotes cell survival by phosphorylating FOXOs at three regulatory sites (Thr32, Ser253, and Ser315), inactivating FOXO3a by its re-localization from the nucleus to the cytoplasm (37). Therefore, an additional mechanism for the observed increase in AML cell death by D2/CA can be a decrease in AKT activity.
Although the specificity of JNK knock-down by siBRAF shown in Fig 4 was confirmed by CRISPR/Cas9 (data not shown), currently it is not clear why BRAF is required for D2/CA-induced cell death enhancement. A signaling connection between BRAF and AKT has been reported (38), so this connection will be investigated in future studies of death enhancement in AML cells with damaged DNA. Additionally, the Apoptosis Signaling Protein 1 (ASK1) is known to bind to both RAF and to JNK, so this may be another potential signaling cross-talk that leads to BIM upregulation in this cell system.
It therefore appears that there seems to be a clear signaling path from JNK to caspase activation and cell death, although the participation of AKT in FOXO modulation resulting in BIM upregulation still remains to be excluded. In contrast, how BRAF signals downstream has multiple potential scenarios discussed above, and these are currently under active investigation.
Highlights.
In AML cells with DNA damage attempted differentiation results in apoptosis instead.
Enhanced cell death is facilitated by an enhanced expression of VDR and BRAF.
The execution of cell death in this setting may be signaled by JNK activation.
Acknowledgments
This study was supported by grant R01CA044722-26 from NIH to GPS.
Abbreviations list
- AP-1
Activator protein 1, c-Jun and dimerization partners
- AKT
Serine/threonine protein kinase B (PKB)
- AML
Acute myeloid leukemia
- AraC
Cytarabine/Arabinocytosine
- ASK1
Apoptosis signal regulating kinase 1
- BCL2
B-cell lymphoma 2
- BIM
Bcl-2-like protein 11
- BNIP3
Bcl2 interacting protein 3
- BRAF
B-Raf proto-oncogene
- CA
Carnosic acid
- CRAF
Raf-1 proto-oncogene
- Crk-L
CRK-like proto-oncogene
- D2
Doxercalciferol (1a-hydroxyvitamin D2; 1-D2)
- DOT1L
DOT like histone lysine methyltransferase
- FAB
French-American British classification of AML subtypes
- FOXO3
Forkhead box protein 3
- H2AX
H2A histone family member X
- HOXA9
Homeobox protein Hox-A9
- IDH1/2
Isocitrate dehydrogenase 1/2
- JNK
c-Jun N-terminal kinase
- JUN
Jun proto-oncogene
- MLL1
Myeloid/lymphoid or mixed-lineage leukemia 1 (MLL1)
- NPM1
Nucleophosmin
- p38MAPK
p38 mitogen-activated protein kinase
- VDD
Vitamin D Derivative
- VDR
Vitamin D Receptor
Footnotes
Disclosure of conflict of interest
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunnberg U, Mohr M, Noppeney R, Dürk HA, Sauerland MC, Müller-Tidow C, Krug U, Koschmieder S, Kessler T, Mesters RM, Schulz C, Kosch M, Büchner T, Ehninger G, Dührsen U, Serve H, Berdel WE. Induction therapy of AML with ara-C plus daunorubicin versus ara-C plus gemtuzumab ozogamicin: a randomized phase II trial in elderly patients. Ann Oncol. 2012;23(4):990–6. doi: 10.1093/annonc/mdr346. [DOI] [PubMed] [Google Scholar]
- 2.Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31(Pt 2):2349–70. doi: 10.1016/j.clinthera.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Löwenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Biemond BJ, Gratwohl A, de Greef GE, Verdonck LF, Schaafsma MR, Gregor M, Theobald M, Schanz U, Maertens J, Ossenkoppele GJ Group D-BCTGfH-OHaSGfCCRSC. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027–36. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A, Liu W, Gliser C, Yang H, Gross S, Artin E, Saada V, Mylonas E, Quivoron C, Popovici-Muller J, Saunders JO, Salituro FG, Yan S, Murray S, Wei W, Gao Y, Dang L, Dorsch M, Agresta S, Schenkein DP, Biller SA, Su SM, de Botton S, Yen KE. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340(6132):622–6. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 5.Pietrak B, Zhao H, Qi H, Quinn C, Gao E, Boyer JG, Concha N, Brown K, Duraiswami C, Wooster R, Sweitzer S, Schwartz B. A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of αHG. Biochemistry. 2011;50(21):4804–12. doi: 10.1021/bi200499m. [DOI] [PubMed] [Google Scholar]
- 6.Rendina AR, Pietrak B, Smallwood A, Zhao H, Qi H, Quinn C, Adams ND, Concha N, Duraiswami C, Thrall SH, Sweitzer S, Schwartz B. Mutant IDH1 enhances the production of 2-hydroxyglutarate due to its kinetic mechanism. Biochemistry. 2013;52(26):4563–77. doi: 10.1021/bi400514k. [DOI] [PubMed] [Google Scholar]
- 7.Sykes DB, Kfoury YS, Mercier FE, Wawer MJ, Law JM, Haynes MK, Lewis TA, Schajnovitz A, Jain E, Lee D, Meyer H, Pierce KA, Tolliday NJ, Waller A, Ferrara SJ, Eheim AL, Stoeckigt D, Maxcy KL, Cobert JM, Bachand J, Szekely BA, Mukherjee S, Sklar LA, Kotz JD, Clish CB, Sadreyev RI, Clemons PA, Janzer A, Schreiber SL, Scadden DT. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell. 2016;167(1):171–86. e15. doi: 10.1016/j.cell.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6(10):1106–17. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kühn MW, Song E, Feng Z, Sinha A, Chen CW, Deshpande AJ, Cusan M, Farnoud N, Mupo A, Grove C, Koche R, Bradner JE, de Stanchina E, Vassiliou GS, Hoshii T, Armstrong SA. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 2016;6(10):1166–81. doi: 10.1158/2159-8290.CD-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hourigan CS, Aplan PD. Accurate Medicine: Indirect Targeting of NPM1-Mutated AML. Cancer Discov. 2016;6(10):1087–9. doi: 10.1158/2159-8290.CD-16-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Wahab O, Patel J, Levine RL. Clinical implications of novel mutations in epigenetic modifiers in AML. Hematol Oncol Clin North Am. 2011;25(6):1119–33. doi: 10.1016/j.hoc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Harrison JS, Studzinski GP. Enhancement of arabinocytosine (AraC) toxicity to AML cells by a differentiation agent combination. