Abstract
Owing to the rapid progress in stem cell research (SCR) and regenerative medicine (RM), society's expectation and interest in these fields are increasing. For effective communication on issues concerning SCR and RM, surveys for understanding the interests of stakeholders is essential. For this purpose, we conducted a large‐scale survey with 2,160 public responses and 1,115 responses from the member of the Japanese Society for Regenerative Medicine. Results showed that the public is more interested in the post‐realization aspects of RM, such as cost of care, countermeasures for risks and accidents, and clarification of responsibility and liability, than in the scientific aspects; the latter is of greater interest only to scientists. Our data indicate that an increased awareness about RM‐associated social responsibility and regulatory framework is required among scientists, such as those regarding its benefits, potential accidents, abuse, and other social consequences. Awareness regarding the importance of communication and education for scientists are critical to bridge the gaps in the interests of the public and scientists. Stem Cells Translational Medicine 2018;7:251–257
Keywords: Science communication; Questionnaire; Stem cell research; Regenerative medicine; Public engagement; Ethical, legal, and social implication
Significance Statement.
This study analyzed the differences between the knowledge that scientists prefer to share with the public and those that actually interest the public. Generally speaking, the public is interested in knowing about the outcome of using regenerative medicine and how to manage the problems that may occur after using such medicine. Further communication between scientists and the public is required to create a common future vision.
Introduction
Successful generation of human induced pluripotent stem cells (iPSCs) in Japan and the U.S. in 2007 gained significant public attention worldwide. Subsequently, news regarding research on iPSCs and iPSC‐derived products captured the media limelight. Interest in iPSCs has influenced both the public and scientists. Terms such as iPSCs and regenerative medicine (RM) are used commonly, and public support for stem cell research (SCR) and RM has increased 1.
The Japanese Ministry of Education, Sports, Culture, Science, and Technology (MEXT) has invested 10 billion yens for promoting research and development of iPSCs over a 5‐year period since March 2008 2. Following the initial generation of iPSCs, SCR, and RM using pluripotent stem cells (PSCs) have become central topics in Japanese science policy. The Japanese authorities have recently changed SCR and RM‐related regulations. The Act on the Promotion of Regenerative Medicine (APRM) and the Act on the Safety of Regenerative Medicine (ASRM) have been implemented, and the Pharmaceutical and Medical Device Act (PMDA) has been revised 3. These three acts are collectively known as the “Regenerative Medicine Three Acts (RMTAs).”
These policies, which primarily address the risky nature of “first‐in‐human,” were implemented after several clinical accidents with legal actions, such as that involving the administration of stem cells without safety approval 4. Issues related to iPSC and RM‐related hype have additionally been highlighted.
Under the recently revised guidelines, the International Society for Stem Cell Research (ISSCR) recommends that the SCR community should promote “accurate, balanced, and responsive public representations” of SCR 5.
Clinical trials of RM using PSCs have recently been initiated in Japan. Dr. Masayo Takahashi's group is undertaking the first‐in‐man clinical trials of iPSC‐derived retinal pigment cells in patients with age‐related macular degeneration 6 under careful monitoring. However, the actual picture of a PSC‐driven RM society is still unknown. Under such circumstances, imagining a future society with RM is challenging. An important question is who should tackle this difficult but creative work?
Since scientifically accurate and adequate information is not always available, for example, regarding self‐entitled “safe stem cell therapy clinics,” Lau et al. (2008) indicated that patients availing such treatments are at risk 7. As a part of the current efforts for solving these issues in Japan, the Ministry of Health, Labor, and Welfare (MHLW) has established certified committees. Any RM‐based clinical plan has to pass through the review process of this committee and mandatory notification has to be submitted 3.
An overview of the ethical, legal, and social implications (ELSIs) of SCR and RM shows that the public concerns remain unanswered. For example, Inoue et al. (2016) showed that the public expressed reservations regarding human‐animal chimera studies 8. Although over 70% respondents have positive and supportive attitude toward promotion of RM‐related research, approximately half of the respondents had negative attitude toward generation of human‐animal chimera. Another research showed that the relationship between education, religion, and medical history of the respondents and their families and attitude toward use of human‐animal chimera is complex and difficult to interpret 9.
