Abstract
Objective
Polymyositis and dermatomyositis (PM/DM) have been implicated in the development of venous thromboembolism (VTE). Previous studies investigating the association between PM/DM and VTE risk had yielded inconsistent findings. The aim of this study was to precisely estimate this association by meta-analysis of all available publications.
Methods
Two investigators independently performed a comprehensive literature search in databases of PubMed, Embase, and the Cochrane Library for eligible studies. The strength for the association was weighed by pooled odds ratios (ORs) with 95% confidence intervals (95% CIs). Stratified analysis and sensitivity analysis were performed for further analysis.
Results
Six studies including 9,045 patients with PM/DM were analyzed. The pooled OR suggested that inflammatory myositis was associated with increased risk of VTE (OR =4.31, 95% CI: 2.55–7.29, P<0.001). Besides, significantly elevated risk of VTE was related with PM and DM, respectively (for PM: OR =6.87, 95% CI: 4.12–11.46, P<0.001; for DM: OR =11.59, 95% CI: 6.54–20.55, P<0.001). In addition, inflammatory myositis could increase the risk of DVT (OR =4.85, 95% CI: 1.38–17.12, P<0.05) and PE (OR =4.74, 95% CI: 2.18–10.30, P<0.05). Sensitivity analysis did not materially alter the pooled results.
Conclusion
Our study shows strong evidence that patients with inflammatory myositis have an increased risk of VTE.
Keywords: polymyositis, dermatomyositis, venous thromboembolism, deep venous thrombosis, pulmonary embolism
Introduction
Polymyositis and dermatomyositis (PM/DM) are the major inflammatory myositis in adults, which are characterized by chronic muscle inflammation/weakness. PM/DM, collectively known as idiopathic inflammatory myositis, are chronic inflammatory disorders and affect the muscles, skin, joints, esophagus, lungs, and heart. The inflammation is predominantly of the endomysium in PM, whereas DM is primarily characterized by perimysial inflammation with a skin rash. Despite advances in the study of immune regulation, genetic predisposition, and environmental factors involved in PM/DM, the pathogenesis of inflammatory myositis remains largely obscure. The presence of chronic inflammation in muscle leads to the fibrotic state that characterizes the end-stage of PM/DM.1 Disorders in the adaptive and innate immune response, hypoxia, and autophagy in the site of tissue inflammation are responsible for the complexity of inflammatory myositis.2–4 The prognosis of PM/DM is extremely poor, with a mortality rate as high as 50%–61%.5 Patients with DM/PM have a reduced quality of life and are at an increased risk for several comorbidities, such as osteoporosis and cardiovascular diseases.2,6,7
For the past few years, the risk of venous thromboembolism (VTE) associated with PM/DM has drawn much attention. VTE primarily consists of pulmonary embolism (PE) and deep venous thrombosis (DVT).8,9 VTE seems to be linked to disease activity in many of the inflammatory rheumatologic diseases, including PM/DM. Rheumatologic conditions are often inflammatory by nature.10 Highly inflammatory conditions may be responsible for hypercoagulability, which thus leads to increased risk of VTE. Previous studies on the association between PM/DM and VTE risk have yielded inconsistent findings. The aim of this study is to investigate this association by meta-analysis of all currently published studies. Stratified analysis by types of disease is also performed for detailed evaluation.
Methods
Search strategy
We searched in databases of PubMed, Embase, and the Cochrane Library from their inception up to August 1, 2017 to identify studies investigating the association between PM/DM and VTE risk. The following search terms were used: (inflammatory myositis, polymyositis, dermatomyositis) and (deep venous thrombosis, pulmonary embolism, venous thromboembolism). Additional studies were identified by screening all reviewing references.
Inclusion criteria and exclusion criteria
Inclusion criteria were as follows: 1) studies on the association between PM/DM and VTE risk; 2) case–control studies or cohort studies; and 3) studies providing data of odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) with 95% confidence intervals (95% CIs). Exclusion criteria were as follows: 1) reviews; 2) animal research; 3) studies with overlapping data; and 4) studies without ORs, RRs, or HRs with 95% CIs.
Data extraction and quality evaluation
Two investigators independently extracted the following data: name of the first author, year of publication, study design, country of origins, ethnicity, adjusted or not, adjusted factors, diagnosis of PM/DM, and RRs, HRs, or ORs with 95% CIs estimating the association between PM/DM and VTE risk. The meta-analysis was conducted in accordance with PRISMA guidelines.11 The quality of each study was scored using the Newcastle–Ottawa Scale. The highest score is 9, and scores between 6 and 9 were considered to indicate a high methodological quality.
