Abstract
The ERK signalling pathway regulates key cell fate decisions in the intestinal epithelium and is frequently dysregulated in colorectal cancers (CRCs). Variations in the dynamics of ERK activation can induce different biological outcomes and are regulated by multiple mechanisms, including activation of negative feedback loops involving transcriptional induction of dual-specificity phosphatases (DUSPs). We have found that the nuclear ERK-selective phosphatase DUSP5 is downregulated in colorectal tumours and cell lines, as previously observed in gastric and prostate cancer. The DUSP5 promoter is methylated in a subset of CRC cell lines and primary tumours, particularly those with a CpG island methylator phenotype (CIMP). However, this epigenetic change alone could not account for reduced DUSP5 expression in CRC cells. Functionally, DUSP5 depletion failed to alter ERK signalling or proliferation in CRC cell lines, and its transgenic overexpression in the mouse intestine had minimal impact on normal intestinal homeostasis or tumour development. Our results suggest that DUSP5 plays a limited role in regulating ERK signalling associated with the growth of colorectal tumours, but that methylation the DUSP5 gene promoter can serve as an additional means of identifying CIMP-high colorectal cancers.
Subject terms: Colorectal cancer, Oncology
Introduction
The ERK signalling pathway couples extracellular and oncogenic signals with molecular networks that control cell proliferation, differentiation, survival, and motility1,2. Activation of the pathway occurs when cell surface receptors such as the EGF receptor (EGFR) induce GTP-loading of RAS, which initiates sequential activation of RAF, MEK and ERK protein kinases. ERKs can phosphorylate a multitude of cytoplasmic and nuclear proteins, the combination of which depends on the duration, magnitude, and site of ERK activation3.
A key mechanism whereby cells control the dynamics of ERK signalling is by inducing negative feedback loops. This involves either direct ERK-mediated phosphorylation of RAF proteins, or transcriptional induction of upstream signalling inhibitors (eg. Sprouty2/4) and/or dual-specificity phosphatases (DUSPs) that directly target ERK4. These systems operate in a cell-specific manner and act at multiple steps of the pathway to maintain signalling flux at levels optimal for normal cell function.
DUSP proteins dephosphorylate tyrosine and serine/threonine residues required for activation of ERK, JNK and p38 mitogen-activated protein kinases (MAPKs)5,6. They contain a C-terminal catalytic domain, a N-terminal domain controlling subcellular localization, and a kinase interacting motif. DUSPs are broadly classified into 3 groups based on their localization: group I DUSPs (DUSPs 1, 2, 4 and 5) are nuclear, group II DUSPs (DUSP 6, 7 and 9) are cytoplasmic, and group III DUSPs (DUSPs 8, 10 and 16) exist in both the nucleus and cytoplasm. Functionally, individual DUSPs often exhibit preference for specific MAPK substrates, with DUSPs 5, 6, and 7 being ERK-selective while DUSP9 prefers ERK over the p38 and JNK MAPKs.
DUSP5 is a nuclear ERK1/2-selective phosphatase induced by ERK signalling in mammalian cells7,8. It also controls ERK pathway activity by anchoring inactive ERK proteins in the nucleus, preventing MEK-mediated reactivation of ERK in the cytoplasm8. In mice, DUSP5 deficiency in the epidermis increases sensitivity to H-Ras-driven papilloma formation in a carcinogen-induced model of skin cancer9.
Consistent with this putative tumour suppressor role, DUSP5 expression is downregulated in prostate and gastric cancers where loss of expression is associated with poor prognosis10–12. In gastric cancer, DUSP5 downregulation was reported to correlate with promoter CpG island methylation, and its re-expression in gastric cancer cell lines reduced nuclear p-ERK levels and cell growth12.
Downregulation of DUSP5 was also recently reported in colorectal cancer (CRC), and shown to be associated with worse outcome10. However, the mechanisms underlying DUSP5 loss in this disease, and whether its loss contributes to aberrant ERK signalling or the initiation and/or progression colorectal cancers has not been established. In this study, we confirm that DUSP5 is significantly downregulated in CRC. Furthermore, we show that the DUSP5 promoter is methylated in a subset of CRCs, specifically those harboring the CpG island methylator phenotype (CIMP). However, promoter methylation alone did not correlate with altered DUSP5 expression in CRC cell lines or primary tumours, suggesting multiple mechanisms contribute to DUSP5 downregulation in this disease. Unexpectedly, knockdown of endogenous DUSP5 in CRC cell lines did not affect ERK signaling or proliferation, while transgenic overexpression of DUSP5 in the mouse intestine had little impact on normal intestinal homeostasis or tumorigenesis.