In the context of the rapid progress and significant impact of SCR and RM, communication regarding advanced science and technology between the scientific community and the society has become a critical science policy issue worldwide 10, 11, 12, 13, 14. An important lesson regarding communication can be learnt from controversies on genetically modified organisms (GMOs). For example, the U.K. government attempted to coordinate and manage the discussion and communication regarding the social aspects of GMOs in 2000. They conducted a variety of communication‐oriented activities such as group interviews, consensus, conference, and public consultations, to understand public interest in GMOs and improve the mutual communication between the scientific community and the public. However, these trials highlighted that the discussions turned into controversies and conflicts, which was the limitation of such communication activities. Although several key questions on GMOs have been shared between the scientific community and the public, the most important lesson learnt was that these activities should have been conducted earlier. In other words, upstream and early stage communication is essential for effective communication on advanced science and technology 15, 16, 17, 18, 19.
This lesson can be adapted in the Japanese context for SCR and RM. Owing to the increased anticipation regarding RM, communication between the scientific community and society on SCR and RM has become a critical issue. Japanese science policies have encouraged communication between the scientific community and the society in general. The Second Science and Technology Basic Plan in 2001 indicated the importance of scientists’ outreach activities to society 20. In addition, The Third Science and Technology Basic Plan in 2006 emphasized the need for mutual communication between scientists and the public 21. According to this trend, activities concerning science communication, such as science café or open laboratories, have increased since 2000 22, 23. More currently, The Fourth Science and Technology Basic Plan in 2011 emphasized the importance of risk communication 24. The latest basic plan in 2016—The Fifth Science and Technology Basic Plan—emphasized mutual communication between scientists and the public with the key word “communication for co‐creation,” particularly in advanced fields such as SCR and RM 25. In summary, discussions concerning SCR and RM, including topics of ELSIs, between the scientific community and the society are urged to ensure the accountability of scientists to society 25. For this purpose, previous studies indicated systemic measures for promoting communication skills of scientists, such as implementation of evaluation systems, infrastructure, and so on 26, 27. The same structural issues were suggested for SCR and RM 28. In addition, to generate a pro‐communication infrastructure for SCR and RM, it is essential to understand the nature of the topics that interest the public and the factors they consider important for the acceptance of RM for effective communication. However, the current lack of knowledge regarding public and scientists’ attitude toward communication on RM is a serious challenge. Therefore, we conducted a survey to examine the attitudes of scientists and the public toward SCR and RM. This work reports a survey in the Japanese context, the findings of which would foster future communication between scientists and the public regarding SCR and RM worldwide.
Materials and Methods
From October 2015 to March 2016, we circulated a survey questionnaire among members of the Japanese Society of Regenerative Medicine (JSRM), who consisted of investigators of stem cell and biomaterials, medical doctors, policymakers, journalists, and so on. We collected 1,115 valid responses, representing a response rate of 22.1%. The public respondents were contacted through a research and monitoring company (the Japan Research Center), and 2,160 valid responses were collected.
We designed multiple choice questions to facilitate comparison between the answers provided by JSRM members and the public (Table 1). Questions were designed based on previous studies of public attitude toward nuclear energy 29, 30. The key questions were “What do you want to know? (What do you want to convey?)” and “What factors are important for your acceptance of RM?” Table 1 shows the rough sketch of our questionnaire.
Table 1.
Questions asked in this survey
| Theme | The public | JSRM members |
|---|---|---|
| Recognition of iPSCs and RM |
Recognition of keywords Opinion for promotion of RM research Expected time of achievement of RM Expected contents of RM after 10 years |
Expected time of achievement of RM Expected contents of RM after 10 years |
| Interesting topics on RM |
What do you want to know? What factors are important for your acceptance of RM? What factors are important for donating your biomaterial? |
What do you want to convey? What factors are important for your acceptance of RM? What factors are important for people to consider donation of their biomaterial? |
| Recognition of social events on RM |
About the RM three acts About the clinical case of RM |
About the RM three acts About the clinical case of RM |
| Demography | Age, gender, education, income, religion, expertise, and so on | |
Gaps in Focus between the Public and Scientists regarding factors considered important for the acceptance of stem cell research and regenerative medicine (RM) (p < .01).
Abbreviations: iPSCs, induced pluripotent stem cells; RM, regenerative medicine.
Results
Gap in Imagination Between JSRM Members and the Public
Approximately 80% of the respondents were supportive of RM and 60.7% expressed that they would (like to) donate their cells or blood.
