Abstract
[Purpose] To investigate the influence of knee immobilization period on recovery of histological damages in the anterior cruciate ligament (ACL) insertion and articular cartilage in rabbits. This knowledge is important for determining the appropriate rehabilitation approach for patients with ligament injuries, fracture, disuse atrophy, and degenerative joint disease. [Materials and Methods] Forty-eight male Japanese white rabbits were divided equally into the remobilization and control groups. The remobilization group had the right knee surgically immobilized, and was divided equally into four subgroups according to the duration of immobilization (1, 2, 4 and 8 weeks). After the immobilization was removed, the rabbits moved freely for 8 weeks. The control group underwent sham operation and followed the same time course as the remobilization group. The chondrocyte apoptosis rate and chondrocyte proliferation rate in the ACL insertion and articular cartilage were analyzed after remobilization. [Results] In the ACL insertion, the remobilization group had a higher chondrocyte apoptosis rate than the control group after 8 weeks of immobilization, and a lower chondrocyte proliferation rate than the control group after 4 and 8 weeks of immobilization. In the articular cartilage, the remobilization group had a lower chondrocyte proliferation rate than the control group after 8 weeks of immobilization. After 8 weeks of remobilization, the ACL insertion and articular cartilage are not completely recovered after 4 and 8 weeks of immobilization, respectively. [Conclusion] Our results suggest that 8 weeks of remobilization will result in recovery of the ACL insertion after 2 weeks of knee immobilization, and recovery of the articular cartilage after 4 weeks of knee immobilization. If 8 weeks of immobilization occurs, a remobilization duration of more than 8 weeks may be necessary.
Keywords: Anterior cruciate ligament insertion, Articular cartilage, Remobilization
INTRODUCTION
Regeneration and/or repair of the anterior cruciate ligament (ACL) insertion and the articular cartilage have been attracting clinical attention1,2,3,4). Joint immobilization is commonly used in the treatment of periarticular fractures and ligament injuries. Immobilization-induced articular cartilage degeneration is caused by decreases in the number of chondrocytes and cartilage extracellular matrix (ECM), changes in cartilage thickness, upregulation of destructive factors in the ECM, and cartilage surface alterations5,6,7,8,9). We previously reported that knee immobilization in rabbits resulted in an increase in chondrocyte apoptosis at 2 and 8 weeks in the ACL insertion and at 4 and 8 weeks in the articular cartilage of the medial tibial condyle, and a decrease in safranin O-stained glycosaminoglycan (GAG) layer thickness from 2 to 8 weeks in the ACL insertion and from 4 to 8 weeks in the articular cartilage10).
Several studies have reported on the reversible or irreversible changes of degenerated articular cartilage in remobilization after immobilization11,12,13). Whether the changes are reversible is dependent on the joint fixing method, the location of the cartilage, and the species of animal; furthermore, the articular cartilage reportedly does not completely recover after remobilization13,14,15). However, the influence of knee remobilization on chondrocyte apoptosis, chondrocyte proliferation, and GAG thickness in the ACL insertion and articular cartilage are unknown. Clarifying the reversibility of degeneration of the ACL insertion and the articular cartilage after immobilization will give insight into the best approach for rehabilitation of immobilized joints. This study aimed to investigate the influence of knee immobilization period on recovery of histological damages in the ACL insertion and articular cartilage in rabbits.
MATERIALS AND METHODS
Forty-eight skeletally immature male Japanese white rabbits (weight range: 2.5–3.0 kg) were used for this study. This study was approved by the Ethical Committee of the Ibaraki Prefectural University of Health Sciences in 2017 (project identification no. 26). The rabbits were maintained in accordance with the guidelines of the Ethical Committee of the Ibaraki Prefectural University of Health Sciences and the National Institutes of Health guidelines for the care and use of laboratory animals (NIH Pub. No. 86-23 Rev. 1985). The rabbits were randomly divided into a remobilization group (n=24) and a control group (n=24).
