Summary
The cell surface antigen prominin‐1 (alias CD133) has gained enormous interest in the past 2 decades and given rise to debates as to its utility as a biological stem and cancer stem cell marker. Important and yet often overlooked knowledge that is pertinent to its physiological function has been generated in other systems given its more general expression beyond primitive cells. This article briefly discusses the importance of particular biochemical features of CD133 with relation to its association with membrane microdomains (lipid rafts) and proper immunodetection. It also draws attention toward the adequate use of detergents and caveats that may apply to the interpretation of the results generated. Stem Cells Translational Medicine 2018;7:155–160
Significance Statement.
Prominin‐1/CD133, the biological function of which remains to be determined exactly, has aroused a great interest as a stem cell and cancer stem cell marker. Some distinctive features like its differential solubility in nonionic detergents and its association with extracellular vesicles have been unveiled in other cellular systems as CD133 is expressed beyond the stem cell compartment. Given the importance of these phenomena for several aspects of stem cell and cancer stem cell biology, this article clarifies some possible misconceptions and presents often overlooked data that should be useful to the design of future studies in the fields of translational medicine and oncology.
Introduction
It is now the 20th anniversary of the first description of the murine and human stem cell antigen prominin‐1 1, 2, 3. Since then, this pentaspan membrane glycoprotein, also known as CD133, has been associated to more than 5,000 publications either as the subject or as a biological tool. Indeed, it rapidly aroused great interest as a cell surface biomarker allowing identification and isolation of stem cells from different tissues or organs and as one of the most prominent and most commonly reported cancer stem cell markers 4, 5. Moreover, CD133 has enabled the unveiling of new features in the biogenesis and maintenance of photoreceptor outer segments since mutations in the PROM1 gene lead to blindness 6, 7. Controversies arose however as to its significance as a selective marker of stem cells and with reference to cancer prognosis and progression, partly in relation with its immunodetection using specific epitopes (e.g., AC133 epitope) 8 and for overlooking in this field key data on its general expression beyond stem cells 1, 9, 10, 11, 12. Similarly, its specific biological features like embedding into specific cholesterol‐based membrane microdomains (referred to as lipid rafts) might have promoted misconceptions. The uncovering of molecular and cellular mechanisms regulating the properties of the stem cells might depend on the proper handling of CD133.
Lipid rafts are considered subdomains at nano/micro scales of plasma membranes where the outer membrane leaflet is enriched in cholesterol and sphingolipids. Lipid‐lipid and lipid‐protein interactions play a major role in their organization 13. Lipid rafts may serve as specific platforms for signal transduction, and hence participate in a wide range of cellular events under healthy and pathological conditions 14. They are also involved in membrane trafficking including membrane budding and cell polarization. Membrane‐associated proteins involved in these biological events show selective affinity for these membrane subdomains. Furthermore, lipid raft‐associated proteins may interact with components of submembraneous cytoskeleton and/or extracellular matrix 15. The partitioning of certain proteins in and out of lipid rafts could underlie certain human diseases including cancers 14, 15.
Biochemically, lipid rafts appear as distinct liquid‐ordered regions of the membrane resistant to extraction in the cold with nonionic detergents, notably Triton X‐100 or zwitterionic detergent CHAPS 16. Recently, Gupta and Banerjee delineated a protocol to isolate CD133+ lipid rafts from cancer stem cells using the nonionic detergent Triton X‐100 17, but unfortunately without providing supporting data or adequate references. In light of our experience with this glycoprotein and its related molecules, we would like to caution against the use of Triton X‐100 for the isolation of CD133+ detergent‐resistant membrane fractions and for the permeabilization step of CD133 immune‐detection in cells or tissues. In view of the widespread utility of CD133 as a cell surface biomarker and the growing interest in lipid rafts in the stem cell and cancer fields, it is important to ascertain appropriate handling in order to generate meaningful data. Therefore, we propose to first remind some distinctive features of CD133 including its biochemical characteristics and then reexamine the detergent resistance of CD133+ lipid rafts to different solubilization conditions.