Results
DUSP5 is downregulated in colorectal cancers (CRCs)
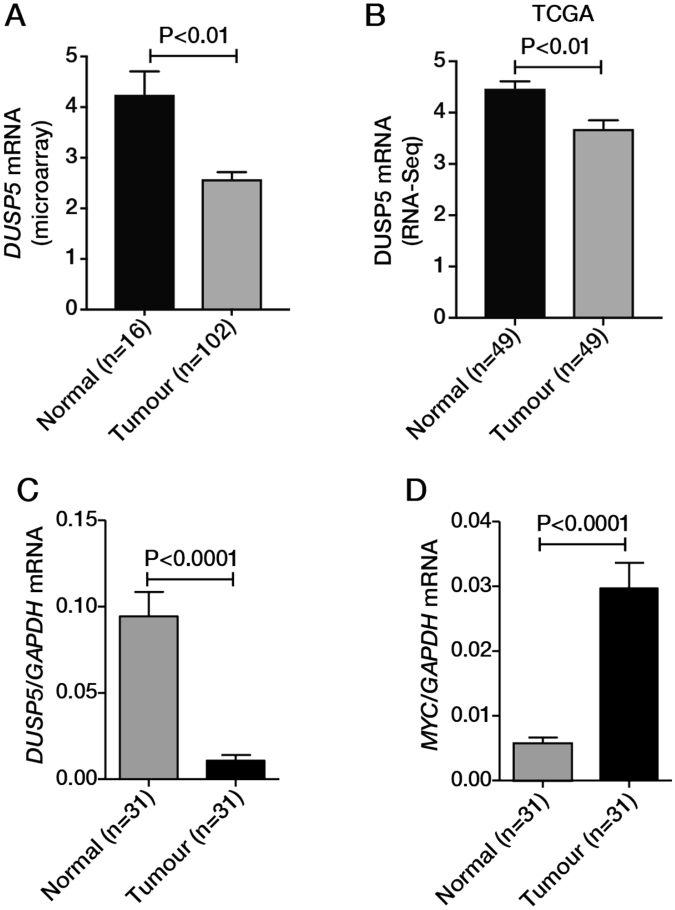
DUSP5 is a putative tumour suppressor gene that is aberrantly expressed in several cancers. To determine if the expression of DUSP5 is altered in CRC, we interrogated an in-house microarray dataset comprising 102 human CRCs and normal colonic tissue from 16 individuals (Fig. 1A), and RNA-seq data from the TCGA of 49 CRCs and matched adjacent normal tissue (Fig. 1B). Both datasets revealed significantly lower DUSP5 expression in CRC compared to normal tissue. This finding was further verified by qPCR analysis using an independent patient cohort consisting of 31 matched pairs of CRCs and normal colonic tissue (Fig. 1C). The tumour and normal colonic origins of the specimens is illustrated by higher levels of MYC in the tumour tissue (Fig. 1D).
Figure 1.
Downregulation of DUSP5 mRNA expression in CRC. DUSP5 mRNA expression in normal colonic mucosa and colorectal tumours determined by analysis of (A) an in-house Affymetrix microarray dataset and (B) RNA-Seq data available from the TCGA. (C) DUSP5 and (D) MYC mRNA expression in colon tumour/normal pairs normalised to GAPDH levels.
DUSP5 is induced by ERK signalling in CRC cell lines
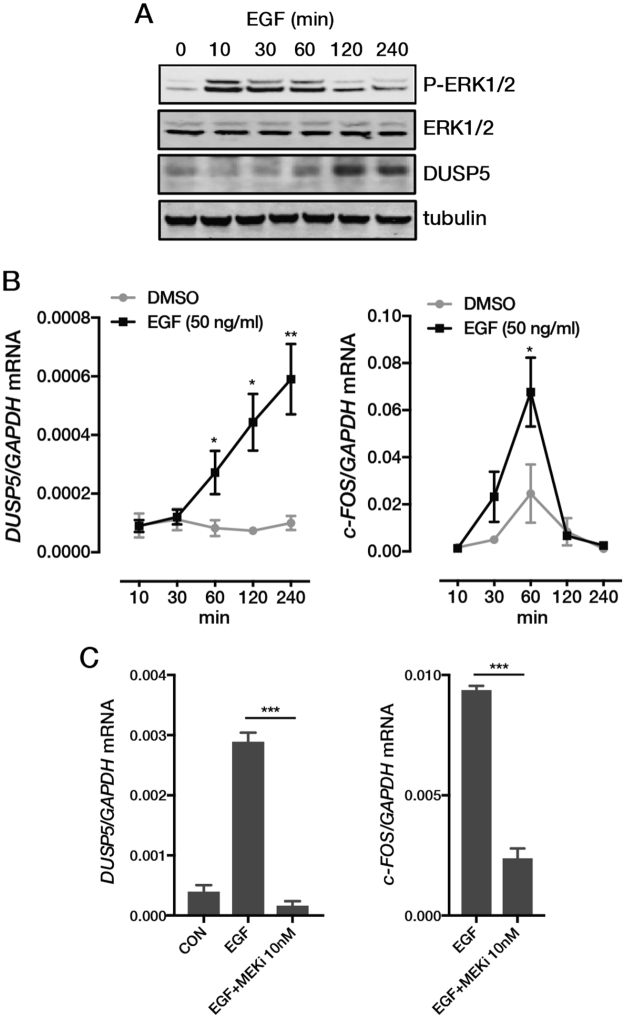
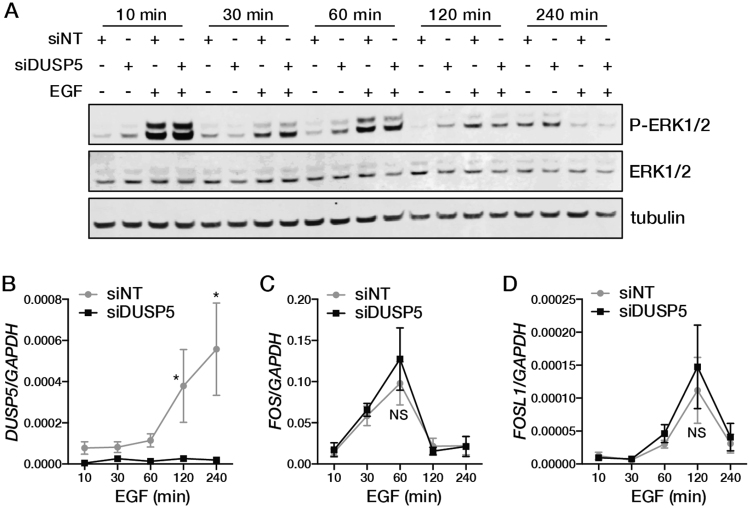
DUSP5 expression is induced by ERK signalling in several cell types6,7. To examine the dynamics of DUSP5 induction in CRC cells, we treated the KRAS/BRAF wild-type CRC cell line LIM1215 with epidermal growth factor (EGF), which induced a transient and robust increase in ERK phosphorylation (Fig. 2A). EGF also invoked a significant increase in DUSP5 protein levels but in a delayed and more prolonged manner compared to ERK phosphorylation, consistent with its role as a negative feedback regulator of this pathway. Examination of these effects at the transcriptional level demonstrated that EGF also induced a delayed but sustained induction of DUSP5, in contrast to the transient induction of the well-established ERK target, FOS (Fig. 2B). EGF induction of DUSP5 and FOS were also strongly attenuated by a MEK inhibitor (trametinib), demonstrating that DUSP5 is a bone fide target of ERK signaling in KRAS/BRAF wild-type CRC cells (Fig. 2C).