In this context, the differences in imagination about RM between JSRM members and the public should be resolved for developing effective communication. Figure 1 shows the responses to “How long it will take to achieve RM with iPS cells?” “Within 10 years” was the most answered item (46.2%). At the same time, 10.0% answered “within 20 years,” 30.6% answered “within several years.” In contrast, only 1.5% answered “one year.”
Figure 1.
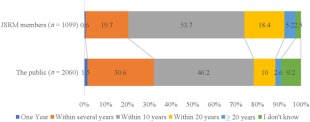
Responses to “How long will it take to achieve regenerative medicine with iPS cells?” Abbreviation: JSRM, Japanese Society of Regenerative Medicine.
Overall, the expectation of the public is slightly shorter than those of the JSRM members. Despite the differences in opinion between JSRM members and the public, the current result shows that the majority of the public concur with the JSRM members, compared with the results of a previous study 31. However, the differences in the imagination of JSRM members and the public are noteworthy.
The differences in expectations from RM within 10 years between JSRM members and the public emphasize this division (Fig. 2). The most numbered answer was “reduce pains and burdens” (public: 45.0%, JSRM members: 50.5%). However, the tendencies varied with other answers; 25.7% of the public and 15.3% of the JSRM members answered “replacement of sick organs,” whereas 18.5% of the public and 7.5% of the JSRM members answered “life‐span extension.” Furthermore, 25.9% JSRM members and 9.0% of the public answered “not much change.”
Figure 2.
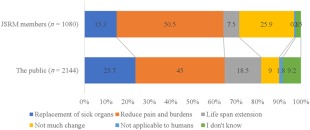
Responses to “Which is a suitable expectation of the progress of regenerative medicine within 10 years?” Abbreviation: JSRM, Japanese Society of Regenerative Medicine.
These results indicate that the expectations triggered by the keyword “regenerative medicine” differed considerably between the public and the scientific community. This also suggests that trials are necessary to reduce these gaps.
Gaps in Focus Between the Scientific Community and the Public
Over 70% of the public expressed that they wished to know more about “risk” and “anticipated new medical care.” These topics were also of high interest to JSRM members (53.2% and 78.9%). Additionally, approximately 60% of the public were interested about the “cost of care.” “Measures for safety” and “responses to medical accidents” were also highly marked (52.6% and 47.3%). Interestingly, significant differences in responses related to the “mechanism of RM,” “medical application and clinical testing,” and “industrial possibilities” were observed between the two groups (p < .01)., with JSRM members more interested on scientific content than the public (Fig. 3).
Figure 3.
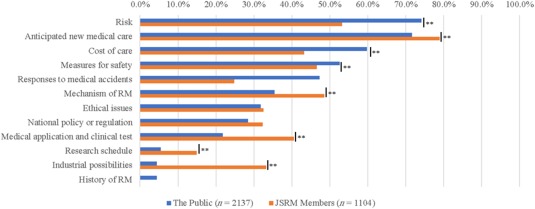
Differences in the response between JSRM members and the public to “What do you want to know?” (for the public) and “What do you want to convey?” (for JSRM members) “Please choose five factors” and “Don't know or other” answers were omitted because of the low ratio. Chi‐square test was conducted; **, p < .01. Abbreviation: RM, regenerative medicine.
Differences were noted regarding factors considered important for the acceptance of SCR and RM (p < .01) (Fig. 4). JSRM members emphasized the importance of scientific validation (55.0%) and necessity of research (36.3%). The public, however, regarded the effectiveness of regulation (50.5%), probabilities of potential risks and accidents (33.5%), and clarification of responsibility and liability (32.2%) as more important factors. In addition, controllability represented a common important point between the two groups (public: 55.7%, JSRM members: 57.6%).
Figure 4.
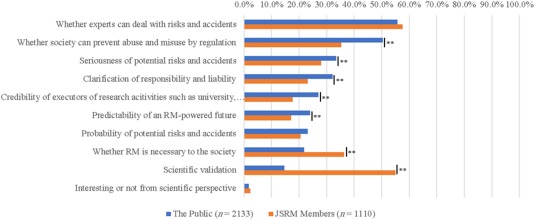
Differences in the response between Japanese Society of Regenerative Medicine members and the public to “What factors are important for your acceptance of regenerative medicine? Please choose three factors.” “Don't know or other” answers were omitted because of the low ratio. Chi‐square test was conducted; **, p < .01. Abbreviation: RM, regenerative medicine.
