Abstract
In the context of medical three-dimensional (3D) printing, in addition to 3D reconstruction from cross-sectional imaging, graphic design plays a role in developing and/or enhancing 3D-printed models. A custom prototype modular 3D model of the liver was graphically designed depicting segmental anatomy of the parenchyma containing color-coded hepatic vasculature and biliary tree. Subsequently, 3D printing was performed using transparent resin for the surface of the liver and polyamide material to develop hollow internal structures that allow for passage of catheters and wires. A number of concepts were incorporated into the model. A representative mass with surrounding feeding arterial supply was embedded to demonstrate tumor embolization. A straight narrow hollow tract connecting the mass to the surface of the liver, displaying the path of a biopsy device’s needle, and the concept of needle “throw” length was designed. A connection between the middle hepatic and right portal veins was created to demonstrate transjugular intrahepatic portosystemic shunt (TIPS) placement. A hollow amorphous structure representing an abscess was created to allow the demonstration of drainage catheter placement with the formation of pigtail tip. Percutaneous biliary drain and cholecystostomy tube placement were also represented. The skills of graphic designers may be utilized in creating highly customized 3D-printed models. A model was developed for the demonstration and simulation of multiple hepatobiliary interventions, for training purposes, patient counseling and consenting, and as a prototype for future development of a functioning interventional phantom.
Keywords: 3D printing, Graphic design, Liver anatomy, Hepatobiliary interventions, Consenting, Medical education
Background
Three-dimensional (3D) printing—also referred to as additive manufacturing or rapid prototyping—allows for the fabrication of a 3D solid object most commonly from a STereoLithography (STL) file via numerous techniques in a layer-by-layer fashion [1]. The applications for 3D printing in the biosciences have burgeoned in the past decade with the creation of an accurate representation of biological tissues and organs as the most widespread and reported clinical use for 3D printing. These 3D-printed objects are increasingly becoming integrated into clinical practice in numerous disciplines ranging from neurosurgery to cardiology for the purposes of surgical/interventional planning and simulation, medical education and training, patient counseling, and research [1, 2]. Despite the recent increase in usage and broadening of the applications for 3D-printed phantoms, there remains a dearth of literature on 3D-printed liver models. As the hepatobiliary system exhibits complex and varied spatial relationships, particularly among vascular and biliary structures, it is ideal for 3D modeling.
Traditionally, training models of the hepatobiliary system have been via virtual reality, computer-generated 3D reconstructions, or through manufactured and animal physical models [3–5]. Virtual reality is an advanced simulation option that allows users to practice hepatobiliary procedures and their complications under life-like conditions with, for example, incorporation of patient respiration and haptic feedback [4]. This technology, however, is constrained by its costliness and its decreased ability to teach spatial relationships among structures secondary to unrealistic sizing and its requirement to be viewed on a 2D monitor despite representing 3D objects. This latter disadvantage also applies to viewing surface-rendered 3D reconstructed models [3]. In contrast, manufactured and animal physical models can display the complex anatomy of the hepatobiliary system in 3D [5]. But again, high cost and lack of reusability, customizability, and reproducibility limit their usefulness. With the advent of 3D printing comes a means to improve upon these previous limitations and create phantoms that exhibit higher fidelity, availability, customizability, and reproducibility, requiring less manufacturing time, while being more cost-effective than existing technologies over the long-term.
As 3D printing of hepatobiliary phantoms is still relatively new, there are only a limited number of studies published on the creation and use of these phantoms (Table 1). Prototypes have been successfully manufactured and applied to the fields of transplant medicine, pediatrics, and surgery. More specifically, patient-specific liver models have been utilized to enhance pre-surgical planning for donor-liver transplantation and for hepatectomy for hepatocellular carcinoma, improving the safety of these procedures [6, 7]. Both procedures require intimate detail of patient-specific anatomy, as there tends to be great variation among biliary and vascular structures. The 3D models were useful in bolstering comprehension of 3D spatial relationships among critical structures. 3D-printed liver models have also been shown to be effective tools in teaching the anatomy of hepatic segments to medical students [8]. The fastest growing and most innovative use for liver phantoms is procedure simulations; thus far, phantom simulations have been reported for laparoscopic choledochal cyst excision surgery and hepatoblastoma resection in pediatric patients and endoscopic ultrasound-guided biliary drainage [9–11]. Despite the fact that 3D-printed models for procedure simulation are ideally suited for interventional radiology, there are currently no reports of a hepatobiliary phantom having been manufactured for this purpose or for specific use in this field. Hence, we set out to create a prototypical, highly customizable, and cost-effective 3D-printed hepatobiliary phantom that could not only showcase the anatomy of this complex system but could also set the stage for the development of advanced functional interventional phantoms that allow for full simulation of a number of hepatobiliary interventions. To this end, the services of a graphic designer were implemented to create initial design and subsequent modifications and enhancements tailored towards the educational goals in mind.
Table 1.