Figure 2.
Induction of DUSP5 by EGFR-ERK signalling in CRC cells. Time-course showing EGF-mediated induction of (A) p-ERK1/2 and DUSP5 protein expression, and (B) DUSP5 and c-FOS gene expression in LIM1215 cells. (C) Induction of DUSP5 and c-FOS mRNA by EGF requires MEK/ERK signalling. LIM1215 cells were stimulated with EGF (50 ng/ml) for 24 h in the absence or presence of the MEK inhibitor (MEKi) trametinib. *P < 0.05, **P < 0.005, *P < 0.0005.
The DUSP5 promoter is methylated in a subset of CRCs
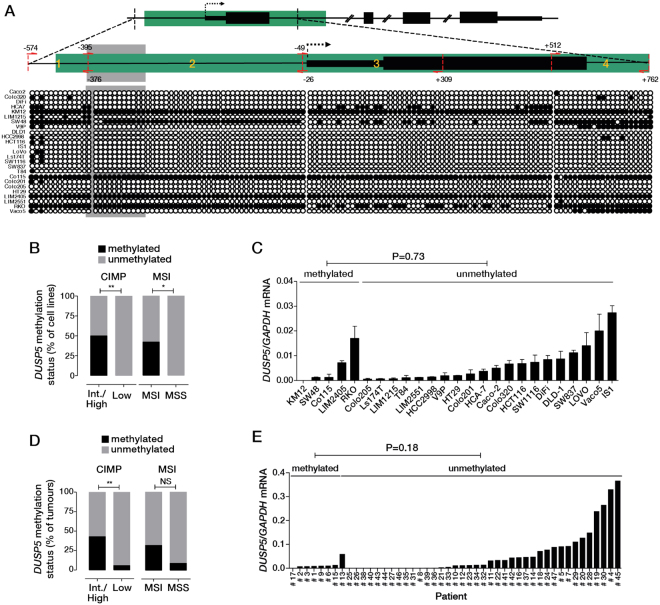
Downregulation of DUSP5 expression in gastric cancer is associated with hypermethylation of a CpG island in its promoter12. To determine if this epigenetic mechanism contributes to downregulation of DUSP5 in CRCs, we used bisulphite conversion and sequencing to interrogate 4 contiguous regions spanning the DUSP5 proximal promoter, exon 1, and the first part of intron 1, in a panel of 25 CRC cell lines (Fig. 3A). The 4 regions were found to be methylated in 5 cell lines (KM12, SW48, Co115, LIM2405 and RKO, Fig. 2B), all of which are microsatellite unstable (P = 0.046, Fisher’s Exact Test) and feature the CpG island methylator high phenotype (CIMP-H) (P = 0.009) (Fig. 3B). However, DUSP5 promoter methylation was not significantly associated with reduced DUSP5 mRNA levels across the cell line panel, with some heavily methylated cell lines (RKO) expressing high levels of DUSP5 (Fig. 3C).
Figure 3.
DUSP5 promoter methylation in CRC cell lines and tumours. (A) Schematic of the DUSP5 gene showing the location of the CpG island (highlighted in green). The transcription start site is indicated by dotted arrow. Zoomed image shows the location of the 4 amplicons investigated for methylation change by bisulphite sequencing. Bottom panel shows the methylation status of specific CpGs (circles) within each amplicon in a panel of 25 CRC cell lines. Open circles indicate non-methylated and closed circles indicate methylated CpGs. KRAS/BRAF wild-type cell lines are shown at the top of the panel (Caco2-V9P), KRAS mutant lines in the middle DLD1-T84) and BRAF mutant lines at the bottom (Co115-Vaco5). (B) Classification of the DUSP5 promoter methylation status of the 25 CRC cell lines relative to CIMP and MSI status. (C) DUSP5 mRNA expression in the 25 CRC cell lines determined by qPCR. (D) Classification of the DUSP5 promoter methylation status of 47 CRC’s according to CIMP and MSI status. (E) DUSP5 mRNA expression in the 47 human CRC’s separated according to DUSP5 promoter methylation status. *P < 0.05, **P < 0.005.
To assess DUSP5 promoter methylation in primary CRCs, we developed a high-resolution melt (HRM) assay applicable to DNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples. The assay interrogates a 51 base pair fragment harboring 9 CpG dinucleotides (Fig. 3A, grey shaded region) within the proximal DUSP5 promoter. After validating its efficacy in discriminating DUSP5 promoter methylation states in cell lines (Fig. S1), we used the HRM assay to evaluate DUSP5 promoter methylation status in 47 CRCs, which included 14 with a CIMP-Intermediate/High (CIMP-I/H) phenotype. DUSP5 promoter methylation was identified in 8/47 cases (17%). As observed in CRC cell lines, the methylation was more frequent in CIMP I/H tumours (6/14) compared to CIMP-Low (2/33) cases (<0.005, Fisher’s exact test) (Fig. 3D), however, was not associated with reduced DUSP5 mRNA levels (Fig. 3E, p = 0.189, unpaired t-test). Collectively, these data indicate that DUSP5 promoter methylation represents a novel marker of CIMP status in CRC but is not the predominant mechanism of DUSP5 repression in these tumours.
