Abstract
The nucleus of the tractus solitarius (NTS) is a brain stem region critical to many physiologic processes and has been implicated in addiction to multiple classes of abused drugs, including alcohol (EtOH). That said, the mechanism by which EtOH modulates NTS neurocircuit activity is not well characterized and has yet to be examined utilizing electrophysiologic methods in mouse models of alcohol use disorders. To begin to address this gap in knowledge, we sought to use whole-cell and cell-attached recordings to determine the mechanism of acute EtOH action on GABAergic and glutamatergic neurotransmission, as well as on action potential firing in the NTS of adult male, EtOH naive mice. Bath application of EtOH (50 mM) significantly enhanced the frequency of spontaneous inhibitory postsynaptic current events, while increasing the amplitude of these events in half of the neurons tested. This finding suggests a presynaptic mechanism of EtOH action on GABAergic transmission in the NTS as well as a postsynaptic mechanism in subsets of NTS neurons. EtOH application was further associated with a significant decrease in action potential firing in most, but not all, NTS neurons tested. EtOH induced a small but significant decrease in spontaneous excitatory postsynaptic current frequency, indicating that EtOH may also inhibit NTS glutamatergic signaling to some degree. Intriguingly, in vivo EtOH exposure (4 g/kg IP) enhanced c-FOS colocalization with tyrosine hydroxylase via immunohistochemical methods, indicating that NTS norepinephrine neurons may be activated by acute EtOH exposure. Although future work is needed, the current data indicate that acute EtOH may enhance GABAergic signaling in local NTS circuits resulting in disinhibition of NTS norepinephrine neurons. Such a finding has important implications in understanding the role of the NTS in the development of alcoholism.
Keywords: Alcoholism, electrophysiology, mechanisms of addiction
Introduction
The nucleus of the tractus solitarius (NTS) is a critical brain stem region involved in the regulation of numerous physiologic activities, such as cardiovascular regulation, central control of autonomic function, and gastric motility, via downstream projections and vagal-vagal reflexes (Andresen and Kunze, 1994; Pilowsky and Goodchild, 2002; Travagli and Anselmi, 2016; Zoccal et al., 2014). Emerging evidence suggests an important role of NTS projections to hypothalamic and limbic regions of the brain in many physiologic processes as well (Lee et al., 2011; Maniscalco and Rinaman, 2017; Rinaman, 2010). Findings further suggest a critical role of NTS in the development of drug preference (Olson et al., 2006) as well as in stress-induced reinstatement of drug seeking behaviors in animal models (Smith and Aston-Jones, 2008). Specifically, it has been postulated that NTS projections, particularly from NTS neurons producing norepinephrine, activate neurons in the bed nucleus of the stria terminalis, which acts to promote seeking behaviors for many drugs of abuse (Smith and Aston-Jones, 2008).
Previous work also suggests that chronic ethanol (EtOH) exposure may similarly enhance the activity of this norepinephrine-initiated relapse pathway in mouse models (Silberman et al., 2013), but the mechanisms by which EtOH modulates NTS activity are not well understood. For instance, intraperitoneal injection of EtOH in rats has been shown to increase c-FOS like immunoreactivity in NTS neurons (Lee et al., 2011; Thiele et al., 2000, 1996). Intraperitoneal EtOH injections also produce overall depressant effects on baroreflex control of heart rate function, which is likely dependent on alteration of NTS activity, may be strain dependent (Wang and Abdel-Rahman, 2004), and can counteract the hypotensive and bradycardiac effects of intra-NTS glutamate injections, via both NMDA and non-NMDA mechanisms (el-Mas and Abdel-Rahman, 1993). Furthermore, intra-NTS microinjections of EtOH can inhibit baroreceptor heart rate responses (Zhang et al., 1989), which is likely due to activation of GABAergic signaling in the region (Varga and Kunos, 1992).
Together, the above studies indicate that acute EtOH may alter NTS function via modulation of neuronal discharge, GABAergic transmission, and/or glutamatergic transmission. However, there have been no conclusive studies to date that directly investigate which of these potential mechanisms EtOH utilizes to modulate NTS function. There is also very limited evidence on the ability of EtOH to modulate NTS activity in mice. The experiments here provide initial findings to bridge this gap in knowledge by examining the effects of acute EtOH administration on mouse NTS brain slices utilizing ex vivo electrophysiology methods. These studies indicate that EtOH enhances GABAergic transmission in NTS neurons via pre- and post-synaptic mechanisms in previously ethanol naïve mice, while having minimal effects on glutamatergic transmission and variable effects on NTS neuron action potential firing rates. Further, IP EtOH injection increased c-FOS expression in NTS tyrosine hydroxylase (TH+) neurons. Together these findings suggest that EtOH may increase local GABAergic transmission in certain NTS neuron subsets, which may lead to disinhibition of NTS TH+ neurons.