Statistical analysis
Data were pooled using standard meta-analysis methods. The pooled RR with corresponding 95% CI was used to assess the association between PM/DM and VTE risk. We evaluated between-study heterogeneity via both χ2 test and I2 test.12 If the P-value for χ2 test was more than 0.10 or the I2 value was less than 50%, the between-study heterogeneity was not significant. Otherwise, the between-study heterogeneity was considered significant when the P-value for χ2 test was less than 0.10 or the I2 value was more than 50%. When the heterogeneity was insignificant, the fixed-effect model was used for analysis.13 When the heterogeneity was significant, the random-effect model was applied.14 Stratified analysis and sensitivity analysis were performed for further analysis. Publication bias was assessed by using funnel plot and Egger’s test.15 All analyses were performed using Stata Version 11.0 (StataCorp, College Station, TX, USA).
Results
Characteristics of studies included in this study
After a comprehensive literature search in PubMed, Embase, and the Cochrane Library databases, 1,025 studies were retrieved. After checking the titles and abstracts, 9 related studies were finally identified.8,16–23 A flowchart is shown in Figure 1. According to the inclusion criteria, 6 independent studies on the association of PM/DM and VTE risk were finally included into our study. Characteristics of all eligible studies are summarized in Table 1. According to types of VTE and inflammatory myositis, 4 publications were divided into several independent studies (Tables S1 and S2). As a result, there were 17 available studies for meta-analysis. Most of the studies were based on cohorts.
Figure 1.
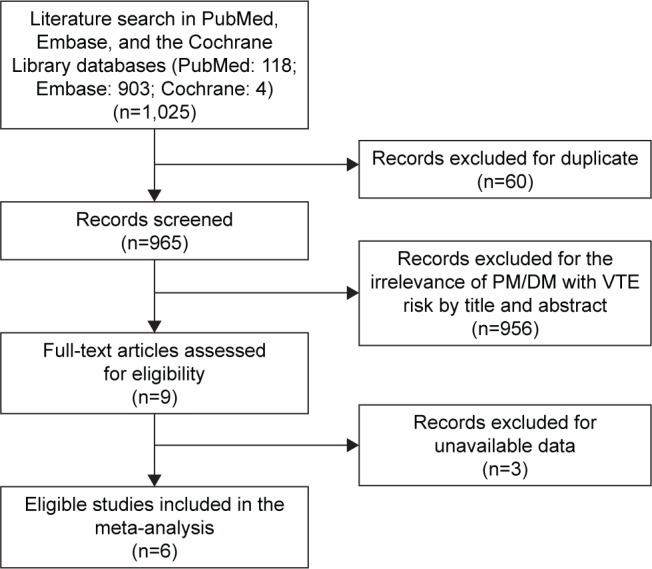
Flow chart for eligible studies.
Abbreviations: DM, dermatomyositis; PM, polymyositis; VTE, venous thromboem-bolism.
Table 1.
Characteristics of all studies
| Study | Country | Study design | Case number | Control number | Case group | Control group | Adjustment | Study quality |
|---|---|---|---|---|---|---|---|---|
| Carruthers et al,20 2016 | Canada | Retrospective cohort | PM, 443; DM, 335 | 8958 | Diagnosed PM/DM from Population Data British Columbia between 1996 and 2010 | General population with age, sex, and entry time matched | Birth year, sex, calendar year of follow-up, relevant risk factors | 9 |
| Chung et al,23 2014 | Taiwan | Retrospective cohort | PM/DM, 2031 | 10155 | Diagnosed PM/DM from the National Health Insurance Research Database between 2000 and 2010 | National Health Insurance beneficiaries matched with age, sex, and year of diagnosis | Age, sex, atrial fibrillation, hypertension, diabetes, hyperlipidemia, CVA, heart failure, lower leg fracture or surgery, cancer, and pregnancy | 9 |
| Bleau et al,19 2014 | Canada | Retrospective cohort | PM, 80; DM, 107 | 7917266 | Pregnant women diagnosed PM/DM from the Health Care Cost and Utilization Project, Nationwide Inpatient Sample between 2003 and 2011 | General pregnant women from the same database | Age | 6 |
| Zoller et al,18 2012 | Sweden | Retrospective cohort | PM/DM, 2122 | 533416 | Diagnosed PM/DM from Swedish national hospital admission database between 1964 and 2008 | General population | Age, sex, period, hospital admission for chronic obstructive pulmonary disease, obesity, alcoholism, and alcohol-related liver disease, coronary heart disease, stroke, hypertension, sepsis, varicose veins, peripheral vascular disease, and congestive heart failure | 7 |
| Johannesdottir et al,22 2012 | Denmark | Case–control | PM/DM, 47 | 147210 | Diagnosed with DVT or PE from Danish national registry database between 1999 and 2009 | General population with sex and age matched from the same database | Age, sex, classic risk factors, other comorbidities, and medication use | 6 |
| Ramagopalan et al,17 2011 | United Kingdom | Prospective cohort study | PM/DM, 6002 | Not available | Diagnosed PM/DM from the English National Hospital Episode Statistics between 1963 and 2008 | Hospitalized patients with minor medical or surgical conditions from the same database | Sex, age in 5-year bands, time-period in single calendar years | 7 |
Abbreviations: DM, dermatomyositis; DVT, deep venous thrombosis; PE, pulmonary embolism; PM, polymyositis.