DUSP5 is not a major regulator of ERK signalling in CRC cells
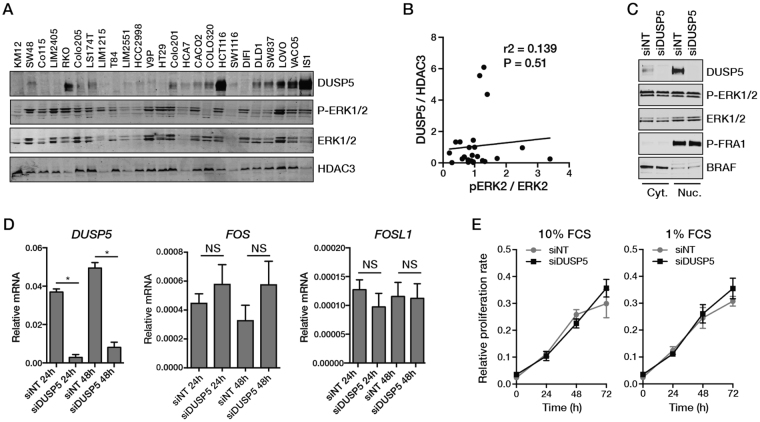
The major functions of DUSP5 described to date are to dephosphorylate nuclear ERK1/2 proteins and to sequester their inactive forms in the nucleus6,8. We therefore assesed whether basal DUSP5 expression correlates with the levels of nuclear p-ERK in a panel of 25 CRC cell lines. Basal levels of DUSP5 varied significantly among the cell lines but displayed no clear relationship with the ratio of phosphorylated to total ERK1/2 in the nucleus (Fig. 4A,B).
Figure 4.
DUSP5 regulation of basal ERK signaling in CRC cells. (A) Relationship between DUSP5 expression and levels of phosphorylated and total ERK1/2 in nuclear extracts of CRC cell lines. HDAC3 was used as loading control. (B) Analysis of the correlation between DUSP5 expression normalized to HDAC3 and the p-ERK2/ERK2 ratio following densitometric analysis of the data shown in (A). (C) Effect of DUSP5 knockdown in RKO cells on cytoplasmic and nuclear levels of phosphorylated and total ERK1/2, and ERK-mediated phosphorylation of the transcription factor FRA1 (FOSL1). BRAF was used as cytoplasmic marker. (D) Effect of DUSP5 knockdown on expression of the ERK-regulated genes FOSL1 and FOS in RKO cells. (E) Effect of DUSP5 knockdown on proliferation of RKO cells grown under normal (10% FCS) or low (1% FCS) serum conditions. *P < 0.05.
DUSP5 levels have been reported to be elevated in some cancers, particularly in the context of BRAF mutations13,14. Studies in DUSP5-deficient MEFs suggest that these high levels of DUSP5 serve to dampen ERK pathway hyperactivation to facilitate transformation by mutant BRAF15. To test if endogenous DUSP5 plays a similar role in CRC cells, we used the BRAF mutant RKO cell line, which expresses high levels of DUSP5 (Fig. 4A) and is dependent on ERK signalling for proliferation (Fig. S2). DUSP5 knockdown in RKO cells did not alter nuclear or cytoplasmic phospho-ERK1/2 levels or phosphorylation of the nuclear ERK substrate FRA1 (Fig. 4C). DUSP5 knockdown also had no effect on ERK-dependent transcriptional induction of FOS or FOSL1 16,17 (Fig. 4D). Finally, DUSP5 depletion did not affect proliferation of RKO cells grown under standard or low-serum conditions (Fig. 4E). These results indicate that endogenous DUSP5 plays a limited role in regulating basal levels of ERK signalling in BRAF mutant CRC cells.
Next, we examined if induction of DUSP5 following EGF stimulation regulates the magnitude or duration of EGF-induced ERK signalling in CRC cells. Knockdown of DUSP5 expression in KRAS/BRAF wild-type LIM1215 cells had no effect on the dynamics of EGF-induced ERK phosphorylation or transcriptional induction of FOS or FOSL1 (Fig. 5A–C). Collectively, our data suggest that although DUSP5 is induced upon ERK pathway activation, it is not a major regulator of ERK signalling in CRC cells.
Figure 5.
DUSP5 regulation of EGF-induced ERK signalling in CRC cells. Effect of DUSP5 knockdown on induction of (A) ERK1/2 phosphorylation, and mRNA levels of (B) DUSP5, (C) FOS, and (D) FOSL1 following treatment of LIM1215 cell with EGF (50 ng/ml).
DUSP5 overexpression in the mouse intestinal epithelium does not alter ERK signalling, intestinal homeostasis or adenoma formation
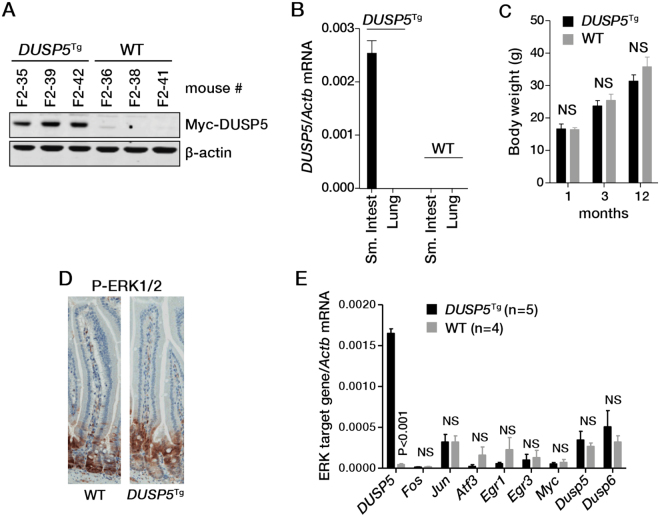
To complement our cell line studies and test the growth suppressive effects of DUSP5 in vivo, we generated a mouse strain in which expression of a human Myc-tagged DUSP5 transgene is driven by the intestinal-specific villin promoter (Vil1-DUSP5 Tg). The functionality of Myc-tagged human DUSP5 has previously been demonstrated in mouse embryo fibroblasts and in vivo studies involving transgene expression in the lymphoid compartment of mice15,18. Overexpression of Myc-DUSP5 specifically in the small intestine was confirmed at the mRNA and protein level by detection of human DUSP5 mRNA and the Myc-tag, respectively (Fig. 6A,B). Vil1-DUSP5 Tg mice were viable, fertile and lacked a discernible phenotype, including no difference in body weight at 1, 3 or 12 months (Fig. 6C). We were unable to detect overexpression of the Myc-DUSP5 protein in the colonic epithelium, in agreement with previous reports that transgene expression driven by the villin promoter is more robust in the mouse small intestine19. All subsequent studies were therefore performed on this tissue.