Methods
Animals
Adult male (>7 weeks old) C57Bl6/J mice were purchased from The Jackson Laboratories. All mice were housed in groups of two to five for the duration of the studies. Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Penn State University Hershey College of Medicine (Hershey, PA).
Immunofluorescent staining and imaging
Mice were injected with EtOH (2 and 4 g/kg) or isovolumic saline (total injection volumes between 250–350 μL depending on weight) and were singly maintained for one hour until perfusion. Mice were then anesthetized with Isofluorane and perfused through the left ventricle of the heart with PBS (15 ml) followed by 4% paraformaldehyde in PBS (15 ml). Brains were removed, postfixed for 24 h at 4°C in the same fixative, and then cryoprotected in PBS with 30% sucrose for an additional 48 h or until ready for sectioning. Forty micrometer semi-horizontal sections of the brainstem containing the NTS (Doyle et al., 2004) were cut on a cryostat (Microm HM 550, Thermo Scientific) and stored in cryoprotectant before fluorescent immunohistochemical staining. Free-floating brain slices containing the NTS were washed in PBS (4 × 10 min), permeabilized with 0.5% Triton X-100 in PBS (30 min), and then blocked with 10% Normal Donkey Serum in PBS containing 0.1% Triton X-100 (1 h); all steps were at room temperature. Sections were then incubated with a rabbit anti-c-FOS antibody (1:200, sc-52, lot K0615, Santa Cruz Biotechnology Inc.) and a goat anti-TH antibody (ab76442, 1:250, lot GR21411-15, Abcam) in blocking solution for 72 h at 4°C. Following the incubation period, the slices underwent PBS washes (4 × 15 min) and were incubated with donkey anti-rabbit Alexa Fluor 488 (1:750, lot#129585, Jackson Immuno Research) and donkey anti-goat Alexa Fluor 594 (1:250, lot#122478, Jackson Immuno Research) fluorescent dye-conjugated secondary antibodies for 24 h at 4°C in PBS with 0.1% Triton X-100. Finally, sections were washed (4 × 10 min), mounted on slides with Prolong Gold Mounting media (Lot#1837730, Invitrogen), and left overnight to dry. Stained NTS sections were z-stack imaged with an Olympus IX81 scanning confocal microscope. Images (2–4 per animal) were analyzed with ImageJ software (NIH) by two researchers blinded to treatment condition independently. Final cell counts on each image were averaged per mouse and used for data analysis. ImageJ was used to autocorrect image brightness and create image overlays when necessary.
Electrophysiology
Mice were perfused with ice-cold sucrose-based artificial cerebrospinal fluid (ACSF) through the left ventricle of the heart under Isofluorane anesthesia. Brains were rapidly removed and two hundred and fifty-micrometer-thick coronal brain slices containing the NTS (−7.3 to −7.8 from Bregma) were prepared from adult male (>7 weeks old) C57Bl6/J mice in ice-cold sucrose ACSF using a Leica VT1200s vibroslicer with ceramic blades (part#7550/1/C, batch#1710, Campden Instruments Limited). Sucrose based ACSF contained the following (in mM): 183 sucrose, 20 NaCl, 0.5 KCl, 1 MgCl2, 1.4 NaH2PO4, 2.5 NaHCO3, and 1 glucose. Following dissection, slices were transferred to a holding chamber with Modified ACSF [containing (in mM): 100 sucrose, 60 NaCl, 2.5 KCl, 1.4 NaH2PO4, 1.1 CaCl2, 3.2 MgCl2, 2 MgSO4, 22 NaHCO3, 20 glucose, 1 ascorbic acid] at 28°C for 20–30 min and then moved to a separate holding chamber with oxygenated standard ACSF [containing (in mM): 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10 glucose, 26 NaHCO3] at 28°C for at least 30 min prior for use in electrophysiology experiments. For electrophysiology recordings, slices were transferred to the submersed recording chamber where they were constantly perfused with standard ACSF at a rate of 2mL/min at room temperature and allowed to equilibrate for at least 15 min prior to recordings. Electrophysiology recordings were made using Clampex 10.7 utilizing a 700B Multiclamp and Digidata 1550A with HumSilencer and analyzed using Clampfit 10.7 (Molecular Devices) or with SutterPatch software utilizing Sutter Integrated Patch Clamp Amplifier and Data Acquisition System (Sutter Instruments). There were no discernable differences in any parameters