In addition, we asked “What is the decisive factor for considering donation of your biomaterials to research?” Although JSRM members regard “protection of privacy” or “pain” as important factors, the public consider “advice from a credible medical doctor,” “achievement in future,” and “sample end‐users?” as important deciding factors for donation (Fig. 5).
Figure 5.
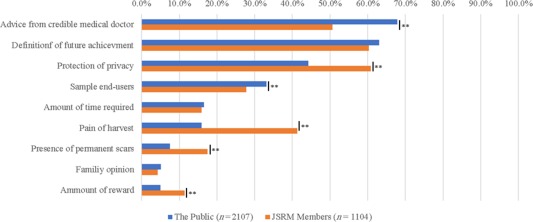
Differences in response between Japanese Society of Regenerative Medicine members and the public to “What is the decisive factor for considering donation of your biomaterials to research? Please choose three factors.” “Don't know or other” answers were omitted because of the low ratio. Chi‐square test was conducted; **, p < .01.
In summary, we observed that JSRM members focused on scientific content and validation, considering the current lack of clinical trials on RM. However, the public's primary interest in SCR and RM was related to the consequences of the potential success of RM, such as the cost of new drugs and the treatment available in case of accidents. The public was also interested in issues regarding post‐accident responsibility and responsiveness, which indicated a pragmatic attitude. These differences between the public and JSRM members should be analyzed since the public expects RM to be a reality in the near future (see also Figs. 1, 2).
In addition, the public regarded “trust” as an important factor for donation (Fig. 5).
Knowledge of Regulatory Frameworks and Social Aspects of RM Among JSRM Members
Figure 6 shows the responses of JSRM members to the following questions: “Are you aware of the Regenerative Medicine Three Acts?” and “Do you know of any cases of fatal clinical accidents related to the administration of stem cell therapies?”; 72.0% of JSRM members answered that they had a knowledge outline on these topics or could explain the Regenerative Medicine Three Acts. However, our results show that a fourth of the surveyed JSRM members lacked basic knowledge of the regulatory framework and policies of their funding source. JSRM members in clinical fields understood regulatory aspects better than those in basic research (p < .01).
Figure 6.
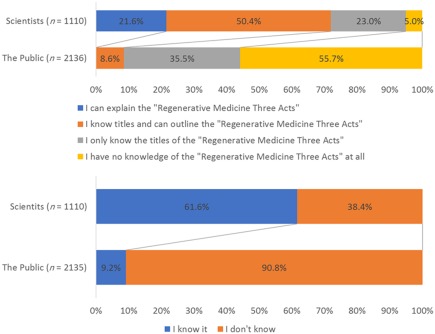
Differences in response between JSRM members and the public about the following questions. (A): Response of Japanese Society of Regenerative Medicine (JSRM) members to “Are you aware of the Regenerative Medicine Three Acts?” (B): Responses of JSRM members to “Do you know any cases of fatal clinical accidents related to the administration of stem cell therapies?”
At the same time, <60% of the respondents were aware of the fatal clinical accident arising from administration of stem cells in 2010 32. Interestingly, no significant difference in terms of this knowledge was observed between basic and clinical scientists.
To address controversies related to the application of RM in society, it is necessary to educate the scientific community regarding the social aspects and regulatory frameworks related to RM.
Discussion
At the international level, Kamenova and Caufield analyzed news articles on clinical trials involving transplantation of embryonic stem cell‐derived neural precursor cells for treatment of spinal damage and observed the emergence of an overall optimistic tone 33. Society's expectation for RM has increased, and RM clinics are now common worldwide; however, the expectations are not based on accurate scientific understanding. The deficit in basic quantitative knowledge due to the lack of scientific information transmission from the scientific community has serious policy implications. Regulatory authorities may be hesitant to enforce actions without a clear understanding of RM 34, 35. Furthermore, a considerable body of news on SCR and RM was broadcast in the Japanese mass media, following the initial implementation of human iPSCs. However, Japanese mass media tended to broadcast a national promotion of SCR and RM, rather than exploring ELSIs 36.