Comparison of 3D printed hepatobiliary models
| Ref | Materials/design of phantom | Cost | Model uses |
|---|---|---|---|
| [6] | –Patient-specific liver phantom containing massive hepatocellular carcinoma and rare anatomic vascular variations –Made with a composite material of zp150 –Printed via a Spectrum z™ 510 3D printer |
Not provided | –Pre-operative planning of virtual hepatectomy, taking into account the patient’s rare vascular variations and depth of tumor invasion –Correct diagnosis of rare anatomic variations of abdominal blood vessels |
| [7] | –6 patient-specific liver models (3 from living donors and 3 LDLT recipients) based on patient’s imaging –Uses TangoPlus/VeroClearPlus for liver parenchyma, TangoBlackPlus/VeroBlue for hepatic veins, and TangoPlus/VeroClear blend for other external vessels –Transparent liver parenchyma and color-coded vascular structures –External vessels and biliary structures attached to liver via permanent adhesive –Printed via Connex350 3D printer |
Not provided, but estimated by Kong et al. to be about $1200/model | –Pre-operative visualization of patient-specific vascular and biliary anatomy |
| [8] | –3 types of liver models displaying hepatic segments (1) without parenchyma, (2) with transparent parenchyma, and (3) with hepatic vessels –3D printed by Spectrum z 510 with high-performance composite powder zp150 –Parenchyma of type 2 model made with transparent jelly wax |
$600 per model | –Anatomical teaching of hepatic segments to medical students |
| [9] | –Dilated biliary system based on MRCP image made of 2-mm thick polycarbonate material –3 access ports created in the bile duct to indicate puncture sites –Bile duct and access ports seen under radiography/EUS –Pumped aerated liquid through added IV tubing to create Doppler effect on U/S –Porcine or bovine liver around access ports for realistic tissue interface –Printed via Viper si2 stereolithography system |
Not provided | –Simulation of EUS-guided biliary drainage (anterograde procedure and choledochoduodenostomy) |
| [10] | –Laparoscopic choledochal surgery model based on hepatic anatomy imaging and standard laparoscopic dimensions –Uses free-standing liver mold made with nylon-based powder and includes a cuboid portal into which disposable components representing ducts and the cyst can be placed –Mold is then used to create multiple models employing T28 silicone mold rubber –Printed via 3D Systems ProJet 660Pro printer |
900€ per mold (or $1002.38) | –Simulation of laparoscopic choledochal cyst excision |
| [11] | –Patient-specific liver model with large hepatoblastoma based on CT images –3D printed via Objet500 Connex3 printer using acrylic UV curable resin –Translucent liver parenchyma and inclusion of hepatic and portal veins and IVC in model |
Not provided | –Assist surgical planning in a pediatric patient through determining respectability and understanding patient’s specific anatomy –Perform surgical simulation for hepatoblastoma treatment |
Methods
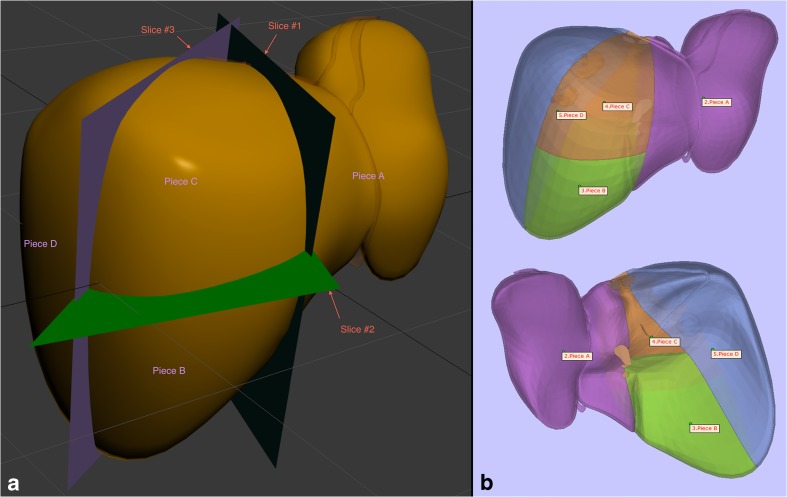
The model comprises two groups of individual parts with each group consisting of four components. One group is the liver surface (Fig. 1), which also depicts the segmental anatomy and is made of transparent resin material (Fig. 2). The other is a series of hollow thin-walled tubular structures composed of polyamide material (specifically nylon), representing the vasculature and biliary system (Fig. 6). A graphic artist, whose services were obtained through an online service (FlatPyramid.com) for freelance graphic designers for a $500 fee, custom-designed the 3D digital model based on design specifications; these were outlined in numerous initial 2D diagrams and figures as well as hand-drawn sketches, explaining the exact detail of the anatomy. For example, the horizontal plane separating the top and bottom rows of liver segments is based on a straight line encompassing the left and right portal veins, and each of the three vertically oriented planes separating the column of segments is created by one of the hepatic veins (Fig. 1a). This 3D digital model was also used for a few other projects, including an interactive virtual tutorial on liver anatomy as well as a smaller 3D-printed model for teaching the segmental anatomy [12]. Based on the needs of the envisioned 8-piece model, multiple subsequent modifications were made to the 3D digital model, such as hollowing of the vasculature and biliary system, creating thickness for each of the liver surface pieces, and fitting of the internal structures through the gap between the liver surface pieces that appose one another at the liver hilum (Fig. 5).
