Abstract
[Purpose] The interaction between the visual and tactile modalities influences on different levels from neural activity, perception, higher cognition to behavior. The aim of this study was to examine how a visual stimulus influences tactile sensitivity depending on temporal asynchrony. [Subjects and Methods] In total, 15 participants took part in this study. They were required to perform a two-alternative forced-choice task regarding whether a tactile pulse was felt. The individual participants’ tactile thresholds were estimated using a repetitive stepwise method. Visual stimuli were simultaneously presented with various temporal gaps (0 ms, ± 50 ms, ± 100 ms, and ± 300 ms), whereas no visual stimulus was presented in the tactile only condition. The tactile thresholds in eight conditions were compared using analysis of variance. [Results] Of the participants, 53.5% showed the most sensitive tactile threshold when presented with a visual stimulus with a short temporal gap, especially when the visual stimulus preceded the tactile one by 50 ms. [Conclusion] The preceding visual stimulus facilitates the perceptual sensitivity of the tactile sensation. Providing sensory stimuli in a multisensory mode benefits perceptual encoding. A pre-attentional mechanism led by a particular sensory modality might work as a perceptual advantage for another modality.
Keywords: Multisensory integration, Stimulus onset asynchrony, Tactile sensitivity
INTRODUCTION
Interactions between the visual and tactile sensory modalities occur at multiple levels in terms of neural activity, perception to higher cognition and behavior1,2,3). According to previous research studies, if neural activity is facilitated, perceptual sensitivity increases and behavioral reaction times become faster through multisensory stimulation4, 5). One example that illustrates perceptual changes via multisensory integration is that the tactile detection threshold becomes more sensitive when tactile stimuli are simultaneously provided with visual stimuli6, 7).
There are some plausible explanations for these perceptual and behavioral changes via multisensory inputs. Among the theoretical explanations, the notion of the pre-attention effect is dominantly advocated by many investigators8); namely, that attention attracted by one modality (i.e., vision) facilitates encoding in another (i.e., touch). However, in the previous research studies, the importance of the timing between the two modalities was not examined in detail, which is critical in order to distinguish whether attention across modalities enhances perceptual sensitivity or whether the effect is based on multisensory integration.
Many studies have investigated the multisensory integration of the visual and auditory senses, but there are few studies of the visual and tactile senses9, 10). Integration of the visual and tactile senses is very notable because the two senses closely cooperate to generate motor action. The tactile threshold is an important basic sensory function for the development of motor action11, 12). However, the question arises of whether such sensory sensitivity can be benefitted when the sensory stimuli are presented with a multisensory complex.
Therefore, the aim of this study was to examine whether multisensory stimulation benefits a unimodal sensory threshold, particularly in the case of visual and tactile sensory integration. This study was designed to investigate whether the timing between the visual and tactile sensations would influence tactile sensitivity.
SUBJECTS AND METHODS
In total, 15 healthy participants took part in this study as paid volunteers. The average age was 23.9 years (age range 18–33) and 8 were males. They were all neurologically healthy and had no particular medical history. They had undergone the Adolescent/Adult Sensory ProfileTM assessment in order to briefly screen their sensitivity of taste/smell, movement, visual, touch, activity level, and auditory stimuli and to check whether they have a normal sensory processing ability13). Only the participants who did not have significant hyper- or hypo-sensitivity to the sensory modalities finally took part in the study. This study was approved by Institutional Review Board of Seoul National University Hospital and the written informed consent was provided to all participants.