Considering the above situation, development of more and effective means of communication between academic societies and the public are urgently required. The present survey showed that the public is more interested in the post‐realization aspects of RM, such as cost of care, countermeasures for risks and accidents, and clarification of responsibility and liability, than in the scientific aspects, whereas the latter is of greater interest only to JSRM members (Figs. 3, 4). Consequently, patients and civilians are exposed to unnecessary risk. In addition, recognition of the social aspects of RM among JSRM members was low (Fig. 2). This gap of communication, if left unattended, might negatively affect everyone. Simultaneously, the “trust” of the public on healthcare providers/developers of RM should be considered while developing strategies to bridge these gaps (Fig. 5). In summary, communication trials concerning RM should be conducted, considering the pragmatic interests of the public. While scientific facts should form the basis of dialogues between the public and JSRM members, there is a need for increasing the awareness on the wider aspects of RM, such as an understanding of factors that may arise after the implementation of RM.
The above‐mentioned point can be related to the current discussion on “Responsible Research and Innovation (RRI)” in European science policy. Recently, the concept of RRI was discussed and applied to science policy, particularly in the European framework Horizon 2020 37. This concept recommends greater communication, engagement, and inclusion of various stakeholders in science and technology innovation. Therefore, RRI has been defined as “responsible innovation means taking care of the future through collective stewardship of science and innovation in the present” 38. The societal impact of scientific research, especially those related to SCR and RM, and the ways in which visions for the future may be shared with society are the focus of such policies 39.
As an example of RRI for RM, a new compensation system for RM therapy was launched in November 2016 by the JSRM and the Mitsui Sumitomo Insurance 40. Donors and recipients in clinical studies, as defined by RMTAs, were covered by the previous compensation system. However, in terms of compensation for RM‐based therapies, only donors have been targeted. The new compensation system aims to provide protection to the recipients. In addition, the JSRM has initiated communication efforts, including questionnaires, surveys, and implementation of lecture meetings and science cafes with funding from the “risk communication model project” by the MEXT. Furthermore, JSRM has initiated “the Regenerative Medicine National Consortium,” which is supported by AMED, for solving various issues related to the conduct and supervision of objective research and for the promotion of all‐Japan RM research. As part of this project, we plan to establish a consultation window for society on matters related to RM 41.
As described above, this change has been implemented partly in response to public interest, and should be continued apace. However, increased recognition of this new scheme within the scientific community is necessary for RRI of RM, although it may be challenging to achieve harmonization between society's interests and RM by maximizing the effect of this new scheme.
Conclusion
As society's interest in RM is increasing, further efforts are required to bridge the difference in knowledge. In contrast, JSRM members’ lack of understanding of the public's social interests regarding RM would generate issues such as communication bias and misunderstanding of RM. Notably, a proportion of the JSRM members’ lacked knowledge regarding the social and regulatory aspects of RM. In Japan, governmental investigations have been conducted for non‐compliance with RM laws, which highlights the importance of RM regulation‐related education. The JSRM has established a system that provides certification qualifications to enhance education for academic members and thereby contribute to quality medical treatment and patient protection 18.
Our data indicate that an increased awareness about RM‐associated social responsibility and regulatory framework is required among scientists, such as those regarding its benefits, potential accidents, abuse, and other social consequences. Accordingly, training programs focusing on RM science policy and regulatory frameworks for scientists are essential.
Author Contributions
R.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.I.: conception and design, manuscript writing; T.I. and A.K.: conception and design; Y.Y.: conception and design, financial support, administrative support, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We thank Kyosuke Mano, and Michi Hamaji (JSRM) for assistance. This study was supported by the Program for Developing Models of Risk Communication in Science and Technology (MEXT), the Highway Program for Realization of Regenerative Medicine (AMED), JSPS KAKENHI (Grant 15H02518, 24720073, and 17K12956), JSPS Topic‐Setting to Advance Cutting‐Edge Humanities and Social Sciences Research Program “Organizations and Society for Responsible Research and Innovation” (OSRRIs), and the GRIPS “Science and Society” Indicator research group.
Contributor Information
Ryuma Shineha, Email: r_shineha@seijo.ac.jp.
Yoshimi Yashiro, Email: yashiro.yoshimi@cira.kyoto-u.ac.jp.