Increased risk of VTE related to inflammatory myositis
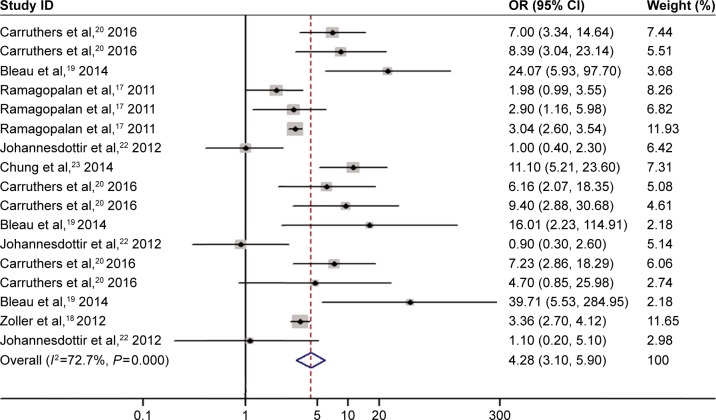
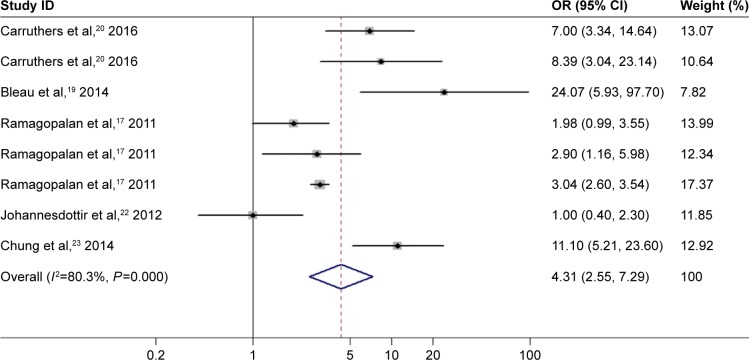
There was significant between-study heterogeneity in pooled analysis of all studies regarding the relationship of PM/DM and VTE-related risk (I2=72.7%, P=0.000; Figure 2). Therefore, the random-effect model was adopted. The pooled OR showed that inflammatory myositis was associated with elevated risk of VTE, PE, and DVT (OR =4.28, 95% CI: 3.10–5.90, P<0.001; Figure 2). We performed sensitivity analysis and subgroup analysis by PM/DM. Sensitivity analysis by sequential omission of single studies further confirmed the pooled result (Figure 3). Subgroup analysis by PM/DM showed that different kinds of inflammatory myositis may be the source of heterogeneity (Figure 4). The pooled OR showed that inflammatory myositis was associated with elevated risk of VTE (OR =4.31, 95% CI: 2.55–7.29, P<0.001; Figure 5).
Figure 2.
Association between inflammatory myositis and risk of VTE, PE and DVT.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio; DVT, deep venous thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Figure 3.
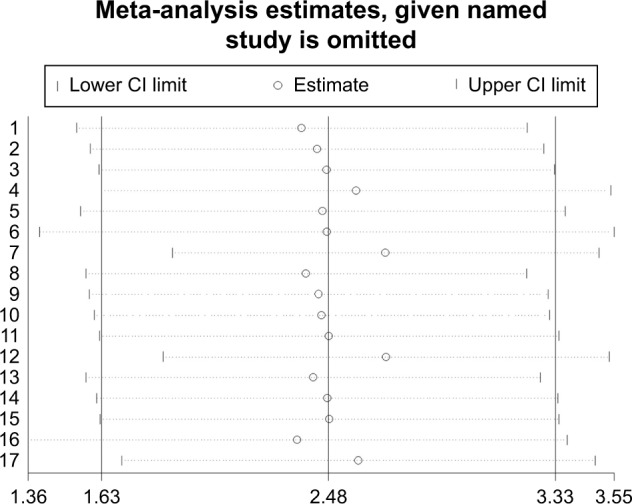
Sensitivity analysis of association between inflammatory myositis and risk of VTE, PE and DVT.