Fig. 1.
Liver surface. a Instructional figure for the graphic designer demonstrating the slice planes dividing the model into 4 pieces based on segmental anatomy. The reflection of the falciform ligament can also be seen, which, in part, demonstrates the delineation of the plane vertically dividing the left lobe of the liver. b Anterior and posterior views of the 4-piece model during quality control prior to 3D printing
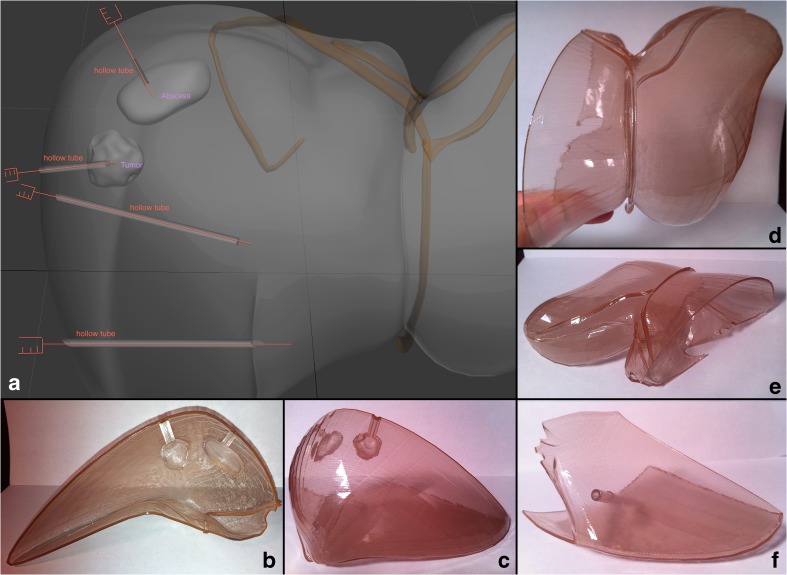
Fig. 2.
Liver surface. a Instructional figure for the graphic designer demonstrating areas for needle tracts, represented as hollow tubes. For ease of design and 3D printability, the hollow tube for demonstrating biliary drain placement was changed to a simple hole on the surface. b, c 3D-printed part of the right hepatic lobe. d, e 3D-printed left hepatic lobe with the falciform ligament reflections. f 3D-printed lower part of the right hepatic lobe with the hollow tube for demonstrating the path of a cholecystostomy tube towards the gallbladder
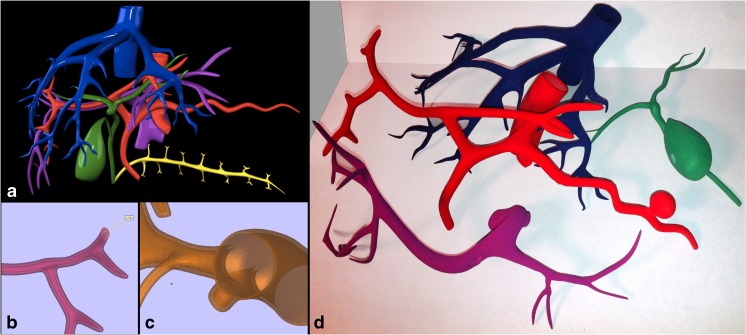
Fig. 6.
Internal structures. a Surface rendering after initial design by the graphic artist. b, c Digital design modification stage of hollowing the internal structures. d Final 3D-printed hollowed models of the hepatic veins, hepatic arteries, portal veins, and hepatobiliary system, including the gallbladder
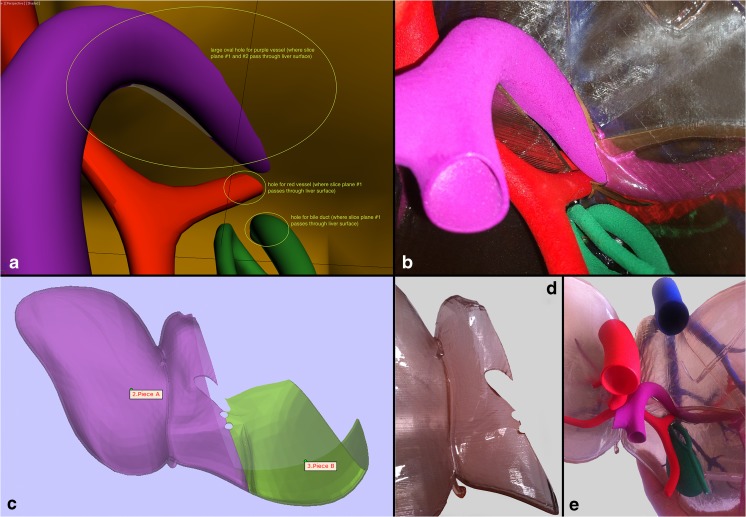
Fig. 5.