CD133, Membrane Protrusions, Extracellular Vesicles, and Lipid Raft Association
A prominent characteristic of CD133 is its restricted association with plasma membrane protrusions irrespective of the nature of cells. In epithelial cells, CD133 is located at the membrane of microvilli and the primary cilium 1, 18 while in nonepithelial cells CD133 is restricted to finger‐like projections that are distributed over the entire surface of human CD34+ hematopoietic stem and progenitor cells 11, 19. Besides membrane protrusions, CD133 is found in endocytic compartments of certain cells such as primitive blood cells and cancer cells 20, 21, 22, 23. Cytoplasmic localization of CD133 in cancer cells might correlate with poor prognosis 24. Therein, CD133 is associated with small membrane vesicles within multivesicular bodies. The physiological factors regulating the shuttling of CD133 between the plasma membrane and intracellular compartments are poorly described, but the ubiquitination of CD133 and/or its interaction with histone deacetylase 6 or syntenin‐1 may be involved 25, 26. It is worth mentioning that CD133 is also associated with extracellular membrane vesicles (EVs) found in various body fluids under healthy or pathological conditions 27, 28. The CD133+ EVs are either budding from microvilli and/or primary cilia as documented in epithelial cells 18, 29 or released upon the fusion of multivesicular bodies with the plasma membrane, as shown in CD34+ primitive blood cells 21. Irrespective of the molecular mechanisms involved, the release of membrane vesicles needs to be considered when the cellular expression of CD133 is assessed.
The specific retention of CD133 in plasma membrane protrusions of polarized epithelial cells such as Madin‐Darby Canine Kidney cells and the budding of CD133+ EVs from human Caco‐2 colon‐carcinoma‐derived cells both require a proper concentration of membrane cholesterol, which directly interacts with CD133 29, 30, 31. Depletion of cholesterol by methyl‐β‐cyclodextrin results in redistribution of CD133 over nonprotruding areas of the plasma membrane and/or stimulation of the release of CD133+ EVs 29, 31. These effects are biochemically correlated with the association of CD133 with detergent‐resistant membranes. Microvilli‐ and EV‐associated CD133 were found to be resistant in a cholesterol‐dependent manner to solubilization in the mild nonionic detergent Lubrol WX, but not in Triton X‐100 29, 31. This observation is in deep contrast with the protocol described by Gupta and Banerjee 17. Insolubility is not related to interaction with the cytoskeleton 31, but may reflect the differential solubilization of components in the inner leaflet of plasma membranes 32. Subtypes of cholesterol‐based membrane microdomain that are preserved upon Lubrol WX, but not Triton X‐100 extraction, are not restricted to epithelial cells but also found in nonepithelial cells 33, 34. Moreover, certain membrane proteins are specifically incorporated into membrane microdomains that are preserved in Triton X‐100 but not Lubrol WX, highlighting the complex organization of the plasma membrane 33 (reviewed in 35). A recent comparative lipidomic and proteomic study of lipid rafts isolated from human platelets using Triton X‐100 versus Lubrol WX has described different profiles of cellular components within the respective fraction of detergent‐resistant membranes, further suggesting that the compatibility of the detergent used with the proteins of interest is to be assessed 36.