Abbreviations: CI, confidence interval; DVT, deep venous thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
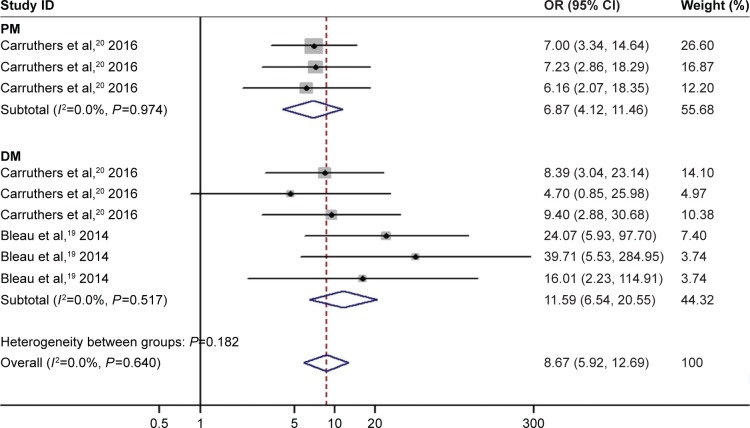
Figure 4.
Subgroup analyses by PM/DM of association between inflammatory myositis and risk of VTE, PE and DVT.
Abbreviations: CI, confidence interval; OR, odds ratio; DVT, deep venous thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Figure 5.
Association between inflammatory myositis and VTE risk.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio; VTE, venous thromboembolism.
Increased risk of VTE related to PM and DM
Heterogeneity analysis did not show evidence for significant between-study heterogeneity when estimating the association of VTE risk with PM and DM, respectively (for PM: I2=0.0%, P=0.974; for DM: I2=0.0%, P=0.64). As suggested by the pooled analysis based on the fixed-effect model, elevated risk of VTE was found to be related to PM (OR =6.87, 95% CI: 4.12–11.46, P<0.001; Figure 4). The pooled OR showed that DM was associated with increased risk of VTE (OR =8.67, 95% CI: 5.92–12.69, P<0.001; Figure 4).
Increased risk of DVT or PE related to inflammatory myositis
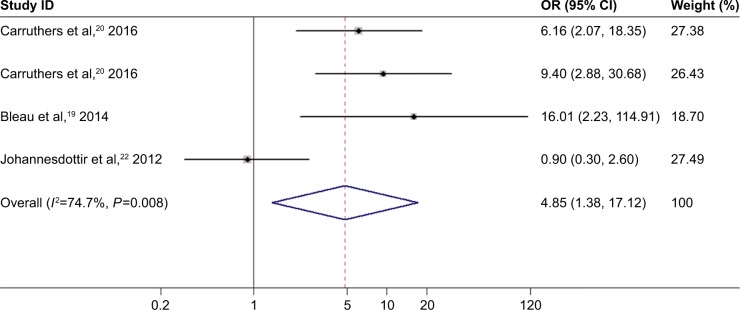
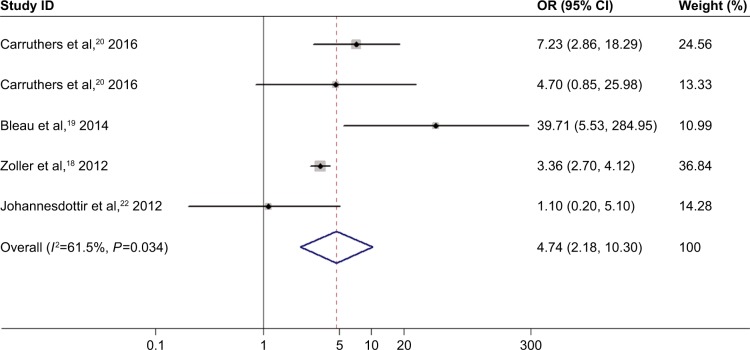
The random-effect model was adopted because significant between-study heterogeneity was observed in studies on the relationship of inflammatory myositis and DVT or PE risk (I2=74.7%, P=0.008 for DVT; I2=61.5%, P=0.034 for PE). Inflammatory myositis was related to significantly elevated risk of DVT (OR =4.85, 95% CI: 1.38–17.12, P<0.05; Figure 6). Elevated risk of PE was associated with inflammatory myositis (OR =4.74, 95% CI: 2.18–10.30, P<0.05; Figure 7).