Hilum of the liver. a Instructional figure for the graphic artist indicating areas where holes had to be incorporated in the surface models to allow for passage of the portal vein, hepatic artery, and the common bile duct, which had to be through the plane of separation between the right and left hepatic lobes to allow for final assembly. b Equivalent close-up view of the final 3D-printed model showing the hilum with the aforementioned structures incorporated. c Digital design stage where the left and part of the right hepatic lobes are shown from the hilum, showing the holes for the internal structures. d The undersurface of the 3D-printed left hepatic lobe showing the holes. e Final assembled 3D-printed model viewed from the hilum
Modification of the graphically designed model was accomplished through use of Autodesk 3D Studio Max, a powerful software that is widely used in graphic design, on a PC-based computer with a quad core 2.66 GHz processor and 16 GB of RAM. At the time of this submission, a freeware full version can be obtained through a student/educator license for educational purposes for up to 3 years. It is important to note that strong processing power and a large amount of RAM is necessary to perform the 3D processing and modification of complex 3D meshes. This is especially crucial in cases where 3D meshes are the result of 3D reconstruction from cross-sectional imaging data; this, in part, explains why this project was not done using those types of 3D meshes (Figs. 3 and 4). The requirement for a high processing power can even further be minimized by working with graphically designed models with a smaller number of faces, but at the cost of smoothness of the surfaces.
Fig. 3.
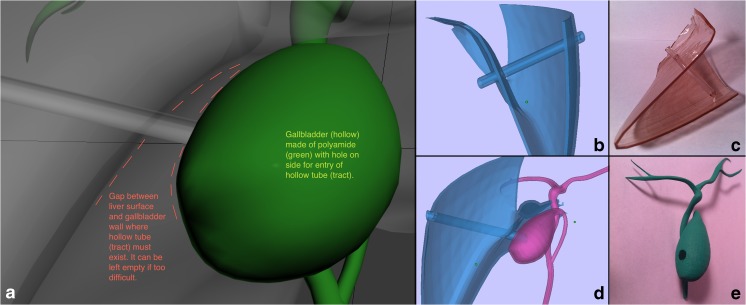
Cholecystostomy tube design. a Instructional figure for the graphic designer describing the course of the hollow tube coursing the liver parenchyma and the gap between gallbladder and liver eventually entering the gallbladder. b, c Lower part of the right hepatic segment during the design stage and final 3D-printed model. d, e Gallbladder as it fits with the liver during the design stage, and the final hollow 3D-printed model with a hole in its surface for the hollow tube representing cholecystostomy tube pathway
Fig. 4.
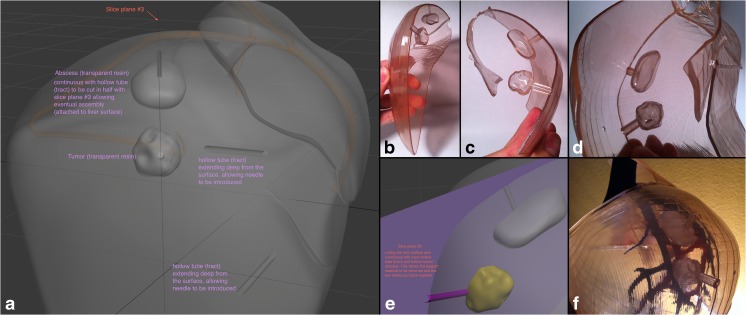
Liver abscess drainage and tumor biopsy tract. a Design instructions to the graphic artist in a view angle parallel to the plane separating the liver surface such that it is also along the tracts dividing them in half. b, c, d 3D-printed liver surface models that contain each half of the tracts, which when coming together, form a complete hollow tube. This affords the ability to easily place and remove a soft object in the site designated for the mass/tumor as well as fluid in the site for the abscess site. e Magnified view of the instructional figure for the graphic designer, showing cut plane passing through the middle of the hollow tubes connecting to the mass and abscess. f View of the final assembled 3D-printed liver model with its contents showing the lateral aspect of the right hepatic lobe with the abscess and mass representative sites seen through the transparent surface
Fitting of models into one another is a very important consideration during the digital design stage; therefore, significant care must be taken to assure proper eventual physical compatibility of the entire model once assembled. For example, the inside structures (i.e., vasculature and hepatobiliary system) have to be fitted through the separation planes between the surface pieces (Fig. 5). Even the representative needle tracts from the surface to the abscess and the mass were designed such that they fall onto the separation plane of each piece, thereby cutting each tract in half (Fig. 4). This would allow placement of fluid in the abscess site to demonstrate or practice creation of a pigtail tip of a catheter or to place an actual soft object in the representative area for a mass to practice going through the steps of performing a biopsy.