CD133 Does Not Associate with Triton X‐100 Insoluble Membrane Fractions
To further validate our earlier data, we have extended our observations with human CD133 expressed by cancer Caco‐2 cells where it is selectively found in microvilli 10 and CD133+ EVs derived from human CD34+ hematopoietic stem and progenitor cells 21. Upon cell lysis at low temperature with Triton X‐100 (at concentration of 0.5%) and differential fractionation into supernatant and pellets, almost all of the Caco‐2 cell‐associated CD133 was recovered as expected in the soluble fraction. In contrast, detergent‐resistant membranes containing CD133 were recovered with Lubrol WX (0.5%) or CHAPS (20 mM) (Fig. 1A). Interestingly, the partition to detergent‐resistant membranes appeared whether cells were grown at a subconfluent state or 10 days postconfluence (i.e., further differentiated). Yet, higher solubilization with both detergents was evidenced at the subconfluent state suggesting membrane reorganization during enterocytic differentiation, that starts in Caco‐2 cells 6–7 days after reaching confluence (Fig. 1A). In CD34+ cell‐derived EVs, CD133 showed the same susceptibility to Triton X‐100 extraction already at 0.1% (Fig. 1B), while CD162 was partly associated with the Triton X‐100‐resistant membrane, as previously reported 37. Flotillins marked also resistance to Triton X‐100 solubilization while moesin, an adapter protein linking the plasma membrane to the cytoskeleton, was associated with the soluble fraction irrespective of the detergent (Fig. 1B). To sum up, human CD133 is mostly, if not completely, extracted from cells and EVs upon solubilization with Triton X‐100. It is also important to keep in mind that different detergents may generate CD133+ detergent‐resistant membranes originating from cellular membranes other than the plasma membrane (e.g., Golgi membranes, endosomes) in variable proportions 31. Protocols including isolation of plasma membrane prior to the solubilization step and alternative detergent‐free isolation of membrane microdomains have been described 38, 39. The present data confirm that if one intends to isolate membrane complexes in relation with CD133, the use of Triton X‐100 is unlikely to be the detergent of choice. Whenever CD133 is found partly associated with Triton X‐100‐resistant membrane complexes it is important to assess the significance of this remaining signal, for instance if a link with the cytoskeleton might justify its association with detergent‐insoluble complexes.
Figure 1.
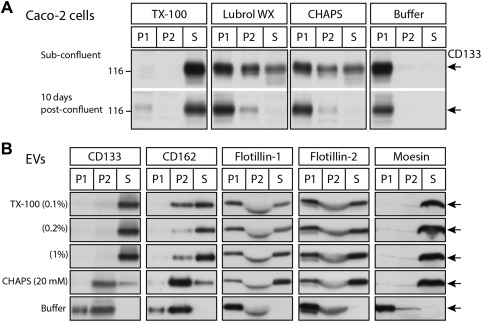
CD133 is soluble in nonionic detergent Triton X‐100 but insoluble in Lubrol WX and zwitterionic detergent CHAPS. (A): Human Caco‐2 cells grown at a subconfluent state or 10 days postconfluence were lysed for 30 minutes on ice in ice‐cold solubilization buffer (150 mM NaCl, 2 mM EGTA, 50 mM TRIS‐HCl, pH 7.5 plus protease inhibitors) containing either 0.5% (wt/vol) Triton X‐100 (TX‐100, Sigma‐Aldrich), 0.5% Lubrol WX (Lubrol 17A17, Serva) or 20 mM CHAPS (AppliChem GmbH, Darmstadt, Germany) or without detergent (Buffer). Lysates were centrifuged at 4°C first at 16,000g (P1 pellet) for 10 minutes and then the resulting supernatant at 100,000g (P2 pellet) for 1 hour. Proteins in the 100,000g supernatant (S) were concentrated by methanol/chlorophorm (4:1) precipitation. All fractions were analyzed by immunoblotting for CD133 after SDS‐PAGE under reducing condition using mouse monoclonal antibody (mAb) clone 80B258 12, followed by the appropriate horseradish peroxidase‐conjugated secondary Ab (Jackson Immunoresearch) and visualized by enhanced chemiluminescence reagents (ECL system, GE Healthcare Life Sciences). (B): EVs released from paramagnetic immuno‐isolated CD34+ hematopoietic stem and