After intravenous barbiturate injection (40 mg/kg), the remobilization group underwent right knee internal fixation using a method similar to that described previously10). After 1, 2, 4 and 8 weeks respectively, (n=6 animals in each subgroup according to the period of immobilization), the fixation devices were removed and the rabbits moved freely for 8 weeks; after 8 weeks, all six animals in each subgroup were euthanized. The control group underwent sham operation and followed the same time course as the remobilization group.
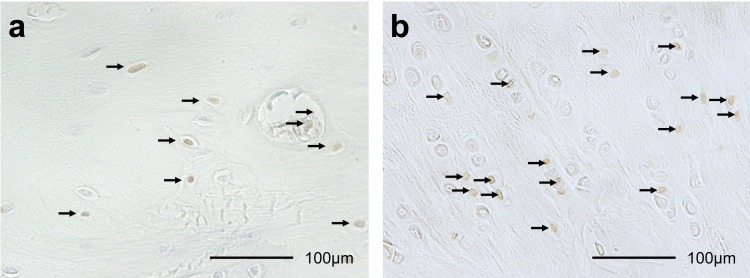
The ACL insertions and articular cartilages of the medial tibial condyle from each animal were fixed in neutral-buffered formalin, decalcified and embedded in paraffin10, 16, 17). The specimens were stained with hematoxylin and eosin and safranin O10, 16, 17). Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick-end labeling (TUNEL) was performed to detect apoptotic cells (Fig. 1a), and proliferating cell nuclear antigen (PCNA) was used to detect proliferating cells (Fig. 1b) using previously described methods10, 16, 17).
Fig. 1.
Histological sections (×400) of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick-end labeling (TUNEL) staining (a), and proliferating cell nuclear antigen (PCNA) staining (b) in ACL insertion after 2 weeks of knee immobilization. Brown cells (arrows) are TUNEL-positive chondrocytes and PCNA-positive chondrocytes. The TUNEL-positive and PCNA-positive rates were calculated by dividing positive cells by the total number of chondrocytes.
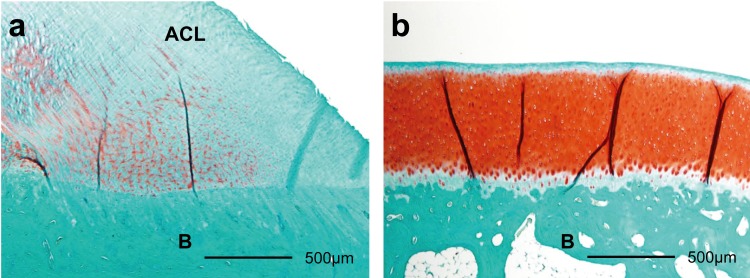
The histomorphometric analysis was performed as described in previous studies10, 16, 17). To define the average thickness of the red GAG-stained areas by safranin O, each red GAG-stained area was divided by the width of the ACL insertion or the articular cartilage (Fig. 2). The TUNEL-positive and PCNA-positive rates were calculated by normalizing to the total number of chondrocytes in the cartilage layer.
Fig. 2.
Histological sections (×40) of Safranin O staining of anterior cruciate ligament (ACL) insertion (a), and articular cartilage (b) in the control group. The red stained areas are the glycosaminoglycan (GAG). To define the average thickness of the red GAG-stained areas, each red GAG-stained area was divided by the width of the ACL insertion or the articular cartilage. ACL: anterior cruciate ligament; B: bone.
The histomorphometric data were compared between groups after each fixation period using Student’s t-tests. To examine the time-dependent histological changes, a one-way analysis of variance (ANOVA) was performed on the data in each group. If ANOVA indicated that the parameters were significantly different between groups, Tukey-Kramer tests were performed. Differences with a p value <0.05 were considered statistically significant.
RESULTS
The results are summarized in Table 1.