Figure 6.
Association between inflammatory myositis and DVT risk.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio; DVT, deep venous thrombosis.
Figure 7.
Association between inflammatory myositis and PE risk.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio; PE, pulmonary embolism.
Publication bias
Begg’s funnel plot was symmetrical (Figure 8). Egger’s test showed no evidence of publication bias (P=0.124).
Figure 8.
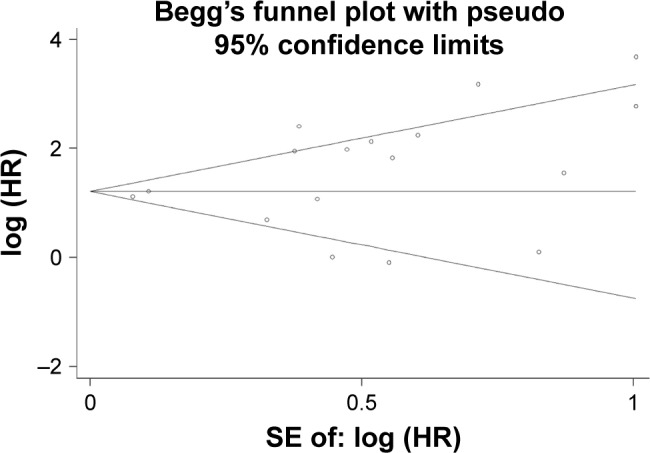
Publication bias risk.
Abbreviations: HR, hazard ratio; SE, standard error of the mean.
Discussion
This meta-analysis showed that inflammatory myositis was related to increased risk of VTE. Our meta-analysis differs from a previous meta-analysis by Lee,24 which missed an important study by Bleau.19 Our meta-analysis found that the elevated risk of VTE was found to be related to not only PM, but also DM, which differed from Lee’s meta-analysis. We conducted subgroup analysis according to different inflammatory myositis (PM/DM), which was not done in Lee’s study. Inflammatory myositis could increase the risk of DVT and PE, respectively. Sensitivity analysis did not materially alter the significant association between PM/DM and the risk of VTE. Taken together, patients with inflammatory myositis are at increased risk of developing VTE.
To the best of our knowledge, increased risk of CVDs has been suggested in a number of autoimmune rheumatic diseases, such as ankylosing spondylitis,25,26 rheumatoid arthritis,21,27 and systemic lupus erythematosus.28,29 VTE is the third most common cardiovascular event following myocardial infarction and stroke. As systemic autoimmune rheumatic diseases, PM and DM are associated with elevated morbidity and mortality, and a high economic burden. The prognosis of these diseases is extremely poor. Current available data on the association between PM/DM and VTE risk are scarce. Rheumatologic conditions are common in PM/DM characterized by inflammation in nature.10 Such systemic inflammation associated with PM/DM may cause hypercoagulability by upregulating procoagulants, downregulating anticoagulants, and suppressing fibrinolysis. Since there is a link between inflammation and VTE, several previous studies have been performed to estimate the association between PM/DM and VTE risk. Nevertheless, the precise association remains largely unknown due to inconsistent findings across diverse studies. Ramagopalan et al17 reported that significantly elevated risk of VTE was found in people with a hospital record of admission for PM/DM. However, they did not assess the association of PM/DM with the risk of DVT and PE, respectively. Higher risk of VTE was demonstrated to be related to DM but not PM, which is suggested by the study by Bleau et al.19 Carruthers et al20 showed detailed evidence that patients with PM/DM had an increased risk of VTE among Caucasians. Those findings are limited because they are based on relative small samples. Besides, previous studies are unadjusted or adjusted for inconsistent confounding factors, such as age, sex, ethnicity, and use of glucocorticoids. Therefore, the statistical power may be insufficient and random errors may be caused in single studies. Meta-analysis takes the advantage of reducing random error and provides more precise estimates, particularly for weak association. This study shows strong evidence for the significant relationship between PM/DM and increased risk of PE and DVT. Nonetheless, some estimates are based on relative small sample size because of insufficient published data. Thus, more studies with enough statistical power are warranted to investigate the correlation of inflammatory myositis with VTE risk.