Creation of adequate space in its designated area for each tubular structure as it passes through the separation plane between the surface pieces can be performed via the “subtraction” Boolean operation of the 3D Studio Max software. Even then, a slight increase in the diameter of the hole needs to be incorporated to allow for proper physical fitting once the components are 3D printed. For the surface parts, the goal is to have smooth transparent objects, for which the color brown was chosen, representing the color of the liver. As for the hollow tubular structures, the goal is to minimize wall thickness while maintaining reasonable sturdiness. The portal system was assigned the color purple, hepatic veins blue, hepatic arteries red, and the biliary system green. The concept of minimum wall thickness is an important consideration in 3D printing and is directly related to the overall size of a given model. Generally speaking, when 3D meshes are designed or 3D reconstructions are performed from imaging, the resultant product consists of surface rendering. Unless an object is being 3D printed with the intent of being completely solid (filled), the design has to be such that the inside is hollowed by creating an arbitrary wall thickness; this means that the inside aspect of the surface has its own faces projecting inwards, just as, for example, a vessel does. The diameter of any given wall depends on the design characteristics and on the material that is to be used during 3D printing. The minimum wall thickness is known for each material, which for polyamide—the material used for the tubular structures—is 1 mm [13]. The 3D printability of a model can be checked using 3D Studio Max’s “STL Check” modifier. This can also be accomplished with freeware software tools, such as Meshlab or Slicer, which allow for some semi-automatic corrections. The Materialise Cloud (cloud.materialise.com) is a valuable service, where numerous solutions for common challenges in 3D printing are made available online. These tools include “STL converter,” “model analyzer,” “model repair,” “parameter extraction,” “wall thickness analyzer,” “scaling,” “triangle reduction,” and “hollowing.”
Transparent resin models (Fig. 2) were constructed via stereolithography, the process of conversion of liquid resin to solid form with an ultraviolet light laser. Polyamide models (Fig. 6) were constructed from a white, very fine, granular powder, resulting in strong, somewhat flexible material that can endure small impacts and resist some pressure while being bent. Selective laser sintering, conversion of powder into solid form using laser, is utilized to build designs with this material, which allows for a high degree of freedom of design compared to all the 3D printing techniques. These models were then spray-painted based on the desired colors. The 3D printing and spray-painting were performed through i.Materialise, a commercial online platform for 3D printing services [13]. The total cost of the eight pieces (four polyamide and four transparent resin) was just under $1000, with each piece ranging from $100–$180. Dimensions for each piece ranged from 3.8 cm for the smallest diameter to 19 cm for the largest. Appropriate wall thickness for the structures was chosen with special consideration of cost-effectiveness, visibility, and durability, with the assistance of engineers at i.Materialise. Eventually, the model was assembled, with the liver surface pieces attached to each other using transparent adhesive tape.
Results
The final 3D-printed model is demonstrated in Fig. 7. A representative mass was embedded at the junction of segments VII and VIII of the liver with its surrounding feeding arterial supply allowing for the demonstration of tumor embolization (Fig. 4). A straight narrow hollow tract connects the mass to the surface of the liver, displaying the path of a biopsy device’s needle (18 gauge) and the concept of needle throw length. The tract can fit an 18-gauge needle. A hollow, walled-off amorphous structure representing an abscess was created inferior to the mass again at the junction of segments VII and VIII, allowing for the demonstration of a drainage catheter (8 French) placement with formation of a pigtail tip (Fig. 4). A connection between the middle hepatic and right portal veins was also created to allow for the demonstration of stent placement during TIPS procedure (Fig. 8). There was also a hole created on the liver surface for displaying percutaneous biliary drain placement, which can extend through the common bile duct (Fig. 4d). Another straight connection was created from the surface of the inferior edge of the liver coursing through the parenchyma into the hollowed gallbladder to exhibit percutaneous cholecystostomy tube placement (Fig. 3).
Fig. 7.
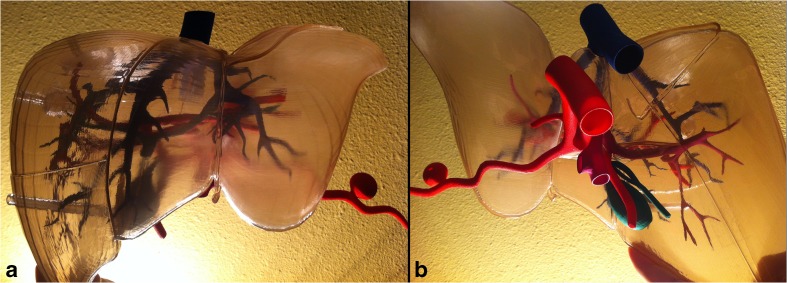
Final assembled 8-piece 3D-printed model of the liver. a Anterior view. b Posteroinferior view
Fig. 8.