Table 1. Histomorphometrical data on recovery from knee immobilization.
| Group | 1 W (n=6) | 2 W (n=6) | 4 W (n=6) | 8 W (n=6) | |||
|---|---|---|---|---|---|---|---|
| Apoptosis rates (%) | ACL | Remobilization | 25.6 ± 12.8 | 15.0 ± 6.2+ | 30.8 ± 17.4 | 38.4 ± 17.9*+ | |
| Control | 17.2 ± 14.6 | 15.5 ± 7.9 | 26.7 ± 20.6 | 16.2 ± 9.8* | |||
| Articular cartilage | Remobilization | 26.2 ± 5.2 | 22.7 ± 12.4 | 22.5 ± 7.8 | 16.0 ± 7.9 | ||
| Control | 25.3 ± 13.9 | 27.3 ± 2.0 | 18.9 ± 12.6 | 23.7 ± 12.8 | |||
| Proliferation rates (%) | ACL | Remobilization | 10.1 ± 7.8 | 11.3 ± 6.1 | 6.1 ± 1.5* | 6.8 ± 4.2* | |
| Control | 10.4 ± 9.9 | 10.6 ± 9.1 | 12.9 ± 5.6* | 14.2 ± 6.1* | |||
| Articular cartilage | Remobilization | 15.1 ± 4.8 | 11.7 ± 8.7 | 10.5 ± 5.5 | 12.4 ± 7.8* | ||
| Control | 18.4 ± 8.8 | 18.4 ± 6.3 | 13.6 ± 9.1 | 25.8 ± 8.8* | |||
| Thickness of GAG-stained areas (μm) | ACL | Remobilization | 258.3 ± 164.3 | 200.6 ± 123.2 | 196.6 ± 192.8 | 231.0 ± 109.1 | |
| Control | 273.5 ± 132.1 | 311.0 ± 186.1 | 288.9 ± 201.5 | 322.2 ± 178.4 | |||
| Articular cartilage | Remobilization | 439.2 ± 206.8 | 501.5 ± 263.0 | 586.1 ± 138.2 | 610.5 ± 160.8 | ||
| Control | 563.3 ± 122.8 | 603.7 ± 134.4 | 551.8 ± 123.7 | 686.0 ± 111.7 | |||
ACL: anterior cruciate ligament; GAG: glycosaminoglycan; W: week. Results are presented as the mean ± SD. *Significant difference between groups in the same week (p<0.05). +Significant difference between weeks in the same group (p<0.05).
In the ACL insertion, the chondrocyte apoptosis rate in the remobilization group was higher than that in the control group after 8 weeks of immobilization (p=0.012); furthermore, in the remobilization group, the apoptosis rate was higher after 8 weeks than 2 weeks of immobilization (p=0.048). In the articular cartilage of the medial tibial condyle, there were no significant between-group differences in the chondrocyte apoptosis rate after any fixation period.
In the ACL insertion, the chondrocyte proliferation rate in the remobilization group was lower than that in the control group after 4 weeks (p=0.008) and 8 weeks of immobilization (p=0.018). In the articular cartilage of the medial tibial condyle, the chondrocyte proliferation rate in the remobilization group was lower than that in the control group after 8 weeks of immobilization (p=0.019).
In the ACL insertion and in the tibial articular cartilage of the medial tibial condyle, there were no significant between-group differences in the thicknesses of GAG-stained areas after any fixation period.