References
- 1. Shineha R, Kawakami M, Kawakami K. Familiarity and prudence of the Japanese public with research into induced pluripotent stem cells, and their desire for its proper regulation. Stem Cell Rev 2010;6:1–7. [DOI] [PubMed] [Google Scholar]
- 2. MEXT . Feature 2. New developments in regenerative medicine and innovative drugs using human iPS cells. White Paper on Science and Technology 2013. Available at: http://www.mext.go.jp/en/publication/whitepaper/title03/detail03/sdetail03/1372880.htm. Accessed on November 19, 2017.
- 3. Konomi K, Tobita M, Kimura K et al. New Japanese initiatives on stem cell therapies. Cell Stem Cell 2015;16:350–352. [DOI] [PubMed] [Google Scholar]
- 4. Ikka T, Fujita M, Yashiro Y. Recent court ruling in Japan exemplifies another layer of regulation for regenerative therapy. Cell Stem Cell 2015;17:507–508. [DOI] [PubMed] [Google Scholar]
- 5.International Society for Stem Cell Research: Guidelines for stem cell research and clinical translation 2016. Available at http://www.isscr.org/docs/default-source/guidelines/isscr-guidelines-for-stem-cell-research-and-clinical-translation.pdf. Accessed November 19, 2017.
- 6. Cyranoski D. Japanese woman is first recipient of next‐generation stem cells. Nature 2014; doi:10.1038/nature.2014.15915 [Google Scholar]
- 7. Lau D, Ogbogu U, Taylor B. Stem cell clinics online: The direct‐to‐consumer portrayal of stem cell medicine. Cell Stem Cell 2008;3:591–594. [DOI] [PubMed] [Google Scholar]
- 8. Inoue Y, Shineha R, Yoshimi Y. Current public support for human‐animal chimera research in Japan is limited, despite high levels of scientific approval. Cell Stem Cell 2016;19:152–153. [DOI] [PubMed] [Google Scholar]
- 9. Shineha R, Inoue Y, Yashiro Y. Analysis of the public attitudes toward human‐animal chimera, The Seijo‐Bungei (The Seijo University arts and literature quarterly) 2017;240:398–416. [Google Scholar]
- 10. U.S. Congress, Committee on Science, House of Representatives One Hundred Fifth . Unlocking our future: Toward a new national science policy 1998. Available at http://www.access.gpo.gov/congress/house/science/cp105-b/science105b.pdf. Accessed on November 19, 2017
- 11. UNESCO World Conference on Science . Science for the twenty‐first century, a new commitment 2000. Available at http://unesdoc.unesco.org/images/0012/001207/120706e.pdf. Accessed on November 19, 2017.
- 12. The House of Lords . Science and Society ‐ Third Report 2000. Available at http://www.parliament.the-stationery-office.co.uk/pa/ld199900/ldselect/ldsctech/38/3801.htm. Accessed on November 19, 2017.
- 13. EU Commission . Science and Society Action Plan 2002. Available at https://ec.europa.eu/research/swafs/pdf/pub_gender_equality/ss_ap_en.pdf. Accessed on November 19, 2017.
- 14. EU Commission . Taking European Knowledge Society seriously 2007. Available at http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.175.660&rep=rep1&type=pdf. Accessed on November 19, 2017.
- 15. Barbagallo F, Nelson J. Report: UK GM dialogue—Separating social and scientific issues. Sci Commun 2005;26:318–325. [Google Scholar]
- 16. Pidgeon NF, Poortinga W, Rowe G et al. Using surveys in public participation processes for risk decision making: The case of the 2003 British GM nation? Public debate. Risk Anal 2005;25:467–479. [DOI] [PubMed] [Google Scholar]
- 17. Rowe G, Horlick‐Jones T, Walls J et al. Difficulties in evaluating public engagement initiatives: Reflections on an evaluation of the UK GM Nation? Public debate about transgenic crops. Public Underst Sci 2005;14:331–352. [Google Scholar]
- 18. Horlick‐Jones T, Walls J, Rowe G et al. On evaluating the GM Nation? Public debate about the commercialisation of transgenic crops in Britain. New Genet Soc 2006;25:265–288. [Google Scholar]
- 19. Horlick‐Jones T, Rowe G, Walls J. Citizen engagement processes as information systems: the role of knowledge and the concept of translation quality. Public Underst Sci 2007;16:259–278. [Google Scholar]
- 20. Cabinet Office . The Second Science and Technology Basic Plan 2001. Available at http://www8.cao.go.jp/cstp/kihonkeikaku/honbun.html. (in Japanese). Accessed on November 19, 2017.