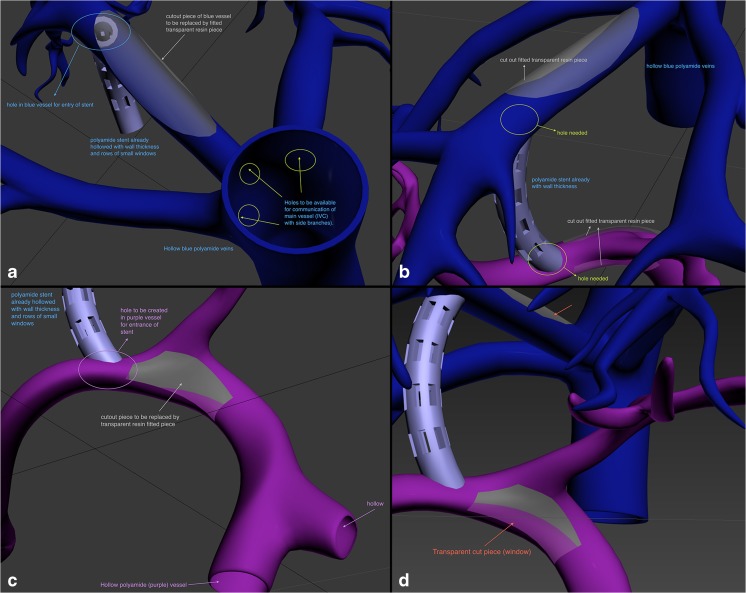
Design instructions for hollowing the hepatic veins and portal veins as well as demonstrating TIPS procedure. a Indicating areas where lumens had to be connected, for example, the lumen of the hepatic veins that need to be connected to the lumen of the inferior vena cava (IVC). b Indicating areas where holes had to be created in the middle hepatic vein and the right portal vein to accommodate each end of the TIPS stent. c, d Design instructions for creating a window in the bifurcation of the portal vein to allow for visibility of the wire during TIPS procedure. The plan for the cut piece areas in the portal vein and the middle hepatic vein that were to be replaced with transparent pieces had to be abandoned due to 3D printability limitations especially given the relatively small size of the structures
Discussion
Graphic designers are professionals that utilize dedicated software in order to create 2D or 3D visual representations in static or animated form. In the setting of 3D printing, the skills of 3D graphic artists may be implemented to develop or modify design, be it for highly detailed and specific anatomic models or for simulation models. Graphic designers have the ability to create a perfected design that is geometrically developed from the initial stages. This allows for much easier modification and manipulation both from a design standpoint and also during preparation for 3D printing, meaning that STL errors are much less likely to arise. Having the ability to start with minimum needed polygons counts allows for faster and more robust processing by 3D software when complicated models are added together. For example, when developing a modular design with a multi-part nature, even if printed separately and only for the purpose of checking compatibility, this aspect provides a great advantage. Furthermore, professional graphic designers are intimately familiar with the features and capabilities of 3D design software, giving them the ability to quickly and efficiently implement the tools in the software.
It is noteworthy to mention that other professionals aside from graphic artists can serve the role of a graphic designer, such as biomedical engineers, radiology technicians, and even radiologists. However, simply being familiar with certain software and using its advanced features efficiently are two different concepts. Communication between graphic designers and physicians or biomedical engineers can at times be challenging, given the potential complexity of the anatomy or design needs. It would be ideal to have face-to-face interactions during communications, but this may not always be possible, as it had not been in our case. Numerous back-and-forth emails, sketches, schematics, and screenshots were exchanged to arrive at the final model. This can be a difficult process to predict when the initial price estimate is being agreed upon. More advanced needs may arise during the design which may require additional time commitment from the graphic artist and therefore incurring further costs. Therefore, being familiar with at least one professional 3D graphic design software at the beginner or intermediate level can somewhat obviate some of that need.
While it is true that a person’s cross-sectional imaging can be modified to meet custom simulator design needs, the perfection and noise-free nature of geometrically developed meshes is at times incomparable. For example, adding wall thickness to reconstructed models can create unwanted crossing and overlapping of edges and planes that eventually may cause numerous STL errors. For designing simulators, there are many occasions where multiple pieces are to be assembled, either due to the nature of design or the need for different materials. Extracting the aortic valve, for example, from the wall of the aorta is much harder when it is reconstructed as a single mesh from a cardiac CT angiogram as opposed to when both have been separately designed by a graphic artist. It is noteworthy to mention that perhaps combining CT/MR segmentation reconstructions, modified if needed, with de novo graphic design meshes can be an ideal approach in order to create a hybrid design.
In this technical note, developing a highly customizable modular 3D-printed liver prototype was discussed, with a graphic designer serving at the core of this process. This model depicts the segmental anatomy of the parenchyma as well as the hepatic vasculature and biliary tree. The components are color-coded for easy identification of structures, and the outer shell is transparent, providing good visibility of internal structures. Interventional radiologists and residents can employ these models to visualize patient-specific anatomy or common variations of anatomy in three dimensions. This becomes of great importance in hepatobiliary interventions, as considerable variation exists within this system. Outside the field of interventional radiology, this model has potential for use in pre-clinical medical education for learning anatomy. It can supplement current anatomy teaching methods by bypassing the limited access that students have to cadavers and providing another avenue for students to learn hepatic segments and the complex spatial relationships of vascular and biliary structures.