progenitor cells were enriched by ultracentrifugation (200,000g) as described 21 prior to their lysis in solubilization buffer containing Triton X‐100 at different concentrations or CHAPS as indicated. Lysates were subjected to differential centrifugation as above. Each fraction was analyzed by immunoblotting for a given protein as specified using the following antibodies: mouse mAb clone KPL‐1 (anti‐CD162; BD Biosciences), mouse mAb clone 18 and 29 (anti‐Flotillin‐1 and −2, respectively; BD Biosciences) and rabbit mAb clone EP1863Y (anti‐moesin; Abcam). Molecular mass marker (kDa) is indicated. Arrows indicate proteins of interest (Note that hematopoietic stem and progenitor cells were collected from healthy donors after informed consent and approval of the local ethics committee [Ethikkommission an der Technischen Universität Dresden; Ethic board no. EK201092004]). Abbreviations: EV, extracellular membrane vesicle; SDS‐PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
CD133, Solubility in Triton X‐100, and Immunodetection
Given that Triton X‐100 is frequently used in immunocyto‐ and histochemistry to permeabilize paraformaldehyde (PFA)‐fixed cells, the high sensitivity of CD133 to solubilization with this detergent prompted us to assess whether this step would interfere with CD133 immunodetection as described for other antigens 40. The immunodetection of CD133 on fixed melanoma FEMX‐I cells 23 was readily impacted upon permeabilization with Triton X‐100 at the lowest concentration (0.1%), while barely affected by saponin (Fig. 2A, top panels). Saponin binds to cholesterol within the cell membrane bilayer, leading to saponin‐cholesterol complexes and transient pores that are large enough to allow the passage of antibodies through the plasma membrane, as evidenced by the visualization of an intracellular pool of CD133 (Fig. 2A, bottom panels, asterisk). Similar data, that is, the selective loss of CD133 immunoreactivity upon Triton X‐100 permeabilization were obtained with Caco‐2 cells (data not shown) indicating that this biochemical phenomenon is not restricted to cells growing as individual units but also occurs in polarized monolayers. The reduction, or even the total removal of CD133 immunoreactivity was confirmed by immunoblotting of the proteins extracted by Triton X‐100 from PFA‐fixed cells. Already at the lowest concentration of Triton X‐100 CD133 was totally solubilized (Fig. 2B, top panel), leaving no detectable level of protein in the corresponding cell extracts (Fig. 2B, bottom panel). On the other hand, a permeabilization buffer containing saponin (at a concentration of 0.2% or 0.15%) solubilized a minute fraction of CD133 from the fixed cells (Fig. 2C, top and bottom panels, respectively). We would like to invite researchers to take these observations into account when analyzing the presence of CD133 alone or its association with other antigenic markers using detergents as permeabilizing agents of PFA‐fixed cells.
Figure 2.
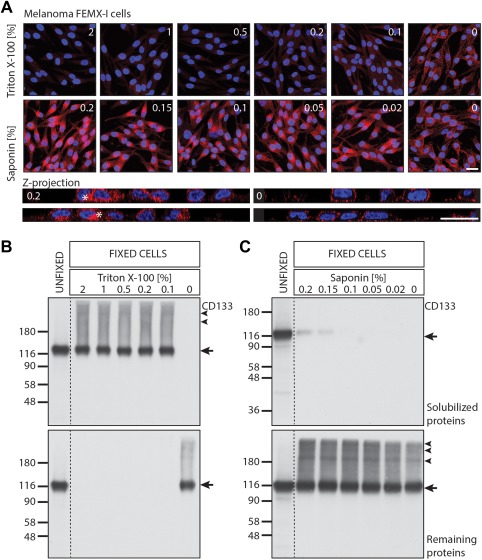
CD133 is extracted by nonionic detergent Triton X‐100 from paraformaldehyde (PFA)‐fixed melanoma FEMX‐I cells. (A): FEMX‐I cells growing on fibronectin‐coated glass coverslips were gently washed with PBS, fixed with 4% PFA for 30 minutes at room temperature and quenched with 50 mM NH4Cl for 10 minutes prior to permeabilization in PBS containing 0.2% gelatin and various concentrations of Triton X‐100 or saponin (AppliChem GmbH) as indicated for 30 minutes. Cells were labeled by indirect immunofluorescence using anti‐CD133/1 antibody (AC133 mAb; Miltenyi Biotec) for 45 minutes followed by Alexa‐555‐conjugated secondary antibody (ThermoFischer Scientific). Nuclei were labeled with 4′,6‐diamidino‐2‐phenylindole (Sigma‐Aldrich). Samples