Figure 6.
Impact of DUSP5 overexpression on ERK signaling in the mouse intestine. (A) Expression of human DUSP5 protein in the intestinal epithelium detected by blotting for the Myc-tag. (B) Expression of human DUSP5 mRNA specifically in the intestinal epithelium of transgenic mice. (C) Body weight of wild-type and Vil-DUSP5 Tg mice determined over time. Effects of DUSP5 overexpression on the (D) intensity and pattern of pERK1/2 staining and (E) expression ERK signalling-regulated genes in the intestinal epithelium. Three mice of each genotype were analysed.
To determine if DUSP5 overexpression alters ERK signalling in this tissue, we assessed p-ERK1/2 levels by immunohistochemistry. No difference in the intensity and pattern of p-ERK1/2 staining was evident in the crypt-villus axis between WT and Vil1-DUSP5 Tg mice (Fig. 6D). Similarly, analysis of intestinal epithelial cells isolated from WT and Vil1-DUSP5 Tg mice revealed no difference in expression of the ERK signalling-regulated genes Fos, Jun, Atf3, Egr1, Egr3, Myc, Dusp5 and Dusp6 (Fig. 6E).
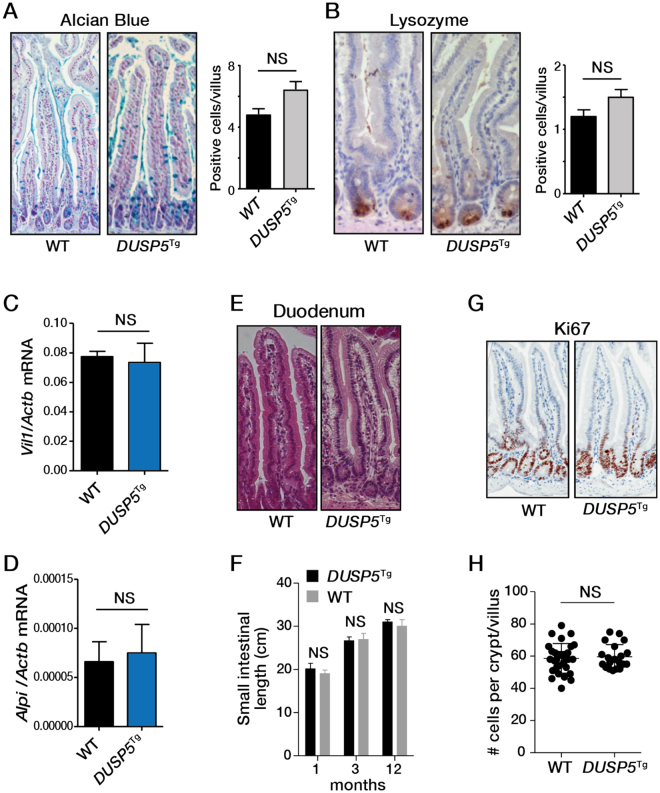
ERK signalling plays an important role in regulating normal cell proliferation and maturation in the mouse intestine20, particularly differentiation along the secretory cell lineage21. However, no significant differences in numbers of goblet (based on Alcian Blue staining) or Paneth cells (based on staining for lysozyme) were detected between WT and Vil1-DUSP5 Tg mice (Fig. 7A,B). DUSP5 overexpression also had no effect on expression of the absorptive lineage markers, villin (Vil1) and alkaline phosphatase (Alpi) (Fig. 7C,D). Similarly, no differences in length of the crypt-villus axis (Fig. 7E), length of the small intestine (Fig. 7F), rate of cell proliferation in the small intestine (Fig. 7G), or cell number along the crypt-villus axis (Fig. 7H) was detected between WT and Vil1-DUSP5 Tg mice (Fig. 7E–H). Collectively, these findings indicate that DUSP5 overexpression has minimal effects on ERK signalling and cell homeostasis in the normal intestinal epithelium.
Figure 7.
Impact of DUSP5 overexpression on intestinal phenotype. Effect of DUSP5 overexpression on cell differentiation as determined by staining for (A) Goblet cell (Alcian Blue) and (B) Paneth cell (lysozyme) markers, and measuring mRNA levels of (C) villin (Vil1) and (D) alkaline phosphatase (Alpi). Effect of DUSP5 overexpression on (E,F) small intestinal length, (G) cell proliferation and (H) crypt cell number (10 crypts per mouse; 3 mice per genotype).