Significant relationship between PM/DM and VTE risk was observed among the Caucasian population. We failed to assess this relationship among other populations, such as Asians and Africans, for lack of relevant publications regarding the risk of VTE in patients with PM/DM. More future studies are warranted to ensure whether such association is also significant among other populations from different origins.
Limitations
First, the pooled findings should be interpreted with caution, although all comparisons were conducted based on adjusted data. The adjusting factors were different in all studies included in our paper. Second, significant between-study heterogeneity was observed in some comparisons. Despite studies estimating the risk of PE as the main source of heterogeneity, some other confounding factors should be considered, including study design, ethnicity, age, sex, duration of disease, and activity of disease. Last but not the least, the effect of PM/DM on VTE risk might be altered by the duration and activity of these diseases. We failed to elucidate this effect due to insufficient available data published up till now. As a result, more independent studies with high quality are encouraged to estimate the influence of the duration and activity of PM/DM in the risk of VTE.
Conclusion
In summary, our study shows the evidence that patients with PM/DM are at elevated risk of venous thromboembolic events, including PE and DVT. In addition, the association of PM/DM with VTE risk warrants further investigation on account of diverse ethnicity and the duration and activity of PM/DM. Although the role of thromboprophylaxis on PM/DM remains unclear and merits further studies, we suggest the physicians should carefully monitor patients with PM/DM for VTE, particularly those with other conventional risk factors.
Supplementary materials
Table S1.
Characteristics of all studies according to types of VTE and inflammatory myositis
| Study | Study design | Ethnicity | Inflammatory myositis | Type of VTE |
|---|---|---|---|---|
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | PM | VTE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | PM | PE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | PM | DVT |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | DM | VTE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | DM | PE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | DM | DVT |
| Chung et al,2 2014 | Retrospective cohort study | Xanthoderm | PM/DM | VTE |
| Bleau et al,3 2014 | Prospective cohort study | Caucasians | DM | VTE |
| Bleau et al,3 2014 | Prospective cohort study | Caucasians | DM | PE |
| Bleau et al,3 2014 | Prospective cohort study | Caucasians | DM | DVT |
| Zoller et al,4 2012 | Prospective cohort study | Caucasians | PM/DM | PE |
| Johannesdottir et al,5 2012 | Case–control study | Caucasians | PM/DM | VTE |
| Johannesdottir et al,5 2012 | Case–control study | Caucasians | PM/DM | DVT |
| Johannesdottir et al,5 2012 | Case–control study | Caucasians | PM/DM | PE |
| Ramagopalan et al,6 2011 | Prospective cohort study | Caucasians | PM/DM | VTE |
| Ramagopalan et al,6 2011 | Prospective cohort study | Caucasians | PM/DM | VTE |
| Ramagopalan et al,6 2011 | Prospective cohort study | Caucasians | PM/DM | VTE |
Abbreviations: DM, dermatomyositis; DVT, deep venous thrombosis; PE, pulmonary embolism; PM, polymyositis; VTE, venous thromboembolism.
Table S2.
Quality evaluation of studies included in this meta-analysis
| Study | Study selectiona | Outcomeb | Comparabilityc | Totald |
|---|---|---|---|---|
| Carruthers et al,1 2016 | 4 | 2 | 3 | 9 |
| Chung et al,2 2014 | 4 | 2 | 3 | 9 |
| Bleau et al,3 2014 | 3 | 1 | 2 | 6 |
| Zoller et al,4 2012 | 4 | 0 | 3 | 7 |
| Johannesdottir et al,5 2012 | 4 | 1 | 1 | 6 |
| Ramagopalan et al,6 2011 | 4 | 0 | 3 | 7 |
Notes:
Maximum 4 stars awarded for cohort representativeness, selection of nonexposed cohort, exposure assessment, and demonstration outcome not present at baseline.
Maximum 3 stars awarded for follow-up length, adequacy of follow-up, and outcome assessment.
Maximum 2 stars awarded for controlling for main confounders.
Studies receiving ≥6 points were considered high quality; a maximum of 9 points could be awarded.