were mounted in Mowiol 4–88 containing the anti‐fading agent 1,4‐diazobicyclo‐[2.2.2]‐octane (Merck‐Millipore). All images were acquired using Leica SP5 upright confocal laser scanning microscope, using identical microscope parameters. Composites of 19–24 optical x‐y sections (top panels) or z‐projections are displayed. Asterisk, intracellular CD133 staining. Scale bar = 25 μm. (B, C): FEMX‐I cells growing on six‐well plates (35 mm) were PFA‐fixed prior to permeabilization with 1.5 ml of buffer containing various concentration of Triton X‐100 (B) or saponin (C) for 25 minutes at room temperature, as above. Permeabilization buffer was collected from fixed cells and solubilized proteins were concentrated by methanol/chlorophorm precipitation. In parallel, the fixed cells were scraped, solubilized in RIPA buffer containing 1% NP‐40, 0.1% SDS, and 0.5% sodium deoxycholate and cell lysates prepared. As control, unfixed cells were extracted. The entire materials recovered in the permeabilization buffer (top panels) and half from cell lysates (bottom panels) were separated by SDS‐PAGE under nonreducing condition, and analyzed by immunoblotting for CD133 using mAb 80B258. Molecular mass markers (kDa) are indicated. Arrow indicates monomeric CD133 while arrowheads potential PFA‐crosslinked oligomers. Abbreviations: PBS, phosphate buffered saline; SDS‐PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Conclusion
The association of CD133 with specific lipid rafts might be of significance in stem cell biology given their implication in signal transduction. This might lead to the concept of “stem cell‐specific lipid rafts” that would hold determinants necessary for maintaining stem cell properties. Their loss might then promote cell differentiation. Several shedding processes of CD133 that occur during differentiation of neural and hematopoietic progenitor cells or cancer cells like asymmetric cell division 41, 42, 43 or the release of EVs might be related to that 10, 21, 27. The release of CD133+ EVs could also mediate communication with the microenvironment, known as stem cell niche 21. As demonstrated recently, specific exchanges of CD133+ lipid rafts mediated by tunneling nanotubes between hematopoietic progenitor cells and leukemic cells may not only promote intercellular communication but also influence the stem cell properties of donor and acceptor cells 44.
It will be of interest to determine the proteome and lipidome of CD133+ Lubrol WX‐resistant membrane complexes associated with stem cells, differentiated cells or cancerous cells as well as of those associated with EVs derived therefrom. Variations in their composition might be instructive about the cellular state and/or an aberrant transformation. Moreover, EVs as sources of biomaterials might allow noninvasive clinical analyses provided that their lipid raft composition reflects that of the cell of interest under physiological and pathological conditions.
Author Contributions
J.K.: conception and design, performed the experiments, data analysis and interpretation, manuscript writing, final approval of manuscript; A.L. and M.B.: provision of study material or patients, manuscript writing, final approval of manuscript; D.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; C.A.F.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Note Added in Proof
This article was published online on 3 November 2017. Minor edits have been made that do not affect data. This notice is included in the online version to indicate an updated file was reposted on 29 January 2018.
Acknowledgments
D.C. was supported by Deutsche Forschungsgemeinschaft (DFG, SFB 655) and Sächsisches Staatsministerium für Wissenschaft und Kunst (#4‐7531.60/29/31). M.B. was supported by DFG (SFB655).