measured when analyzing recordings between the two systems. Whole-cell, voltage-clamp recordings of AMPA receptor-mediated spontaneous excitatory postsynaptic currents (sEPSCs) were made at −70 mV and pharmacologically isolated by the addition of 25 μM picrotoxin to the standard ACSF utilizing a K-Gluconate based pipette solution (in mM: 135 K+-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 Na-ATP, 0.4 Na-GTP, pH 7.2–7.3, 280–290 mOsmol). Whole-cell voltage clamp recordings of GABA mediated spontaneous inhibitory postsynaptic currents (sIPSCs) were made at −70 mV and pharmacologically isolated by the addition of 3 mM kynurenic acid to block AMPA- and NMDA-mediated transmission utilizing a CsCl based pipette solution (in mM: 115 CsCl, 5 NaCl, 10 TEA-Cl, 10 HEPES, 1.1 EGTA, 4 Na-ATP, 0.3 Na-GTP, 10 glucose, pH 7.2–7.3, 280–290 mOsmol). Cells were allowed to equilibrate to whole-cell configuration for 3–5 min before recordings began and access resistance was monitored continuously. Those experiments in which the access resistance changed by >20% were not included in the data analyses. Cell-attached recordings were made without the addition of pharmacologic blockers to the standard ACSF utilizing the K-Gluconate based pipette solution with a driving potential set at −40 mV. Only neurons with steady basal action potential firing rates above 0.2Hz were included in these experiments.
Statistical analyses
Statistical analyses were performed using Microsoft Excel 2010 and GraphPad Prism 7. Graphpad Prism 7 and Microsoft Powerpoint 2010 were used for figure preparation. Specifically, when determining whether EtOH had a significant effect on electrophysiologic recordings, a Student’s paired t-test was used, comparing the baseline value to the experimental value. For cell-attached action potential recordings, peak was calculated as the furthest negative value from baseline, half-width was calculated as the time between rise and falling periods at the height of 50% of the action potential peak. Rise time was calculated as the slope of the line from 10–90% of action potential peak from baseline and decay time was calculated as the slope of the line from 90–10% of the peak back to baseline. For IHC, one-way ANOVA was used followed by post-hoc analysis controlling for false discovery rate to determine the significance of specific comparisons between groups. All values throughout the study are presented as mean±SEM.
Reagents
Ethanol (95%) was purchased from PHARMCO-AAPER (lot#C16H25001). All other reagents used were purchased from Sigma-Aldrich or Fischer Scientific unless otherwise noted in the text.
Results
EtOH enhances GABAergic transmission in the NTS
We performed whole-cell patch clamp electrophysiology recordings of spontaneous inhibitory postsynaptic currents (sIPSCs) and spontaneous excitatory postsynaptic currents (sEPSCs) in NTS neurons from two cohorts of ethanol naïve adult male C57Bl6/J mice. Neurons were recorded for an eight-minute baseline period prior to bath application of 50 mM EtOH for ten minutes. Table 1 shows basal properties of neurons tested. In testing GABAergic signaling, 50 mM EtOH significantly enhanced sIPSC frequency (64.3±20.6% increase from baseline, n=6 neurons from 4 mice, p<0.05, Fig 1) while having no significant effect on sIPSC amplitude (64.7±33.1% increase from baseline, n=6, p>0.05). However, when sIPSC amplitude data was further examined by a median split analysis (median=50.5% increase from baseline) it was revealed that EtOH significantly enhanced sIPSC amplitude in 3 out of 6 cells tested (134.3±21.2% increase from baseline, n=3, p<0.05). The three cells above the median were from three different mice. Further analysis revealed a significant positive correlation between the magnitude of 50 mM EtOH induced changes to sIPSC frequency and the magnitude of sIPSC amplitude changes (Pearson’s r=0.94, p<0.05, n=6, Fig 1D). In testing AMPA-mediated glutamatergic transmission, 50 mM EtOH produced a small but significant decrease in sEPSC frequency (14.9±6.0% decrease from baseline, n=6 neurons from 3 mice, p<0.05, Fig 2) while having no significant effect on sEPSC amplitude (6.4±7.6 % decrease from baseline, n=6, p>0.05).
Table 1.