In addition to its ability to help teach hepatobiliary anatomy, this model has the added benefit of having hollow internal structures, which, in turn, enables catheters and tubing to be passed through it. To our knowledge, this is the first 3D-printed phantom of its kind that can be utilized for the practice, planning, and eventually, simulation of a number of diagnostic and therapeutic hepatobiliary interventional procedures. These include stent placement during TIPS, hepatic artery embolization, abscess drainage, catheter placement with formation of a pigtail tip, and percutaneous cholecystostomy tube placement. This model also allows users to conceptually understand needle throw length and to visualize a splenic artery aneurysm. Having the opportunity to engage in advanced pre-interventional planning and procedure simulation/practice has important implications for patient care, as they lead to better outcomes following procedures with fewer complications and shorter patient recovery times [6, 11].
Patients have also been shown to be more satisfied with their care when providers employ these models when counseling them; in comparison to using no visual aids or the patient’s imaging, utilizing patient-specific 3D-printed phantoms helps patients better understand their disease processes and the surgeries that will be performed on them [14]. Similarly, interventional radiologists may use 3D-printed models to explain complex anatomy to patients, explain how guidance is used, and allay their concerns about the accuracy of needle or catheter placement. Overall, this model is multi-faceted in the roles it can serve in interventional radiology and has the ability to further meet the needs of users by being highly customizable. While its versatility is arguably its greatest advantage, the model also benefits from being easily reproducible and reusable and for being cost-effective. To this last point, note that while the model is comparable in price to other 3D-printed models, it will become even more cost-effective as the technology of 3D printing improves and becomes more widespread.
While there are multiple advantages to this model, as have been laid out in this report, there are some limitations to both using 3D printing, particularly as related to the graphic design stage, and to our model specifically. In terms of 3D printing, the initial graphic design of complicated 3D meshes requires advanced skills. Since most healthcare providers never encounter the need for such skills, it is probably best that the initial design be either done by professional 3D graphic artists or obtained from commercial online repositories that provide 3D models, such as TurboSquid.com. Subsequently, making changes to such models is possible. Based on the needs and the skill level of the user, there are numerous powerful 3D design software available, freeware or proprietary, including Autodesk 3DS Max, Autodesk 123D, Freeform Modeling Plus from 3D Systems, Google SketchUp, 3dtin, Tinkercad, Rhino, ZBrush, and Maya. Choosing the ideal software can be difficult, but trial versions are always available along with an extensive array of tutorials.
One of the greatest challenges in design is creating 3D models that can truly exist in the real physical world. There is a long list of requirements pertaining to this subject, which is beyond the scope of this technical note, but we will briefly mention two of the most common errors that may arise: “non-manifold” and “mixed normals” errors. Manifoldness refers to the fact that models cannot be “open,” i.e., containing “holes” not part of the intended design; all vertices must eventually be connected to create a solid form. In other words, an object needs to essentially be “watertight” to exist in the real world. Models additionally cannot contain coincident edges and overlapping faces. Furthermore, faces must all be appropriately facing inwards or outwards; this refers to the concept of normals, which are vectors that are perpendicular to each face of the surface of a 3D mesh. These potential errors are often impossible to spot through visual inspection of the outside of the model alone and therefore require special software, such as 3D Studio Max, Meshlab, Blender, Slicer, NetFabb, Spaceclaim, or Materialise. Materialise has an array of software especially useful in medical 3D printing; Mimics is used primarily for image segmentation, Magics for STL cleanup, 3Matic for STL modifications, and inPrint for quick, streamlined, and simpler medical 3D printing, especially for cardiac and orthopedic applications.
More specific to our model, its assembly is slightly cumbersome, as the outer shell components had to be put together using transparent adhesive tape. The model also exhibits some physical vulnerability, as there was breakage of the component connecting the hepatic vein and portal vein used for TIPS simulation during the 3D processing step. Another limitation is that this model’s percutaneous biliary drain placement simulation is not completely realistic, as access is generally obtained from a much more peripheral part of the biliary tree. The model’s absence of a liver parenchyma is another downside, as it restricts opportunity for tactile feedback. These shortcomings can be addressed in the future, especially with multi-material 3D printers (e.g., the Stratasys Objet Connex J750), which can simultaneously print up to seven materials, one of which is only support material and not a modeling material [15]. But cost would still likely be a limitation. Perhaps molding techniques, while using material such as silicone, may be an alternative consideration.
In addition to overcoming these previous shortcomings, future directions will also include developing fixtures and clasps and/or areas for magnet placement to allow for easy assembly and disassembly. Another likely change is the substitution of the materials that compose the internal structures with ones that allow for greater flexibility while still maintaining the desired luminal diameter and wall thickness. Furthermore, to simulate contrast injection and thus allow for a more accurate simulation of interventional procedures, a future endeavor will be to attach a pump system to internal structures to allow for continuous flow of fluid. A challenge would be developing a replaceable access site where multiple needle entries can be performed. The creation of a multimodality-compatible phantom is also another likely future step [16]. The ultimate direction of this technology is the production of patient-specific hepatobiliary models that allow users to practice and plan interventional procedures pre-operatively and to become familiar with the patient’s unique anatomy. Of note, while this model has great potential and is quite versatile, a future prototype will need to be devised to eventually measure its efficacy and validity in teaching trainees as well as to determine its true clinical utility.