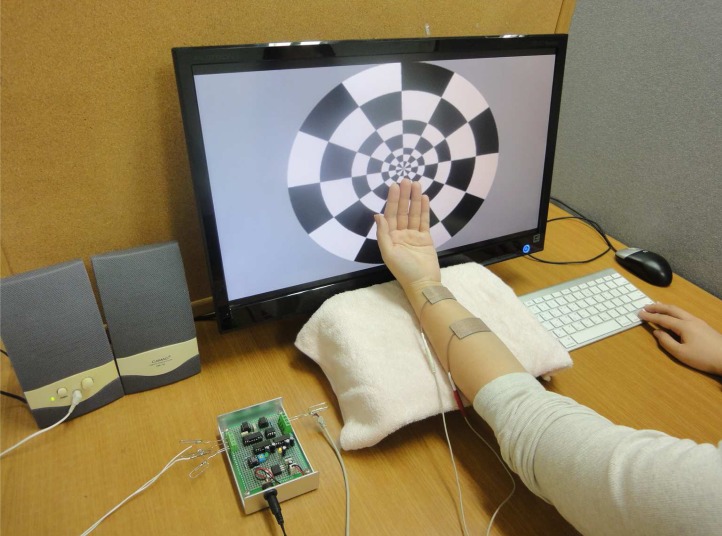
First, a tactile stimulus was presented using an electrical stimulator that was made in our laboratory. The stimulator is based on a voltage-to-current converter circuit generating bipolar current pulses and it is controlled using MATLAB (Mathworks Inc., MA, USA). For the tactile stimulus, once a pulse (frequency 1 Hz, impedance 10 kΩ) is evoked, a tapping sensation is felt through the median nerve 1 cm above the wrist crease. An on-offset checkerboard was presented as the visual stimulus on an LG 23-inch LCD monitor (resolution 1,920 × 1,080, refresh rate 60 Hz). The visual stimulus was synchronized with the tactile stimulus using a MATLAB program (Fig. 1). The safety issues related to the tactile and visual stimuli on the human body were approved by the ethical committee before the experiment.
Fig. 1.
Scene of the experiment. The visual stimulus shows on the monitor and the tactile stimulus is given to the left forearm.
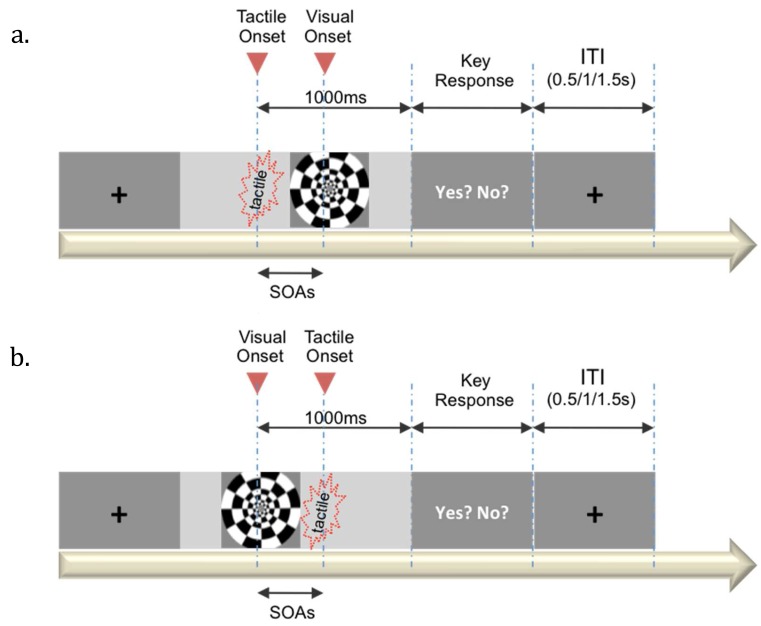
In order to adjust the individually different tactile thresholds, a pilot test of the threshold setup was conducted. The individual tactile threshold was estimated using a repetitive stepwise method. Eleven pre-determined pulse intensities were repeated 10 times each and pulses at various intensities were randomly presented. Then, participants indicated whether the stimulus was felt or not by pressing the “Y” key on the keyboard for yes and the “N” key for no. If they felt it at all 10 times at a certain pulse intensity, the lowest intensity was set as their individual tactile threshold. Once the individual threshold was set, six points of the tactile sub-threshold intensity were determined as the stimulus conditions. The six points of the sub-threshold intensity were 0%, 20%, 40%, 60%, 80%, and 100% of the pre-set individual threshold. Each threshold level was repeated for 20 trials. An on-offset checkerboard flickered in synchronization with the tactile stimulus with various stimulus onset asynchronies (SOA; 0 ms, ± 50 ms, ± 100 ms, and ± 300 ms), whereas no visual stimulus was presented in the tactile only condition (Fig. 2). The duration of the visual stimulus was 10 ms. Participants were required to perform a yes-or-no two-alternative forced choice task regarding whether the tactile pulse was felt.