DISCUSSION
After 8 weeks of remobilization, the ACL insertion and articular cartilage did not completely recovered after 4 and 8 weeks of immobilization, respectively. In contrast, the ACL insertion and articular cartilage recovered after 2 and 4 weeks of immobilization, respectively. Therefore, the chondrocytes in the ACL insertion and articular cartilage may be in the process of recovery. That is, more than 8 weeks of remobilization may be needed for chondrocytes in the ACL insertion and articular cartilage to recover from 8 weeks of immobilization. Conversely, if the remobilization period is less than 8 weeks, the change in the chondrocytes caused by immobilization may be greater and may appear earlier than that after 8 weeks of remobilization. There were greater histological changes in the chondrocyte apoptosis and chondrocyte proliferation in the ACL insertion than in the articular cartilage. This may have occurred because the cartilage layer in the ACL insertion is thinner and contains fewer chondrocytes than that in the articular cartilage, and so the ACL insertion may have been more strongly influenced by the immobilization and thus the recovery was delayed10).
Chondrocytes in the ACL insertion and articular cartilage can recover once apoptosis increases or cell proliferation decreases. The chondrocyte apoptosis rate and chondrocyte proliferation rate in the ACL insertion and articular cartilage recovered to the same level as the control group after 8 weeks of remobilization in rabbits that had undergone 2 weeks of immobilization. A previous study reported that the apoptosis of chondrocytes caused by unloading was decreased by reloading in rat bone18). Furthermore, administration of a drug (salubrinal) reportedly increased the chondrocyte proliferation and reduced the chondrocyte apoptosis under various compressive stresses on the temporomandibular joint in rats19). These previous findings indicate that chondrocyte apoptosis and chondrocyte proliferation cannot be irreversible, as they can be improved by changing the mechanical environment or administering medicines.
Regarding the synthesis of GAG, the ACL insertion and articular cartilage can recover from 8 weeks of knee immobilization after 8 weeks of remobilization. The GAG thicknesses in the ACL insertion and cartilage layer did not significantly differ between both groups after any fixation period after 8 weeks of remobilization. However, a previous study reported that the articular cartilage GAG concentration did not recover in dogs that underwent unilateral hindlimb immobilization for 11 weeks followed by remobilization for 50 weeks13). Moreover, articular cartilage degeneration was found in rats that underwent unilateral knee immobilization for 8 weeks followed by remobilization for 8 weeks, depending on the location of the articular cartilage14). Although the degree of degeneration depends on the fixation method, the location of the cartilage, and the species of the animal, articular cartilage cannot completely recover even after remobilization11,12,13,14,15). Even though the thickness of the GAG recovered after remobilization in the present study, other cartilage matrix constituents and/or mechanical properties of the cartilage layer may have been changed. If the duration of immobilization is longer than 8 weeks or the duration of remobilization is shorter than 8 weeks, the GAG thickness may be smaller than that found in the present study. In particular, long periods of immobilization may result in an irreversible change in the cartilage layer.
Clinically, knowledge of the histological changes that occur in the ACL insertion and articular cartilage after remobilization is important for determining the appropriate rehabilitation approach for patients with ligament injuries, fracture, disuse atrophy, and degenerative joint disease. Our results suggest that 8 weeks of remobilization will result in recovery of the ACL insertion after 2 weeks of knee immobilization, and recovery of the articular cartilage after 4 weeks of knee immobilization. If 8 weeks of immobilization occurs, a remobilization duration of more than 8 weeks may be necessary. Moreover, this knowledge may help in devising better rehabilitation protocols for ligament injuries and/or periarticular fractures that control and limit chondrocyte apoptosis and chondrocyte proliferation. Further immunohistochemical analysis of other components of the ECM in cartilage layers and mechanical analyses are necessary to clarify the details of remobilization after immobilization.