Basic electrophysiologic properties of NTS neurons utilized for whole-cell patch clamp recordings.
| Membrane capacitance | Membrane resistance | Basal sIPSC frequency | Basal sIPSC amplitude | Basal sEPSC frequency | Basal sEPSC amplitude |
|---|---|---|---|---|---|
| 37.9±4.0 pF | 821.3±110.4 MΩ | 0.77±0.14 Hz | −20.7±10.8 pA | 7.1±2.0 Hz | −16±2.9 pA |
| n=12 neurons |
n=12 neurons |
n=6 neurons |
n=6 neurons |
n=6 neurons |
n=6 neurons |
Figure 1. EtOH enhances GABAergic transmission in the NTS.
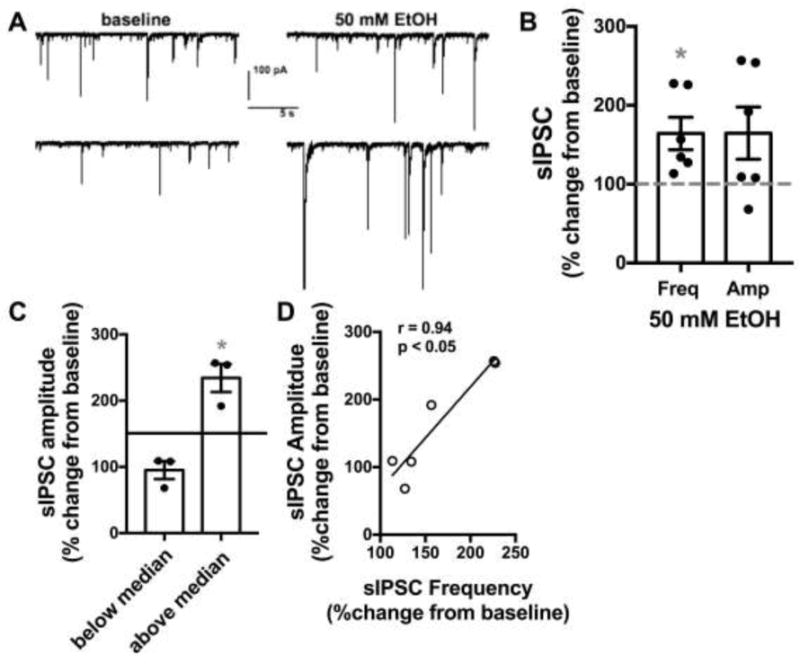
A) Exemplar traces from one neuron tested showing the effects of 50 mM EtOH on sIPSCs compared to baseline. Top and bottom traces are continuous but separated into two lines due to space constraints. B) Bar graph summarizing the effects of EtOH on sIPSC frequency and amplitude normalized as percent change from baseline. Black circles are individual data points. Dashed line indicates baseline level, normalized to 100%. Asterisk indicates significant difference from baseline. C) Bar graph summarizing the median split of sIPSC amplitude data from B. Data is expressed as change from normalized baseline (100%). Black circles are individual data point. Solid line indicates median. Asterisk indicates a significant difference from baseline. D) Correlation examining the magnitude of EtOH induced changes to sIPSC frequency vs amplitude, expressed as percent changes from normalized baselines.
Figure 2. EtOH has modest effects on glutamatergic transmission in the NTS.
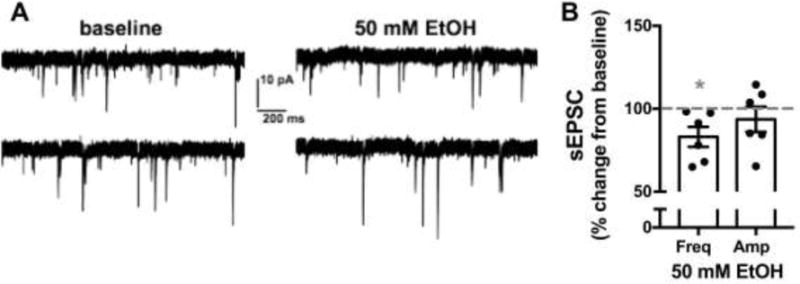
A) Exemplar traces from one neuron tested showing the effects of 50 mM EtOH on sEPSCs compared to baseline. Top and bottom traces are continuous but separated into two lines due to space constraints. showing the effects of 50 mM EtOH on sEPSCs compared to baseline. B) Bar graph summarizing the effects of EtOH on sEPSC frequency and amplitude normalized as percent change from baseline. Black circles are individual data points. Dashed line indicates baseline level, normalized to 100%. Asterisk indicates significant difference from baseline.