Fig. 2.
Design of the experiment. The eight stimulus onset asynchrony conditions were (a) tactile-first conditions with a stimulus onset asynchronies (SOAs) of 50 ms, 100 ms, and 300 ms, and (b) the visual-first conditions with SOAs of 50 ms, 100 ms, and 300 ms. In addition, there were a visual and tactile synchronous condition and a tactile only condition. ITI: Inter-Trial Interval.
The hit rates of the tactile detection were estimated for the six points of the tactile sub-threshold intensity and then the data were fit using a sigmoid function. It was possible to compare the detection threshold among the eight SOA conditions. The 50% tactile threshold for the detection sensitivity was defined as the threshold at which the participant hit 50% of the given tactile stimuli. Each value of the 50% tactile threshold was drawn from the sigmoid fitted curve. Then, each condition was analyzed according to the SOA using a one-way analysis of variance in MATLAB.
RESULTS
The lowest tactile threshold which means the most sensitive in tactile reception varied across the participants. Of the 15 participants, 53.3% (N=8) showed that the tactile threshold was the lowest when the visual stimulus preceded the tactile stimulus by 50 ms (VT50).
The tactile threshold of the 15 participants was averaged at each SOA condition (Table 1). The highest mean threshold was 0.67 at the Tactile Only condition (provided the tactile stimuli without a visual one), and TV300 (tactile stimulus comes first 300 ms than the visual one) and VT300 (visual stimulus comes first 300 ms than the tactile one) followed. The mean threshold of TV300 and VT 300 were 0.66 and 0.65, respectively. The lowest mean threshold was 0.59 at the VT50 condition where the visual stimulus came first 50 ms than the tactile one. The VT50 condition differed from the other conditions in the post-hoc tests (LSD) (mean difference=0.079, standard error=0.043, p=0.07). However, it was not statistically significant.
Table 1. Tactile threshold according to 8 different SOA conditions.
| SOA conditions | Minimum (RI) | Maximum (RI) | Mean (RI) | Std. deviation | Variance |
|---|---|---|---|---|---|
| Tactile only | 0.53 | 0.83 | 0.67 | 0.08 | 0.01 |
| VT300 | 0.55 | 0.84 | 0.65 | 0.08 | 0.01 |
| VT100 | 0.37 | 0.83 | 0.60 | 0.12 | 0.01 |
| VT50 | 0.39 | 0.80 | 0.59 | 0.13 | 0.02 |
| Sync | 0.36 | 0.94 | 0.61 | 0.14 | 0.02 |
| TV50 | 0.46 | 0.84 | 0.62 | 0.11 | 0.01 |
| TV100 | 0.45 | 0.98 | 0.63 | 0.15 | 0.02 |
| TV300 | 0.52 | 0.83 | 0.66 | 0.11 | 0.01 |
*Relative Intensity (RI) was used for threshold measure.
DISCUSSION
The primary sensory perception in terms of both the detection and discrimination is important for the motor action. Especially, the visual and tactile sensory integration is necessary to generate the motor action projected for the object and visual surroundings11, 12). Clinically, it senses that the multisensory inputs benefit the motor planning and action, but it is difficult to draw the hypothetical testing under a rigorous experimental setting. In this study, attempts were made to investigate the tactile threshold changes when the multisensory mode is provided in accordance with the onset asynchronies between them. The timing between a tactile and visual sensation is important for changing the tactile threshold.
According to the results of this study, the tactile sense was most sensitive when the visual stimulus preceded the tactile stimulus with a SOA of 50 ms. The viewpoint of the pre-attention effect in previous studies, which is that attention drawn by one sensory modality facilitates the encoding of another sensory stimulus, is consistent with these results8). The change in the detection sensitivity at a certain time implies that attention is attracted across sensory modalities and that it enhanced perceptual sensitivity.