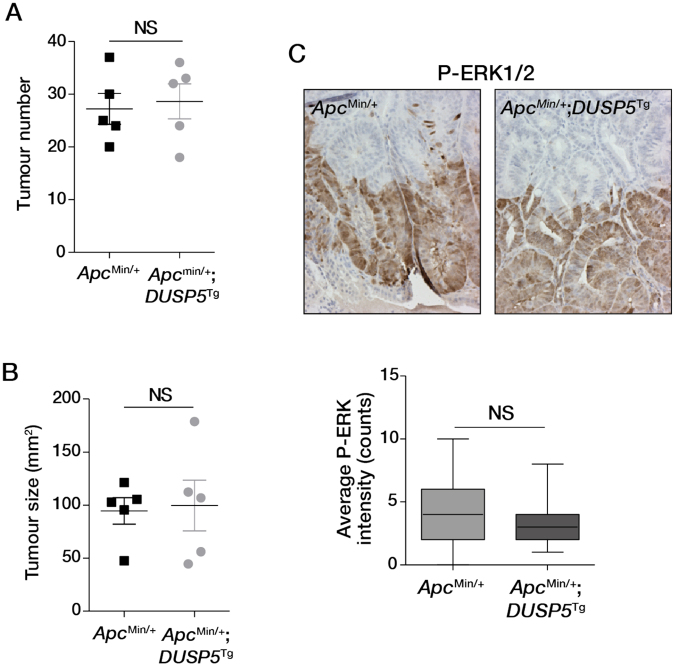
Finally, previous work has shown that DUSP5 exhibits tumour suppressive activity in a mouse model of carcinogen-induced skin tumorigenesis9. To determine the tumour suppressive effects of DUSP5 in the intestinal epithelium in vivo, we crossed Vil1-DUSP5 Tg with Apc Min/+ mice. Adenoma formation in Apc Min/+ mice requires ERK signalling22 and this model is widely used to investigate mechanisms regulating Apc mutation-initiated intestinal adenoma formation23. Apc Min/+ mice developed the expected number of intestinal adenomas at 4 months of age, which was not affected by DUSP5 overexpression (Fig. 8A). Similarly, DUSP5 overexpression had no impact on tumour size (Fig. 8B) or the pattern and intensity of pERK staining in the adenomas (Fig. 8C). These findings indicate that DUSP5 overexpression does not exert tumour suppressive activity in a mouse model of intestinal tumorigenesis.
Figure 8.
Impact of DUSP5 overexpression on intestinal adenoma formation. Effect of DUSP5 overexpression on the (A) number and (B) size of intestinal tumours that develop upon Apc loss. (C) DUSP5 overexpression does not alter the pattern or intensity of pERK1/2 staining in intestinal adenomas. Five mice of each genotype were analysed.
Discussion
In this study we provide clear evidence of DUSP5 downregulation in CRCs compared to normal colonic tissue. We show that the DUSP5 promoter is methylated in both CRC cell lines and patient tumours, but in contrast to previous findings in gatric cancer, our data indicate that this epigenetic mechanism alone is insufficient to account for downregulation of DUSP5. Notably, DUSP5 promoter methylation occurs predominantly in CIMP-high CRCs, where multiple genes and loci are coordinately methylated24. Thus, DUSP5 may be a novel addition to the panel of genes preferentially methylated in this tumour subset.
While the lack of correlation between DUSP5 promoter methylation and gene expression was unexpected, this observation is consistent with genomic analyses indicating that the majority of genes methylated in CRC are not altered in expression25. In fact, we found that some cell lines with extensive methylation of the DUSP5 promoter, such as RKO, feature high DUSP5 expression, likely due to strong ERK pathway activation driven by mutant BRAF. DUSP5 overexpression has been documented in several tumour types and cell lines harboring BRAF mutations13,14. Recent studies using DUSP5-deficient MEFs indicate that the elevated DUSP5 levels function to prevent senescence by limiting ERK pathway activation to a level that promotes cell proliferation and transformation15. In contrast to these data, we found that knockdown of endogenous DUSP5 did not alter proliferation, p-ERK levels or phosphorylation of the nuclear-localised ERK substrate FRA1 in BRAF mutant CRC cells. Furthermore, transgenic overexpression of DUSP5 in the mouse intestinal epithelium did not alter basal p-ERK levels or ERK-regulated gene expression, cell differentiation, proliferation or adenoma formation, processes that require activation of this pathway20,22. Importantly, our findings are consistent with the lack of any discernible phenotype of DUSP5 knockout mice that has been previously reported, other than the differential response to DMBA challenge9.
Our findings raise the possibility that other DUSP family members or negative feedback regulators, play more dominant roles in regulating the ERK pathway on the normal intestinal epithelium and in CRC. Indeed, previous studies have identified an important role for DUSP4 in regulating nuclear ERK phosphorylation in intestinal epithelial cells and in CRC cell lines26. It was also reported that DUSP6 induction by p53 reduces global phospho-ERK levels in HCT116 CRC cells27.
Collectively, our data indicate that DUSP5 is not a major negative regulator of ERK signalling in the intestinal epithelium and has limited tumour suppressive activity in CRC. Promoter methylation of DUSP5 in these cancers may be a passenger event, which differs from its role in gastric cancer cells. It however remains possible that DUSP5 may regulate the functions of the pathway in contexts other than those examined in the present study. Notably, DUSP5 downregulation in CRC has been shown to be adversely prognostic in advanced but not early stage disease, where it is associated with metastasis and markers of epithelial-mesenchymal transition10. Further studies are thus needed to determine if DUSP5 contributes to regulation of the ERK pathway in more advanced stages of colorectal tumorigenesis.
Methods
Cell lines and treatments
Colorectal cancer cell lines were maintained at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle’s Minimal Essential Medium Nutrient Mixture F-12 (DMEM/F-12) supplemented with 10% foetal calf serum (FCS) and penicillin (100 units/mL)/streptomycin (100ug/mL), all supplied by Invitrogen (Carlsbad, CA, USA).
Quantitative real-time PCR
RNA was extracted using the High Pure RNA Isolation Kit from Roche (Penzberg, Germany) according to the manufacturer’s instructions. The quality, purity and amount of RNA isolated was analysed using the Nanodrop 1000 Spectrophotometer from Thermo Fischer Scientific (Waltham, MA, USA). The High Pure Paraffin Kit (Roche) was used to extract total RNA from FFPE human specimens. RNA Samples were reverse transcribed into cDNA using the Transcriptor first strand cDNA Synthesis Kit (Roche). Gene expression was measured by quantitative real time PCR (qPCR) using the 7500 Fast and Viia 7 Real-Time PCR Systems from Life Technologies (Carlsbad, CA, USA). Gene expression changes were compared relative to expression of a house keeping gene (GAPDH or β-actin) using the 2−ΔCt method.