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison JS, Wang X, Studzinski GP. The role of VDR and BIM in potentiation of cytarabine-induced cell death in human AML blasts. Oncotarget. 2016;7(24):36447–60. doi: 10.18632/oncotarget.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Harrison JS, Studzinski GP. BRAF signals to pro-apoptotic BIM to enhance AraC cytotoxicity induced in AML cells by Vitamin D-based differentiation agents. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher R, Collins S, Trujillo J, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54(3):713–733. [PubMed] [Google Scholar]; Blood. 2016;128(25):2871. doi: 10.1182/blood-2016-10-748780. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2005;19(12):2232–40. doi: 10.1038/sj.leu.2404009. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Zhang Y, Zhou X, Ji W, Zhao J, Wei L, Li Y. Establishment and evaluation of an in vitro method for neutrophil extracellular trap generation and degradation. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30(9):986–8. [PubMed] [Google Scholar]
- 18.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–20. doi: 10.1038/cdd.2011.96. Epub 2011/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93(16):1224–33. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- 20.Sunters A, Fernández de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278(50):49795–805. doi: 10.1074/jbc.M309523200. Epub 2003/10/03. [DOI] [PubMed] [Google Scholar]
- 21.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–51. doi: 10.1038/onc.2008.301. Epub 2008/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YR, Wang XP, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation - Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271(50):31929–36. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Posner GH, Danilenko M, Studzinski GP. Differentiation-inducing potency of the seco-steroid JK-1624F2-2 can be increased by combination with an antioxidant and a p38MAPK inhibitor which upregulates the JNK pathway. J Steroid Biochem. 2007;105(1–5):140–9. doi: 10.1016/j.jsbmg.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XN, Studzinski GP. Inhibition of p38MAP kinase potentiates the JNK/SAPK pathway and AP-1 activity in monocytic but not in macrophage or granulocytic differentiation of HL60 cells. J Cell Biochem. 2001;82(1):68–77. doi: 10.1002/Jcb.1141. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Wang XN, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D-3. J Cell Biochem. 2003;89(6):1087–101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- 26.Buitrago CG, Ronda AC, de Boland AR, Boland R. MAP kinases p38 and JNK are activated by the steroid hormone 1 alpha,25(OH)(2)-vitamin D-3 on the C2C12 muscle cell line. J Cell Biochem. 2006;97(4):698–708. doi: 10.1002/jcb.20639. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson S, Ronai Z. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11(5):447–60. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volk A, Li J, Xin J, You D, Zhang J, Liu X, Xiao Y, Breslin P, Li Z, Wei W, Schmidt R, Li X, Zhang Z, Kuo PC, Nand S, Chen J. Co-inhibition of NF-κB and JNK is synergistic in TNF-expressing human AML. J Exp Med. 2014;211(6):1093–108. doi: 10.1084/jem.20130990. Epub 2014/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagadinou ED, Ziros PG, Tsopra OA, Dimas K, Kokkinou D, Thanopoulou E, Karakantza M, Pantazis P, Spyridonidis A, Zoumbos NC. c-Jun N-terminal kinase activation failure is a new mechanism of anthracycline resistance in acute myeloid leukemia. Leukemia. 2008;22(10):1899–908. doi: 10.1038/leu.2008.192. [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Evans S, Yan B, Povsic TJ, Tapson V, Goldschmidt-Clermont PJ, Dong C. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation. 2008;118(21):2156–65. doi: 10.1161/CIRCULATIONAHA.108.787200. Epub 2008/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Essafi A, Fernández de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24(14):2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 32.Ekoff M, Kaufmann T, Engström M, Motoyama N, Villunger A, Jönsson JI, Strasser A, Nilsson G. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110(9):3209–17. doi: 10.1182/blood-2007-02-073957. Epub 2007/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Li X, Wu J, Li J, Zhang L, Xiong T, Tang J, Qu Y, Mu D. Involvement of the JNK/FOXO3a/Bim Pathway in Neuronal Apoptosis after Hypoxic-Ischemic Brain Damage in Neonatal Rats. PLoS One. 2015;10(7):e0132998. doi: 10.1371/journal.pone.0132998. Epub 2015/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Chaanine AH, Jeong D, Liang L, Chemaly ER, Fish K, Gordon RE, Hajjar RJ. JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis. 2012;3:265. doi: 10.1038/cddis.2012.5. Epub 2012/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC, Lam EW. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66(1):212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 36.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–86. doi: 10.1016/j.bbamcr.2011.03.010. Epub 2011/03/31. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Hong A, Kong X, Koya RC, Song C, Moriceau G, Hugo W, Yu CC, Ng C, Chodon T, Scolyer RA, Kefford RF, Ribas A, Long GV, Lo RS. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov. 2014;4(1):69–79. doi: 10.1158/2159-8290.CD-13-0279. Epub 2013/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]