EtOH has variable effects on spontaneous action potential firing in NTS neurons To examine if acute EtOH modulates action potential (AP) firing, we performed cell-attached electrophysiology recordings of NTS neurons from ethanol naive adult male C57Bl6/J mice. Neurons were recorded for an eight-minute baseline period prior to bath application of 50 mM EtOH for ten minutes. Basal AP firing rates were 2.1±0.9 Hz. 50 mM EtOH did not significantly alter AP firing rates (14.6 + 7.2% decrease from baseline, n=7 neurons from 4 mice, p>0.05, Fig 3.), nor did 50 mM EtOH alter the peak amplitude, halfwidth, rise time, or decay time of APs (p>0.05 for all measures.). Median split (median=15.6% decrease from baseline) of AP frequency change data revealed that 50 mM EtOH significantly inhibited AP firing in 5 out of 7 cells tested (24.3±4.9 % decrease from baseline, n=5, p<0.05). We further correlated EtOH induced changes in AP frequency with basal AP frequency and EtOH induced changes in amplitude, half-width, rise time, or decay time and found no significant correlations (data not shown).
Figure 3. EtOH predominantly inhibits action potential firing in the NTS.
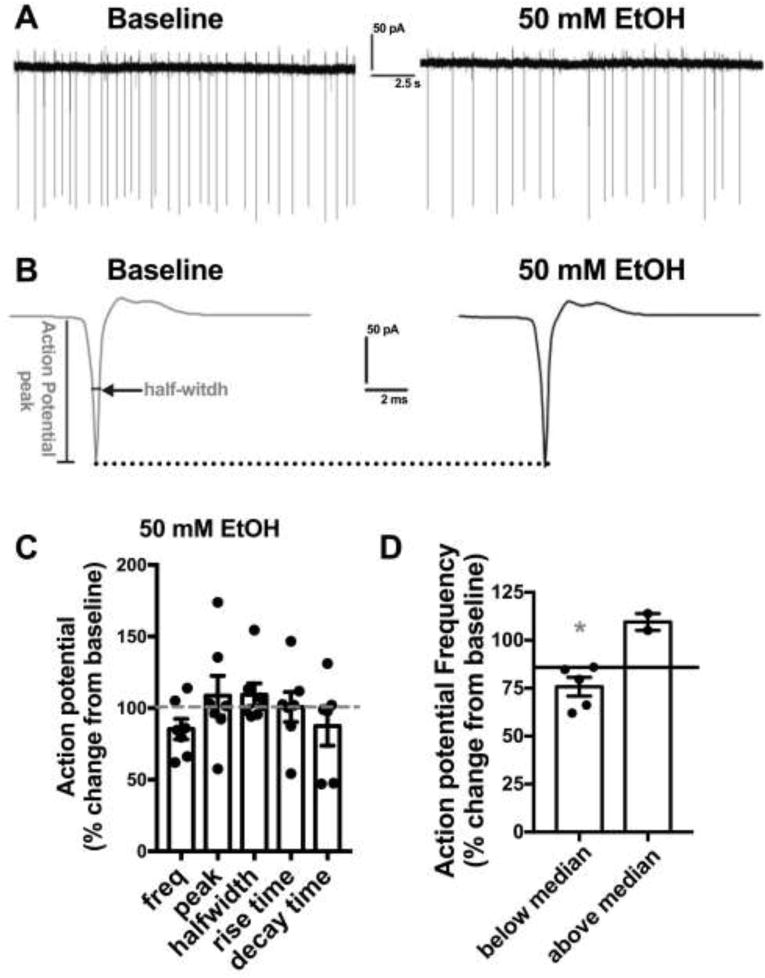
A) Exemplar traces showing the effects of 50 mM EtOH on action potential firing compared to baseline. B) Exemplar traces showing averaged action potential waveform from experiment in A. Determination of action potential peak and half-width are shown. Dotted line shows no change in peak between baseline and EtOH C) Bar graph summarizing the effects of EtOH on action potential frequency and kinetics normalized as percent change from baseline. Black circles are individual data points. Dashed line indicates baseline level, normalized to 100%. D) Bar graph summarizing the median split of action potentiation frequency data from B. Data is expressed as change from normalized baseline (100%). Black circles are individual data points. Solid line indicates group median. Asterisk indicates a significant difference from baseline.