References
- 1.Carruthers EC, Choi HK, Sayre EC, Avina-Zubieta JA. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis. 2016;75(1):110–116. doi: 10.1136/annrheumdis-2014-205800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung WS, Lin CL, Sung FC, Lu CC, Kao CH. Increased risk of venous thromboembolism in patients with dermatomyositis/polymyositis: a nationwide cohort study. Thromb Res. 2014;134(3):622–626. doi: 10.1016/j.thromres.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Bleau N, Patenaude V, Abenhaim HA. Risk of venous thromboembolic events in pregnant patients with autoimmune diseases: A Population-Based Study. Clin Appl Thromb Hemost. 2016;22(3):285–291. doi: 10.1177/1076029614553023. [DOI] [PubMed] [Google Scholar]
- 4.Zoller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379(9812):244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 5.Johannesdottir SA, Schmidt M, Horvath-Puho E, Sorensen HT. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10(5):815–821. doi: 10.1111/j.1538-7836.2012.04666.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
The study was funded by the PhD financial support of Weifang Medical University.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dalakas MC. Inflammatory disorders of muscle: progress in polymyositis, dermatomyositis and inclusion body myositis. Curr Opin Neurol. 2004;17(5):561–567. doi: 10.1097/00019052-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Danieli MG, Gelardi C, Guerra F, Cardinaletti P, Pedini V, Gabrielli A. Cardiac involvement in polymyositis and dermatomyositis. Autoimmun Rev. 2016;15(5):462–465. doi: 10.1016/j.autrev.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Amato AA, Greenberg SA. Inflammatory myopathies. Continuum (Minneap Minn) 2013;19(6 Muscle Disease):1615–1633. doi: 10.1212/01.CON.0000440662.26427.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briani C, Doria A, Sarzi-Puttini P, Dalakas MC. Update on idiopathic inflammatory myopathies. Autoimmunity. 2006;39(3):161–170. doi: 10.1080/08916930600622132. [DOI] [PubMed] [Google Scholar]
- 5.Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep. 2012;14(3):275–285. doi: 10.1007/s11926-012-0249-3. [DOI] [PubMed] [Google Scholar]
- 6.Bernatsky S, Panopalis P, Pineau CA, Hudson M, Pierre Y, St, Clarke AE. Healthcare costs of inflammatory myopathies. J Rheumatol. 2011;38(5):885–888. doi: 10.3899/jrheum.101083. [DOI] [PubMed] [Google Scholar]
- 7.Lee CW, Muo CH, Liang JA, Sung FC, Hsu CY, Kao CH. Increased osteoporosis risk in dermatomyositis or polymyositis independent of the treatments: a population-based cohort study with propensity score. Endocrine. 2016;52(1):86–92. doi: 10.1007/s12020-015-0756-x. [DOI] [PubMed] [Google Scholar]
- 8.Selva-O’Callaghan A, Fernandez-Luque A, Martinez-Gomez X, Labirua-Iturburu A, Vilardell-Tarres M. Venous thromboembolism in patients with dermatomyositis and polymyositis. Clin Exp Rheumatol. 2011;29(5):846–849. [PubMed] [Google Scholar]
- 9.Nowak M, Krolak-Nowak K, Sobolewska-Wlodarczyk A, Fichna J, Wlodarczyk M. Elevated risk of venous thromboembolic events in patients with inflammatory myopathies. Vasc Health Risk Manag. 2016;12:233–238. doi: 10.2147/VHRM.S75308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23(12):2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Diaz J, Garcia-Sosa I, Sanchez-Guerrero J. Thrombosis in systemic lupus erythematosus and other autoimmune diseases of recent onset. J Rheumatol. 2009;36(1):68–75. doi: 10.3899/jrheum.071244. [DOI] [PubMed] [Google Scholar]
- 17.Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379(9812):244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 19.Bleau N, Patenaude V, Abenhaim HA. Risk of venous thromboembolic events in pregnant patients with autoimmune diseases: A Population-Based Study. Clin Appl Thromb Hemost. 2016;22(3):285–291. doi: 10.1177/1076029614553023. [DOI] [PubMed] [Google Scholar]
- 20.Carruthers EC, Choi HK, Sayre EC, Avina-Zubieta JA. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis. 2016;75(1):110–116. doi: 10.1136/annrheumdis-2014-205800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak B, Madej M, Luczak A, Malecki R, Wiland P. Disease activity, oxidized-LDL fraction and anti-oxidized LDL antibodies influence cardiovascular risk in rheumatoid arthritis. Adv Clin Exp Med. 2016;25(1):43–50. doi: 10.17219/acem/29847. [DOI] [PubMed] [Google Scholar]