References
- 1. Weigmann A, Corbeil D, Hellwig A et al. Prominin, a novel microvilli‐specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non‐epithelial cells. Proc Natl Acad Sci USA 1997;94:12425–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yin AH, Miraglia S, Zanjani ED et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997;90:5002–5012. [PubMed] [Google Scholar]
- 3. Miraglia S, Godfrey W, Yin AH et al. A novel five‐transmembrane hematopoietic stem cell antigen: Isolation, characterization, and molecular cloning. Blood 1997;90:5013–5021. [PubMed] [Google Scholar]
- 4. Grosse‐Gehling P, Fargeas CA, Dittfeld C et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: Limitations, problems and challenges. J Pathol 2013;229:355–378. [DOI] [PubMed] [Google Scholar]
- 5. Kim Y, Kaidina AM, Chiang JH et al. Cancer stem cell molecular markers verified in vivo. Biomed Khim 2017;62:228–238. [DOI] [PubMed] [Google Scholar]
- 6. Gurudev N, Florek M, Corbeil D et al. Prominent role of prominin in the retina. Adv Exp Med Biol 2013;777:55–71. [DOI] [PubMed] [Google Scholar]
- 7. Goldberg A, Moritz O, Williams D. Molecular basis for photoreceptor outer segment architecture. Prog Retin Eye Res 2016;55:52–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med (Berl) 2008;86:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maw MA, Corbeil D, Koch J et al. A frameshift mutation in prominin (mouse)‐like 1 causes human retinal degeneration. Hum Mol Genet 2000;9:27–34. [DOI] [PubMed] [Google Scholar]
- 10. Florek M, Haase M, Marzesco AM et al. Prominin‐1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res 2005;319:15–26. [DOI] [PubMed] [Google Scholar]
- 11. Corbeil D, Röper K, Hellwig A et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem 2000;275:5512–5520. [DOI] [PubMed] [Google Scholar]
- 12. Karbanová J, Missol‐Kolka E, Fonseca AV et al. The stem cell marker CD133 (Prominin‐1) is expressed in various human glandular epithelia. J Histochem Cytochem 2008;56:977–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sezgin E, Levental I, Mayor S et al. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol 2017;18:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000;1:31–39. [DOI] [PubMed] [Google Scholar]
- 15. Nicolson GL. Cell membrane fluid‐mosaic structure and cancer metastasis. Cancer Res 2015;75:1169–1176. [DOI] [PubMed] [Google Scholar]
- 16. Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protoc 2007;2:2159–2165. [DOI] [PubMed] [Google Scholar]
- 17. Gupta VK, Banerjee S. Isolation of lipid raft proteins from CD133+ cancer stem cells. Methods Mol Biol 2017;1609:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubreuil V, Marzesco AM, Corbeil D et al. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin‐1. J Cell Biol 2007;176:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freund D, Bauer N, Boxberger S et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: Effects on proliferation and clonogenicity. Stem Cells Dev 2006;15:815–829. [DOI] [PubMed] [Google Scholar]
- 20. Saigusa S, Tanaka K, Toiyama Y et al. Immunohistochemical features of CD133 expression: Association with resistance to chemoradiotherapy in rectal cancer. Oncol Rep 2010;24:345–350. [DOI] [PubMed] [Google Scholar]
- 21. Bauer N, Wilsch‐Bräuninger M, Karbanová J et al. Haematopoietic stem cell differentiation promotes the release of prominin‐1/CD133‐containing membrane vesicles–a role of the endocytic‐exocytic pathway. EMBO Mol Med 2011;3:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campos B, Zeng L, Daotrong PH et al. Expression and regulation of AC133 and CD133 in glioblastoma. Glia 2011;59:1974–1986. [DOI] [PubMed] [Google Scholar]