In vivo EtOH enhances c-FOS expression in NTS TH+ neurons
We next sought to examine the ability of acute EtOH to alter NTS function when administered in vivo. Previous reports indicate that EtOH can enhance c-FOS-like immunoreactivity in NTS TH+ neurons in rats. Therefore, we administered saline or EtOH (2 and 4 g/kg intraperitoneal) in EtOH-naive adult male C57Bl6/J mice (5–6 mice per group). One hour following injection, mice were euthanized and examined for c-FOS and TH expression in NTS via immunohistochemistry (Fig 4A). Overall, c_FOS+ cells count were 15.3± 4.1 for saline, 31.4±5.9 for 2g/kg, and 32.3±7.8 for 4g/kg injections. Although standard one-way ANOVA revealed a trend for increased cFOS+ cell counts in the NTS of EtOH exposed mice, this trend did not reach significance (F(2,14)=2.71, p=0.10, Fig 4B). We then examined the percentage of TH+ neurons that co-expressed c-FOS. Overall, the percentage of TH+ neurons that coexpressed c-FOS were: saline 17.9±7.6; 2g/kg 12.4 ±2.2; 4g/kg 40.7±10.2. One-way ANOVA revealed a significant effect of EtOH on c-FOS/TH colocalization in the NTS (F(2, 14)=4.29, p=0.0352). Post-hoc analysis controlling for false discovery rate revealed a significant increase in c-FOS/TH colocalization in the 4g/kg group compared to both the saline and 2g/kg groups.
Figure 4. Effects of EtOH on c-FOS/TH colocalization.
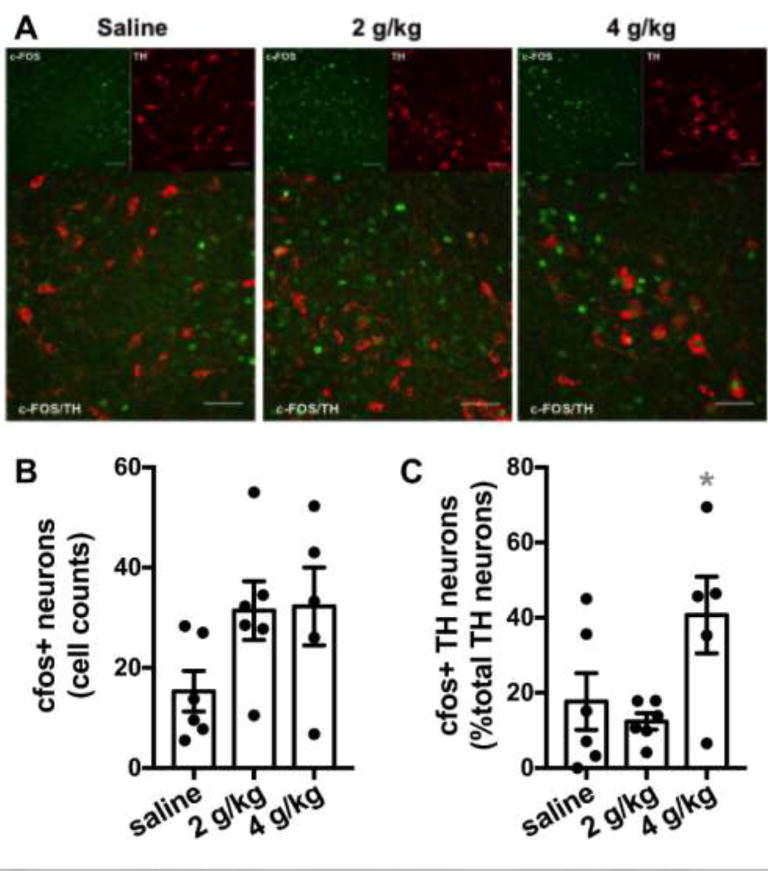
A) Exemplar images of NTS slices prepared from saline, 2 g/kg EtOH and 4 g/kg EtOH injected mice utilized for immunohistochemistry. White scale bars indicate 50 microns. B) Bar graph summarizing the effects of treatment on total number of c-FOS+ cell counts. Black circles are individual data points. C) Bar graph summarizing the effects of treatment on percent of TH+ neurons colabeled with c-FOS. Black circles are individual data points. Asterisk indicates significant differences from all other groups.
Discussion
The NTS is critical for numerous physiologic processes and has been implicated in stress-induced reinstatement to drug seeking behaviors in animal models of addiction, including alcoholism. However, the mechanisms by which acute EtOH modulates NTS neuronal activity and neurotransmission are not well understood. The findings here indicate that acute EtOH increases presynaptic GABAergic release in NTS neurons, with potential postsynaptic effects in a subset(s) of as yet unknown neuronal population(s). This appears to be associated with a decrease in action potential firing, which again seems to be specific to some NTS subpopulations. While EtOH does not seem to have any dramatic effects on NTS glutamatergic signaling, acute EtOH injection in vivo increases c-FOS expression in NTS TH+ neurons.