Bisulfite sequencing
Genomic DNA was extracted from colorectal cancer cell lines using the DNeasy Blood & Tissue Kit from QIAGEN (Hilden, Germany). Genomic DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tumour tissue from patients with CRC using the QIAmp DNA FFPE Tissue Kit (QIAGEN, Hilden). Extracted DNA (500–1000ng) was bisulfite treated and purified using the EpiTect Bisulfite Kit (QIAGEN)). Converted DNA was eluted in 20 μl, of which 4 μl was used to amplify 4 individual regions of the DUSP5 CpG island spanning the proximal promoter and 5′ intragenic region for PCR using Platinum Taq DNA polymerase (Invitrogen). PCR products were Sanger-sequenced by the Australian Genome Research Facility (AGRF, Melbourne, Australia). Sequences of converted DNA were evaluated using the BiQ Analyzer software28. Primers used were as follows: F1, 5′-TTAAATATATAGAAAAGTGGAGAAAATAGT-3′ and R1, 5′-ACCAAAACCCCAAAAAAATC-3′, to amplify a region spanning −574 to −346 nucleotides upstream of the TSS. F2, 5′-GATTTTTTTGGGGTTTTGGT-3′ and R2, 5′-TAAAACCAAATATAAATATTTCCCC-3′ to amplify a region spanning −395 to −26nt upstream of the TSS. F3, 5′-GGGAAATATTTATATTTGGTTTTATATGG-3′ and R3, 5′-CCTCCTTACGAAACATCTTAC-3′ to amplify a region spanning −49 to +309nt of the TSS. F4, 5′-GGTGGTGTTGGATTAGGGTAGT-3′ and R4, 5′-AAACCACAAAATACAAACTCCTACAA-3′ to amplify a region spanning +512 to +726 nucleotides downstream of the TSS.
High-resolution melting methylation analysis
Bisulfite-converted genomic DNA extracted from CRC cell lines or from cores of FFPE tumour tissue from patients with CRC were subjected to HRM using the MeltDoctor HRM Master Mix (Thermo Fischer Scientific) according to the manufacturer’s instructions. A sequence spanning nucleotides −396 to −303 upstream of the TSS was analyzed. The primers used were: DUSP5-HRM-F 5′-CGGATTTTTTTGGGGTTTTGGT-3′ and DUSP5-HRM-R 5′-GATCCGACCTTCAACTTCAC-3′. The use of human CRC samples was performed in accordance with relevant guidelines and regulations of the Austin Health Human Research Ethics Committee. All patients gave written informed consent for use of their tumour material for research purposes.
Western blotting
Total protein was extracted from cells in RIPA buffer (50 mM Tris-HCL pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 0.1% SDS, 5 mM EGTA). Cytosolic protein was extracted in hypotonic lysis buffer (10 mM Tris-HCL pH 7.5, 10 mM KCl, 1.5 mM MgCl2), following which nuclei were lysed in RIPA buffer. All buffers were supplemented with a cocktail of protease (cOmplete, Roche) and phosphatase (PhosSTOP, Roche) inhibitors. Protein concentrations were determined using the Bradford protein assay from Bio-Rad (Hercules, CA, USA). Samples were mixed with Reducing Agent and NuPAGE LDS Sample Loading Buffer (both from Invitrogen), and 20–50 µg protein resolved on NuPAGE Novex 4–12% Bis-Tris Gels (Invitrogen). Protein transfer to PVDF membranes was performed using the Invitrogen iBlot Transfer system. Antibodies used were anti-ERK1/2 (9107, Cell Signalling Technology, 1:2000), anti-pERK1/2 (4370, Cell Signalling Technology, 1:1000), anti-αTubulin (sc-8035, Santa Cruz, 1:1000), anti-DUSP5 (sc-393801, Santa Cruz, 1:1000), anti-pFRA1 (5841, Cell Signalling, 1:1000), anti-HDAC3 (AB16047, Abcam, 1:10000), anti-BRAF (sc-9002, Santa Cruz, 1:1000), anti-MYC-tag (ab9106, Abcam, 1:8000) and anti-β-actin (A5316, Sigma, 1:10000). Secondary antibodies used were fluorescent-labelled goat anti-mouse (IRdye680CW, LI-COR, 1:15000) and goat anti-rabbit (IRdye800CW, LI-COR, 1:15000). Signal was detected using the Odyssey imaging system (Li-Cor), and fluorescent intensities quantified using the Odyssey Infrared Imaging System software.
Generation of Vil1-DUSP5Tg mice
The myc-tagged coding sequence of human DUSP5 was amplified from the expression plasmid pSG5-DUSP58, kindly provided by SM Keyse) using the forward primer BsiWI-DUSP5: TACGTACGGCGAATTCATGAAGGTC and the reverse primer MluI-DUSP5: TCACGCGTAGATCTGGATCCTTACAG, which introduced a BsiW I and a Mlu I restriction site at the 5′, and 3′ end of the PCR product, respectively. These sites were used to clone the PCR product into the expression vector pBSKSVillinSV40polyA (kindly provided by Sylvie Robine), which contains a 9 kb fragment of the murine villin gene promoter to generate pBSKS-Vil1-DUSP5. This construct was used for pronuclear injections (Transgenic Animal Service, University of Queensland). Transgenic founder animals were bred with C57Bl/6 mice to generate heterozygous Vil1-DUSP5 Tg mice. All animal experiments were carried out in accordance with protocols approved by the Austin Health Animal Ethics Committee.