- 23. Rappa G, Mercapide J, Anzanello F et al. Wnt interaction and extracellular release of prominin‐1/CD133 in human malignant melanoma cells. Exp Cell Res 2013;319:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Lin P, Ming Y et al. The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer 2017;17:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mak AB, Nixon AM, Kittanakom S et al. Regulation of CD133 by HDAC6 promotes beta‐catenin signaling to suppress cancer cell differentiation. Cell Rep 2012;2:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karbanová J, Laco J, Marzesco AM et al. Human prominin‐1 (CD133) is detected in both neoplastic and non‐neoplastic salivary gland diseases and released into saliva in a ubiquitinated form. PLoS One 2014;9:e98927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marzesco AM, Janich P, Wilsch‐Bräuninger M et al. Release of extracellular membrane particles carrying the stem cell marker prominin‐1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 2005;118:2849–2858. [DOI] [PubMed] [Google Scholar]
- 28. Huttner HB, Janich P, Kohrmann M et al. The stem cell marker prominin‐1/CD133 on membrane particles in human cerebrospinal fluid offers novel approaches for studying central nervous system disease. Stem Cells 2008;26:698–705. [DOI] [PubMed] [Google Scholar]
- 29. Marzesco AM, Wilsch‐Bräuninger M, Dubreuil V et al. Release of extracellular membrane vesicles from microvilli of epithelial cells is enhanced by depleting membrane cholesterol. FEBS Lett 2009;583:897–902. [DOI] [PubMed] [Google Scholar]
- 30. Corbeil D, Röper K, Hannah MJ et al. Selective localization of the polytopic membrane protein prominin in microvilli of epithelial cells ‐ a combination of apical sorting and retention in plasma membrane protrusions. J Cell Sci 1999;112:1023–1033. [DOI] [PubMed] [Google Scholar]
- 31. Röper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol‐based lipid micro‐domains in the apical plasma membrane. Nat Cell Biol 2000;2:582–592. [DOI] [PubMed] [Google Scholar]
- 32. Delaunay JL, Breton M, Trugnan G et al. Differential solubilization of inner plasma membrane leaflet components by Lubrol WX and Triton X‐100. Biochim Biophys Acta 2008;1778:105–112. [DOI] [PubMed] [Google Scholar]
- 33. Drobnik W, Borsukova H, Böttcher A et al. Apo AI/ABCA1‐dependent and HDL3‐mediated lipid efflux from compositionally distinct cholesterol‐based microdomains. Traffic 2002;3:268–278. [DOI] [PubMed] [Google Scholar]
- 34. Won JS, Im YB, Khan M et al. Lovastatin inhibits amyloid precursor protein (APP) beta‐cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts. J Neurochem 2008;105:1536–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corbeil D, Marzesco AM, Fargeas CA et al. Prominin‐1, a distinct cholesterol–binding membrane protein, and the organisation of the apical plasma membrane of epithelial cells. Subcell Biochem 2010;51:399–423. [DOI] [PubMed] [Google Scholar]
- 36. Rabani V, Davani S, Gambert‐Nicot S et al. Comparative lipidomics and proteomics analysis of platelet lipid rafts using different detergents. Platelets 2016;27:634–641. [DOI] [PubMed] [Google Scholar]
- 37. Shao B, Yago T, Setiadi H et al. O‐glycans direct selectin ligands to lipid rafts on leukocytes. Proc Natl Acad Sci USA 2015;112:8661–8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogiso H, Taniguchi M, Okazaki T. Analysis of lipid‐composition changes in plasma membrane microdomains. J Lipid Res 2015;56:1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Macdonald JL, Pike LJ. A simplified method for the preparation of detergent‐free lipid rafts. J Lipid Res 2005;46:1061–1067. [DOI] [PubMed] [Google Scholar]
- 40. Hannah MJ, Weiss U, Huttner WB. Differential extraction of proteins from paraformaldehyde‐fixed cells: Lessons from synaptophysin and other membrane proteins. Methods 1998;16:170–181. [DOI] [PubMed] [Google Scholar]
- 41. Kosodo Y, Röper K, Haubensak W et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J 2004;23:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fargeas CA, Fonseca AV, Huttner WB et al. Prominin‐1 (CD133) from progenitor cells to human diseases. Future Lipidol 2006;1:213–225. [Google Scholar]
- 43. Lathia JD, Hitomi M, Gallagher J et al. Distribution of CD133 reveals glioma stem cells self‐renew through symmetric and asymmetric cell divisions. Cell Death Dis 2011;2:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reichert D, Scheinpflug J, Karbanová J et al. Tunneling nanotubes mediate the transfer of stem cell marker CD133 between hematopoietic progenitor cells. Exp Hematol 2016;44:1092–1112. [DOI] [PubMed] [Google Scholar]