EtOH is known to increase GABAergic transmission in many brain regions through a variety of pre- and post-synaptic mechanisms (Breese et al., 2006; Lowery-Gionta et al., 2015; Roberto et al., 2003; Silberman et al., 2008). It is perhaps not surprising that the data here also show significant effects of EtOH on NTS GABAergic transmission. Specifically, there was an increase in sIPSC frequency, suggesting EtOH enhances presynaptic GABAergic transmission in the NTS. Additionally, EtOH significantly enhanced sIPSC amplitude in 50% of cells tested, suggesting EtOH may have additional postsynaptic effects in specific neuronal populations, particularly in neurons that had the highest increases in sIPSC frequency. The selective postsynaptic effects of EtOH on a subset of NTS neurons is not entirely surprising given that the NTS contains very heterogeneous populations of neurons. Overall, the finding that EtOH enhances NTS GABAergic transmission is in accordance with previous work suggesting intra-NTS injection of EtOH enhances GABAergic signaling to inhibit baroreceptor heart rate response (Varga and Kunos, 1992; Zhang et al., 1989) and provides two potential mechanisms by which this may occur (increase in presynaptic GABA release and/or enhanced function of postsynaptic GABA receptors). There were minimal effects of acute EtOH on glutamatergic transmission in the NTS. However, since repeated chronic ethanol exposure and withdrawal has been shown to cause an imbalance of inhibitory and excitatory transmission in many brain regions (Diana et al., 2003; Pleil et al., 2015)-which may be a critical factor in the development of alcoholism-future research will be need to determine how chronic EtOH exposure alters the balance of excitatory and inhibitory transmission in the NTS.
Acute ethanol was also shown to inhibit AP firing of the majority of NTS neurons tested. This is most likely related to the strong enhancement of inhibitory GABAergic neurotransmission seen during acute EtOH application. However, even in this limited sample not all neurons responded to EtOH with a reduction in AP firing rates and there was a trend toward increased c-FOS staining (a standard marker of neuronal activity) in the NTS following in vivo EtOH exposure. Ethanol has been shown to modulate AP firing in cell type specific manner in other brain regions (Herman and Roberto, 2016). As mentioned above, the NTS contains a very heterogeneous collection of neuronal cell types and future research will be needed to determine which of these cell types are inhibited or activated following acute and chronic EtOH exposure.
A clue as to at least one neuronal type that responds to acute EtOH with increasing activation was determined in the studies with in vivo EtOH exposure. Here, colocalization of c-FOS was enhanced in TH+ NTS neurons. These findings are in accordance with previous experiments in rats (Lee et al., 2011; Thiele et al., 2000). Assuming that enhanced c-FOS/TH colocalization indicates that EtOH has increased the activity of these neurons, the findings here suggest that NTS TH+ neurons may be uniquely EtOH sensitive compared to other NTS neuronal subtypes. NTS TH+ neurons have been implicated in both initial preference to opiates (Olson et al., 2006) as well as stress-induced reinstatement to drug seeking behaviors (Smith and Aston-Jones, 2008), potentially suggesting that this neuronal population is sensitive to numerous classes of abused drugs. The data presented here suggest that the ability of EtOH to enhance c-FOS activity in NTS TH neurons is not likely to be due to modulation of glutamatergic transmission, as sEPSCs were minimally inhibited in our preparation. Previous studies indicate that EtOH may inhibit NMDA and non-NMDA glutamatergic transmission in the NTS (el-Mas and Abdel-Rahman, 1993). The findings here are in support of these previous studies, but this mechanism likely does not play a role in NTS TH+ neuron activation.
Together, these findings indicate that acute EtOH alters NTS TH+ neuron activity via either a direct or indirect mechanism. The data here cannot confirm nor reject the hypothesis that EtOH may directly alter NTS TH+ neurons, however, such a mechanism would disregard the data here showing the ability of EtOH to inhibit AP firing in most neurons and the strong facilitatory effects of EtOH on NTS GABAergic transmission. Therefore, a more likely scenario is that EtOH may enhance GABAergic transmission onto other GABAergic neurons within the NTS, which in turn would prevent subsequent GABA release onto NTS TH+ neurons. The net result would be an EtOH-induced disinhibition of NTS TH+ neurons. A similar mechanism of EtOH-induced disinhibition of catecholaminergic neurons has been suggested to occur in the ventral tegmental area (Xiao et al., 2007; Xiao and Ye, 2008; c.f. Theile et al., 2011). Future work will be necessary to determine if acute EtOH effects on NTS TH+ neurons are due to disinhibition, direct activation, or potentially both. It will also be important to determine if chronic EtOH exposure may impact either of these potential mechanisms.