- 21. Cabinet Office . The Third Science and Technology Basic Plan 2006. Available at http://www.mext.go.jp/a_menu/kagaku/kihon/06032816/001/001.pdf. (in Japanese). Accessed on November 19, 2017.
- 22. Matsuda K. Science cafe in Japan: A report of the poster exhibition and the workshop about science cafe in science Agora 2007. Jap J Sci Commun 2007;3:3–15. [Google Scholar]
- 23. Nakamura M. Science café: Its scope and challenge. J Sci Tech Stud 2008;5:31–43. [Google Scholar]
- 24. Cabinet Office . The Fourth Science and Technology Basic Plan 2016. Available at http://www.mext.go.jp/component/a_menu/science/detail/__icsFiles/afieldfile/2011/08/19/1293746_02.pdf. (in Japanese). Accessed on November 19, 2017.
- 25. Cabinet Office . The Fifth Science and Technology Basic Plan 2016. Available at http://www8.cao.go.jp/cstp/kihonkeikaku/5honbun.pdf. (in Japanese). Accessed on November 19, 2017.
- 26. Shineha R, Kawakami M, Kato K. The life science researchers’ attitudes toward science communication‐motivation, hurdle, and way of promotion. Jpn J Sci Commun 2009;6:17–32. (in Japanese) [Google Scholar]
- 27. Japan Science and Technology Agency . Report of questionnaire concerning science communication of scientists 2013. Available at https://www.jst.go.jp/csc/mt/mt-static/support/theme_static/csc/pdf/csc_fy2013_03.pdf. (in Japanese). Accessed on November 19, 2017.
- 28. Shineha R, Inoue Y, Ikka T et al. Science communication in regenerative medicine: implications for role of the academic society and science policies. Regen Ther 2017;7:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashi C, Morikawa S. Attitude toward nuclear power plant and its public relations. J Inst Nucl Saf Syst 1994;1:93–158. [Google Scholar]
- 30. Atsuko K, Chikio H. The Japanese attitude towards nuclear power generation‐ changes as seen through time series. J Inst Nucl Saf Syst 1999;6:2–22. [Google Scholar]
- 31. Caulfield T, Sipp D, Murry CE et al. Confronting stem cell hype. Science 2016;352:776–777. [DOI] [PubMed] [Google Scholar]
- 32. Cyranoski D. Korean deaths spark inquiry: Cases highlight the challenge of policing multinational trade in stem‐cell treatments. Nature 2010;468:485. [DOI] [PubMed] [Google Scholar]
- 33. Kamenova K, Caulfield T. Stem cell hype: Media portrayal of therapy translation. Sci Transl Med 2015;7:278–282. [DOI] [PubMed] [Google Scholar]
- 34. Berger I, Ahmad A, Bansal A et al. Global distribution of businesses marketing stem cell‐based interventions. Cell Stem Cell 2016;19:158–162. [DOI] [PubMed] [Google Scholar]
- 35. Turner L, Knoepfler P. Selling stem cells in the USA: Assessing the direct‐to‐consumer industry. Cell Stem Cell 2016;19:154–157. [DOI] [PubMed] [Google Scholar]
- 36. Shineha R. Attention to stem cell research in Japanese mass media: Twenty‐year macrotrends and the gap between media attention and ethical, legal, and social issues. East Asian Sci Technol Soc 2016;10:229–246. [Google Scholar]
- 37. European Commission . Responsible Research & Innovation 2014. Available at https://ec.europa.eu/programmes/horizon2020/en/h2020-section/responsible-research-innovation. Accessed on November 19, 2017.
- 38. Stilgoe J, Owen R, Macnaghten P. Developing a framework for responsible innovation. Res Policy 2013;42:1568–1580. [Google Scholar]
- 39. UK Research Council . Pathway to Impact 2016. Available at http://www.rcuk.ac.uk/innovation/impacts/. Accessed on November 19, 2017.
- 40.The Japanese Society for Regenerative Medicine. 2017. Available at https://www.jsrm.jp/certification/ (in Japanese). Accessed on November 19, 2017.
- 41.Japanese Society for Regenerative Medicine. Available at https://www.jsrm.jp/insurance/clinicalresearch/ Accessed on November 19, 2017.