Acknowledgements
The authors would like to acknowledge Bahareh Sianati, MD, for assisting in gathering references.
Abbreviations
- 3D
Three-dimensional
- STL
STereoLithography or Standard Tessellation Language
- TIPS
Transjugular intrahepatic portosystemic shunt
Contributor Information
Ramin Javan, Phone: (202) 715-5212, Email: rjavan@mfa.gwu.edu.
Merissa N. Zeman, Phone: (202) 715-5212, Email: zmerissa@gwmail.gwu.edu
References
- 1.Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK, George E, Wake N, Caterson EJ, Pomahac B, Ho VB, Grant GT, Rybicki FJ. Medical 3D printing for the radiologist. Radiographics. 2015;35:1965–1988. doi: 10.1148/rg.2015140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannopoulos AA, Steigner ML, George E, Barile M, Hunsaker AR, Rybicki FJ, Mitsouras D. Cardiothoracic applications of 3-dimensional printing. J Thorac Imaging. 2016;31:253–272. doi: 10.1097/RTI.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng N, Tao H, Fang C, Fan Y, Xiang N, Yang J, Zhu W, Liu J, Guan T, Fang C, Xiang F. Individualized preoperative planning using three-dimensional modeling for Bismuth and Corlette type III hilar cholangiocarcinoma. World J Surg Oncol. 2016;14:44. doi: 10.1186/s12957-016-0794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villard PF, Vidal FP, Cenydd L, Holbrey R, Pisharody S, Johnson S, Bulpitt A, John NW, Bello F, Gould D. Interventional radiology virtual simulator for liver biopsy. Int J CARS. 2014;9:255–267. doi: 10.1007/s11548-013-0929-0. [DOI] [PubMed] [Google Scholar]
- 5.Sekhar A, Sun MR, Siewert B. A tissue phantom model for training residents in ultrasound-guided liver biopsy. Acad Radiol. 2014;21:902–908. doi: 10.1016/j.acra.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Xiang N, Fang C, Fan Y, Yang J, Zeng N, Liu J, Zhu W. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int J Clin Exp Med. 2015;8:18873–18878. [PMC free article] [PubMed] [Google Scholar]
- 7.Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Three-dimensional print of liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304–1310. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 8.Kong X, Nie L, Zhang H, Wang Z, Ye Q, Tang L, Huang W, Li J. Do 3D printing models improve anatomical teaching about hepatic segments to medical students? A randomized controlled study. World J Surg. 2016;40:1969–1976. doi: 10.1007/s00268-016-3541-y. [DOI] [PubMed] [Google Scholar]
- 9.Dhir V, Itoi T, Fockens P, Perez-Miranda M, Khashab MA, Seo DW, Yang AM, Lawrence KY, Maydeo A. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: creation of “Mumbai EUS” stereolithography/3D printing bile duct prototype (with videos) Gastrointest Endosc. 2015;81:440–446. doi: 10.1016/j.gie.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Burdall OC, Makin E, Davenport M, Ade-Ajayi N. Three-dimensional printing to simulate laparoscopic choledochal surgery. J Pediatr Surg. 2016;51:828–831. doi: 10.1016/j.jpedsurg.2016.02.093. [DOI] [PubMed] [Google Scholar]
- 11.Souzaki R, Kinoshita Y, Ieiri S, Hayashida M, Koga Y, Shirabe K, Hara T, Maehara Y, Hashizume M, Taguchi T. Three-dimensional liver model based on preoperative CT images as a tool to assist in surgical planning for hepatoblastoma in a child. Pediatr Surg Int. 2015;31:593–596. doi: 10.1007/s00383-015-3709-9. [DOI] [PubMed] [Google Scholar]
- 12.Javan R, Herrin D, Tangestanipoor A. Understanding Spatially Complex Segmental and Branch Anatomy Utilizing 3D Printing: Liver, Lung, Prostate, Coronary Arteries, and Circle of Willis. Academic Radiology. 2016;23(9):1183–1189. doi: 10.1016/j.acra.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 13.i. Materialise.com. <http://www.i.materialise.com>, Accessed April 9, 2016
- 14.Bernhard JC, Isotani S, Matsugasumi T, Duddalwar V, Hung AJ, Suer E, Baco E, Satkunasivam R, Djaladat H, Metcalfe C, Hu B, Wong K, Park D, Nguyen M, Hwang D, Bazargani ST, de Castro Abreu AL, Aron M, Ukimura O, Gill IS. Personalized 3D printed model of kidney and tumor anatomy: a useful tool for patient education. World J Urol. 2016;34:337–345. doi: 10.1007/s00345-015-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratasys.com. <http://www.stratasys.com/j750>, Accessed Jan 30, 2017
- 16.Javan R, Bansal M, Tangestanipoor A. A prototype hybrid gypsum-based 3D printed training model for CT-guided spinal pain management. Journal of Computer Assisted Tomography. 2016;40(4):626–631. doi: 10.1097/RCT.0000000000000415. [DOI] [PubMed] [Google Scholar]