In conclusion, the results of these studies represent the initial characterization of the mechanisms by which acute EtOH modulates neurocircuitry in the NTS, a brain region that may be critical for alcoholism. Overall, the results here indicate that acute EtOH may have multiple cell-type and synapse-specific effects that may work in concert to contribute to the development of alcoholism. Future work will be necessary to delineate potential neuron and circuit specific effects of acute and chronic EtOH, and to determine the behavioral relevance of this circuit in addictive behaviors in alcoholism. These findings may also be of relevance to the study of addiction to other drugs of abuse.
Highlights.
EtOH enhances pre- and post-synaptic GABAergic transmission in the NTS
EtOH has minimal effects on NTS glutamatergic transmission
EtOH decreases action potential firing in a majority of neurons
EtOH enhances c-FOS/tyrosine hydroxylase colocalization in the NTS
EtOH may disinhibit NTS TH neurons by increasing GABA signals between interneurons
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant number AA022937]
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AP
action potential
- EtOH
ethanol
- NTS
nucleus of the tractus solitarius
- PBS
phospho-buffered saline
- sEPSC
spontaneous excitatory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andresen MC, Kunze DL. Nucleus Tractus Solitarius—Gateway to Neural Circulatory Control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the Gabamimetic Profile of Ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Brodie M, Muntoni A, Puddu MC, Pillolla G, Steffensen S, Spiga S, Little HJ. Enduring Effects of Chronic Ethanol in the CNS: Basis for Alcoholism. Alcohol Clin Exp Res. 2003;27:354–361. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods. 2004;137:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- el-Mas MM, Abdel-Rahman AA. Role of NMDA and non-NMDA receptors in the nucleus tractus solitarius in the depressant effect of ethanol on baroreflexes. J Pharmacol Exp Ther. 1993;266:602–10. [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2016;21:72–86. doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Craddock Z, Rivier C. Brain stem catecholamines circuitry: Activation by alcohol and role in the hypothalamic-pituitary-adrenal response to this drug. J Neuroendocrinol. 2011;23:531–541. doi: 10.1111/j.1365-2826.2011.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional Alterations in the Dorsal Raphe Nucleus Following Acute and Chronic Ethanol Exposure. Neuropsychopharmacology. 2015;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Rinaman L. Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–20. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–88. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–8. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:950–60. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: Role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008 doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience. 2011;172:94–103. doi: 10.1016/j.neuroscience.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Cubero I, van Dijk G, Mediavilla C, Bernstein IL. Ethanol-induced c-fos expression in catecholamine- and neuropeptide Y-producing neurons in rat brainstem. Alcohol Clin Exp Res. 2000;24:802–9. doi: 10.1111/j.1530-0277.2000.tb02059.x. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. c-Fos Induction in Rat Brainstem in Response to Ethanol- and Lithium Chloride-Induced Conditioned Taste Aversions. Alcohol Clin Exp Res. 1996;20:1023–1028. doi: 10.1111/j.1530-0277.1996.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389–401. doi: 10.1038/nrgastro.2016.76. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Kunos G. Inhibition of baroreflex bradycardia by ethanol involves both GABAA and GABAB receptors in the brainstem of the rat. Eur J Pharmacol. 1992;214:223–232. doi: 10.1016/0014-2999(92)90122-K. [DOI] [PubMed] [Google Scholar]
- Wang X, Abdel-Rahman AA. An association between ethanol-evoked enhancement of c-jun gene expression in the nucleus tractus solitarius and the attenuation of baroreflexes. Alcohol Clin Exp Res. 2004;28:1264–1272. doi: 10.1097/01.alc.0000137299.04112.c2. [pii] [DOI] [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: Role of μ-opioid receptors. Neuroscience. 2008;153:240–248. doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjević K, Ye JH. Effects of ethanol on midbrain neurons: Role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abdel-Rahman AA, Wooles WR. Impairment of baroreceptor reflex control of heart rate but not sympathetic efferent discharge by central neuroadministration of ethanol. Hypertension. 1989;14:282–292. doi: 10.1161/01.HYP.14.3.282. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Furuya WI, Bassi M, Colombari DSA, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol. 2014 doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]