Immunohistochemistry
Immunohistochemistry was performed on 5 μm FFPE sections. Antigen was retrieved using Dako Target Retreival Citrate buffered solution (Agilent Technologies, CA, USA), and sections incubated with anti-pERK1/2 (4370, Cell Signalling Technology, 1:100) and anti-Ki67 (MA5–14520, Thermo Fischer Scientific, 1:150) antibodies overnight at 4 °C. Sections were incubated with Dako EnVision + System HRP-conjugated anti-rabbit secondary antibody (K4011, Agilent Technologies) for 30 minutes at room temperature and subjected to chromagen-staining (3, 3′-diaminobenzidine, DAB; Agilent Technologies). Sections were counterstained with hematoxylin, scanned using a ScanScope XT system (Aperio), and analyzed using ImageScope ver.12.1.0.5029 (Aperio).
Statistical methods
All values shown are mean ± standard error of mean. For all analyses, significance level was set at p < 0.05.
Data availability
Additional data and reagents relating to the manuscript will be made available upon request.
Electronic supplementary material
Acknowledgements
Funding for this project was provided by the National Health and Medical Research Council (NHMRC) of Australia (1026555), an Australian Research Council Future Fellowship (FT0992234) to JMM, a NHMRC Senior Research Fellowship (1046092) to JMM, Ludwig Cancer Research, and the Operational Infrastructure Support Program, Victorian Government, Australia. Fiona Chionh was supported by a NHMRC PhD scholarship.
Author Contributions
J.M., L.T. and A.D. conceived and coordinated the study. Study design: L.T., R.N., J.M., A.D., D.A. and N.T. Experimental procedures and data processing: L.T., R.N., R.W., A.C., S.O., I.L., M.D., F.C., D.B., O.S. Manuscript writing: L.T., J.M. and A.D. All authors read and approved the final manuscript. Identification and provision of BRAF mutant colorectal cancer cases: D.W., C.M.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/10/2023
A Correction to this paper has been published: 10.1038/s41598-023-29328-y
Contributor Information
Amardeep S. Dhillon, Email: amardeep.dhillon@onjcri.org.au
John M. Mariadason, Email: john.mariadason@onjcri.org.au
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20176-9.
References
- 1.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 3.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Lake D, Correa SA, Muller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73:4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. doi: 10.1096/fasebj.14.1.6. [DOI] [PubMed] [Google Scholar]
- 6.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs) Semin Cell Dev Biol. 2016;50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucharska A, Rushworth LK, Staples C, Morrice NA, Keyse SM. Regulation of the inducible nuclear dual-specificity phosphatase DUSP5 by ERK MAPK. Cell Signal. 2009;21:1794–1805. doi: 10.1016/j.cellsig.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Mandl M, Slack DN, Keyse SM. Specific inactivation and nuclear anchoring of extracellular signal-regulated kinase 2 by the inducible dual-specificity protein phosphatase DUSP5. Mol Cell Biol. 2005;25:1830–1845. doi: 10.1128/MCB.25.5.1830-1845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rushworth LK, et al. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci USA. 2014;111:18267–18272. doi: 10.1073/pnas.1420159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan X, et al. Dual specificity phosphatase 5 is a novel prognostic indicator for patients with advanced colorectal cancer. Am J Cancer Res. 2016;6:2323–2333. [PMC free article] [PubMed] [Google Scholar]
- 11.Cai C, et al. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int J Clin Exp Med. 2015;8:4186–4194. [PMC free article] [PubMed] [Google Scholar]
- 12.Shin SH, Park SY, Kang GH. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am J Pathol. 2013;182:1275–1285. doi: 10.1016/j.ajpath.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Montero-Conde C, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratilas CA, et al. V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA. 2009;106:4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidger AM, et al. Dual-specificity phosphatase 5 controls the localized inhibition, propagation, and transforming potential of ERK signaling. Proc Natl Acad Sci USA. 2017;114:E317–E326. doi: 10.1073/pnas.1614684114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casalino L, De Cesare D, Verde P. Accumulation of Fra-1 in ras-transformed cells depends on both transcriptional autoregulation and MEK-dependent posttranslational stabilization. Mol Cell Biol. 2003;23:4401–4415. doi: 10.1128/MCB.23.12.4401-4415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basbous J, Chalbos D, Hipskind R, Jariel-Encontre I, Piechaczyk M. Ubiquitin-independent proteasomal degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilizer. Mol Cell Biol. 2007;27:3936–3950. doi: 10.1128/MCB.01776-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovanen PE, et al. T-cell development and function are modulated by dual specificity phosphatase DUSP5. J Biol Chem. 2008;283:17362–17369. doi: 10.1074/jbc.M709887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 20.de Jong PR, et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat Commun. 2016;7:11551. doi: 10.1038/ncomms11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuberger J, et al. Shp2/MAPK signaling controls goblet/paneth cell fate decisions in the intestine. Proc Natl Acad Sci USA. 2014;111:3472–3477. doi: 10.1073/pnas.1309342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackstadt R, Sansom OJ. Mouse models of intestinal cancer. J Pathol. 2016;238:141–151. doi: 10.1002/path.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moarii M, Boeva V, Vert JP, Reyal F. Changes in correlation between promoter methylation and gene expression in cancer. BMC Genomics. 2015;16:873. doi: 10.1186/s12864-015-1994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cagnol S, Rivard N. Oncogenic KRAS and BRAF activation of the MEK/ERK signaling pathway promotes expression of dual-specificity phosphatase 4 (DUSP4/MKP2) resulting in nuclear ERK1/2 inhibition. Oncogene. 2013;32:564–576. doi: 10.1038/onc.2012.88. [DOI] [PubMed] [Google Scholar]
- 27.Piya S, et al. DUSP6 is a novel transcriptional target of p53 and regulates p53-mediated apoptosis by modulating expression levels of Bcl-2 family proteins. FEBS Lett. 2012;586:4233–4240. doi: 10.1016/j.febslet.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Bock C, et al. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21:4067–4068. doi: 10.1093/bioinformatics/bti652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data and reagents relating to the manuscript will be made available upon request.