Again, the tactile threshold can become more sensitive when the visual stimulus is presented with a brief temporal gap; 50 ms between the two modalities is suggested by the results of this study. However, a longer onset asynchrony between the two sensory modalities disadvantaged each modality. The tactile threshold was less sensitive when there was more than a 300 ms temporal asynchrony between the two stimuli regardless of the stimuli order. From the pre-attentional theory perspective, more than a 300 ms gap between the preceding and following attentional cues is not effective for encoding sensory information and it further interferes with the information processing instead. According to Posner’s inhibition of return effect, spatially deprived attention delays the sensory encoding of another spatial area, but this can also be similarly explained in terms of the temporal dimension of the multisensory mode14). Attention that was previously inhibited by a sensory stimulus degrades the perceptual sensitivity of another modality.
It was apparent that the hit rate of the tactile stimuli increases when it is provided with the visual stimuli with a brief temporal gap, however, there is still the possibility that the false-alarm would also increase on the other hand15). Unfortunately, ‘visual only’ condition which there were no tactile stimuli and only visual stimuli were provided was not included in this experimental design. It was not possible to check as to whether the rate of false-alarm would increase or not, which is the limitation of this study.
In future studies, it is necessary to investigate whether the perceptual advantage of the multisensory mode with a short temporal gap is consistent with neural activity using electroencephalogram/event-related potential experiments. Furthermore, how visual and tactile sensory integration influences higher level motor actions and behaviors needs to be examined for the clinical use in rehabilitation therapy.
REFERENCES
- 1.Taylor-Clarke M, Kennett S, Haggard P: Vision modulates somatosensory cortical processing. Curr Biol, 2002, 12: 233–236. [DOI] [PubMed] [Google Scholar]
- 2.Newport R, Rabb B, Jackson SR: Noninformative vision improves haptic spatial perception. Curr Biol, 2002, 12: 1661–1664. [DOI] [PubMed] [Google Scholar]
- 3.Ro T, Wallace R, Hagedorn J, et al. : Visual enhancing of tactile perception in the posterior parietal cortex. J Cogn Neurosci, 2004, 16: 24–30. [DOI] [PubMed] [Google Scholar]
- 4.Kennett S, Taylor-Clarke M, Haggard P: Noninformative vision improves the spatial resolution of touch in humans. Curr Biol, 2001, 11: 1188–1191. [DOI] [PubMed] [Google Scholar]
- 5.Tipper SP, Lloyd D, Shorland B, et al. : Vision influences tactile perception without proprioceptive orienting. Neuroreport, 1998, 9: 1741–1744. [DOI] [PubMed] [Google Scholar]
- 6.Shams L, Kamitani Y, Shimojo S: Illusions. What you see is what you hear. Nature, 2000, 408: 788. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RM, Burton PC, Ro T: Visually induced feelings of touch. Brain Res, 2006, 1073-1074: 398–406. [DOI] [PubMed] [Google Scholar]
- 8.Mirams L, Poliakoff E, Brown RJ, et al. : Vision of the body increases interference on the somatic signal detection task. Exp Brain Res, 2010, 202: 787–794. [DOI] [PubMed] [Google Scholar]
- 9.Colonius H, Diederich A: The optimal time window of visual-auditory integration: a reaction time analysis. Front Integr Nuerosci, 2010, 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giard MH, Peronnet F: Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci, 1999, 11: 473–490. [DOI] [PubMed] [Google Scholar]
- 11.Laszlo JI: The role of visual and kinaesthetic cues in learning a novel skill. Aust J Psychol, 1968, 20: 191–196. [Google Scholar]
- 12.Chase RA, Rapin I, Gilden L, et al. : II Sensory feedback influences on keytapping motor tasks. Q J Exp Psychol, 1961, 13: 153–167. [Google Scholar]
- 13.Brown CE, Dunn W: Adolescent/Adult Sensory Profile: user’s manual. San Antonio: Psychological Corporation, 2002. [Google Scholar]
- 14.Posner MI, Rafal RD, Choate LS, et al. : Inhibition of return: neural basis and function. Cogn Neuropsychol, 1985, 2: 211–228. [Google Scholar]
- 15.Macmillan NA, Douglas CC: Detection theory: a user’s guide, 2nd ed. New Jersey: Lawrence Erlbaum Associates, 2004. [Google Scholar]