In conclusion, histological changes caused by 4 weeks of immobilization in the ACL insertion and 8 weeks of immobilization in the articular cartilage remained after 8 weeks of remobilization. After 8 weeks of remobilization, the cartilage layers cannot be completely recovered in the ACL insertion after 4 weeks of knee immobilization and in the articular cartilage after 8 weeks of knee immobilization.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by a Grant-in-Aid for Encouragement for Young Scientists from Ibaraki Prefectural University of Health Sciences, 2015. We thank Kelly Zammit, BVSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
REFERENCES
- 1.Mutsuzaki H, Sakane M, Fujie H, et al. : Effect of calcium phosphate-hybridized tendon graft on biomechanical behavior in anterior cruciate ligament reconstruction in a goat model: novel technique for improving tendon-bone healing. Am J Sports Med, 2011, 39: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 2.Dong Y, Zhang Q, Li Y, et al. : Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci, 2012, 13: 13605–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Wei X, Gao J, et al. : Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: an experimental study in a rat model of osteoarthritis. Int J Mol Sci, 2016, 17: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorzi AR, Amstalden EM, Plepis AM, et al. : Effect of human adipose tissue mesenchymal stem cells on the regeneration of ovine articular cartilage. Int J Mol Sci, 2015, 16: 26813–26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagiwara Y, Ando A, Chimoto E, et al. : Changes of articular cartilage after immobilization in a rat knee contracture model. J Orthop Res, 2009, 27: 236–242. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara Y, Ando A, Chimoto E, et al. : Expression of collagen types I and II on articular cartilage in a rat knee contracture model. Connect Tissue Res, 2010, 51: 22–30. [DOI] [PubMed] [Google Scholar]
- 7.Ando A, Hagiwara Y, Tsuchiya M, et al. : Increased expression of metalloproteinase-8 and -13 on articular cartilage in a rat immobilized knee model. Tohoku J Exp Med, 2009, 217: 271–278. [DOI] [PubMed] [Google Scholar]
- 8.Leong DJ, Gu XI, Li Y, et al. : Matrix metalloproteinase-3 in articular cartilage is upregulated by joint immobilization and suppressed by passive joint motion. Matrix Biol, 2010, 29: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai M, Aoyama T, Ito A, et al. : Alteration of cartilage surface collagen fibers differs locally after immobilization of knee joints in rats. J Anat, 2015, 226: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutsuzaki H, Nakajima H, Wadano Y, et al. : Influence of knee immobilization on chondrocyte apoptosis and histological features of the anterior cruciate ligament insertion and articular cartilage in rabbits. Int J Mol Sci, 2017, 18: E253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schollmeier G, Sarkar K, Fukuhara K, et al. : Structural and functional changes in the canine shoulder after cessation of immobilization. Clin Orthop Relat Res, 1996, (323): 310–315. [DOI] [PubMed] [Google Scholar]
- 12.Setton LA, Mow VC, Müller FJ, et al. : Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage, 1997, 5: 1–16. [DOI] [PubMed] [Google Scholar]
- 13.Haapala J, Arokoski JP, Hyttinen MM, et al. : Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res, 1999, (362): 218–229. [PubMed] [Google Scholar]
- 14.Nagai M, Ito A, Tajino J, et al. : Remobilization causes site-specific cyst formation in immobilization-induced knee cartilage degeneration in an immobilized rat model. J Anat, 2016, 228: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando A, Suda H, Hagiwara Y, et al. : Reversibility of immobilization-induced articular cartilage degeneration after remobilization in rat knee joints. Tohoku J Exp Med, 2011, 224: 77–85. [DOI] [PubMed] [Google Scholar]
- 16.Basso N, Heersche JN: Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone, 2006, 39: 807–814. [DOI] [PubMed] [Google Scholar]
- 17.Wen J, Jiang Y, Zhang C, et al. : The protective effects of salubrinal on the cartilage and subchondral bone of the temporomandibular joint under various compressive mechanical stimulations. PLoS One, 2016, 11: e0155514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutsuzaki H, Nakajima H, Wadano Y, et al. : Influence of gradual elongation to the patella tendon insertion in rabbits. Int J Mol Sci, 2014, 15: 14835–14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutsuzaki H, Nakajima H, Wadano Y, et al. : Influence of mechanical unloading on histological changes of the patellar tendon insertion in rabbits. Knee, 2015, 22: 469–474. [DOI] [PubMed] [Google Scholar]