- 22.Johannesdottir SA, Schmidt M, Horvath-Puho E, Sorensen HT. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10(5):815–821. doi: 10.1111/j.1538-7836.2012.04666.x. [DOI] [PubMed] [Google Scholar]
- 23.Chung WS, Lin CL, Sung FC, Lu CC, Kao CH. Increased risk of venous thromboembolism in patients with dermatomyositis/polymyositis: a nationwide cohort study. Thromb Res. 2014;134(3):622–626. doi: 10.1016/j.thromres.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Song GG. Idiopathic inflammatory myopathy and the risk of venous thromboembolism: a meta-analysis. Rheumatol Int. 2017;37(7):1165–1173. doi: 10.1007/s00296-017-3735-0. [DOI] [PubMed] [Google Scholar]
- 25.Heslinga SC, Van den Oever IA, Van Sijl AM, et al. Cardiovascular risk management in patients with active ankylosing spondylitis: a detailed evaluation. BMC Musculoskelet Disord. 2015;16:80. doi: 10.1186/s12891-015-0532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med. 2015;163(6):409–416. doi: 10.7326/M14-2470. [DOI] [PubMed] [Google Scholar]
- 27.Pujades-Rodriguez M, Duyx B, Thomas SL, et al. Rheumatoid arthritis and incidence of twelve initial presentations of cardiovascular disease: a population record-linkage cohort study in England. PLoS One. 2016;11(3):e0151245. doi: 10.1371/journal.pone.0151245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tselios K, Sheane BJ, Gladman DD, Urowitz MB. Optimal monitoring for coronary heart disease risk in patients with systemic Lupus erythematosus: a systematic review. J Rheumatol. 2016;43(1):54–65. doi: 10.3899/jrheum.150460. [DOI] [PubMed] [Google Scholar]
- 29.Gusetu G, Pop D, Pamfil C, et al. Subclinical myocardial impairment in SLE: insights from novel ultrasound techniques and clinical determinants. Med Ultrason. 2016;18(1):47–56. doi: 10.11152/mu.2013.2066.181.zdr. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Characteristics of all studies according to types of VTE and inflammatory myositis
| Study | Study design | Ethnicity | Inflammatory myositis | Type of VTE |
|---|---|---|---|---|
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | PM | VTE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | PM | PE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | PM | DVT |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | DM | VTE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | DM | PE |
| Carruthers et al,1 2016 | Retrospective cohort study | Caucasians | DM | DVT |
| Chung et al,2 2014 | Retrospective cohort study | Xanthoderm | PM/DM | VTE |
| Bleau et al,3 2014 | Prospective cohort study | Caucasians | DM | VTE |
| Bleau et al,3 2014 | Prospective cohort study | Caucasians | DM | PE |
| Bleau et al,3 2014 | Prospective cohort study | Caucasians | DM | DVT |
| Zoller et al,4 2012 | Prospective cohort study | Caucasians | PM/DM | PE |
| Johannesdottir et al,5 2012 | Case–control study | Caucasians | PM/DM | VTE |
| Johannesdottir et al,5 2012 | Case–control study | Caucasians | PM/DM | DVT |
| Johannesdottir et al,5 2012 | Case–control study | Caucasians | PM/DM | PE |
| Ramagopalan et al,6 2011 | Prospective cohort study | Caucasians | PM/DM | VTE |
| Ramagopalan et al,6 2011 | Prospective cohort study | Caucasians | PM/DM | VTE |
| Ramagopalan et al,6 2011 | Prospective cohort study | Caucasians | PM/DM | VTE |
Abbreviations: DM, dermatomyositis; DVT, deep venous thrombosis; PE, pulmonary embolism; PM, polymyositis; VTE, venous thromboembolism.
Table S2.
Quality evaluation of studies included in this meta-analysis
| Study | Study selectiona | Outcomeb | Comparabilityc | Totald |
|---|---|---|---|---|
| Carruthers et al,1 2016 | 4 | 2 | 3 | 9 |
| Chung et al,2 2014 | 4 | 2 | 3 | 9 |
| Bleau et al,3 2014 | 3 | 1 | 2 | 6 |
| Zoller et al,4 2012 | 4 | 0 | 3 | 7 |
| Johannesdottir et al,5 2012 | 4 | 1 | 1 | 6 |
| Ramagopalan et al,6 2011 | 4 | 0 | 3 | 7 |
Notes:
Maximum 4 stars awarded for cohort representativeness, selection of nonexposed cohort, exposure assessment, and demonstration outcome not present at baseline.
Maximum 3 stars awarded for follow-up length, adequacy of follow-up, and outcome assessment.
Maximum 2 stars awarded for controlling for main confounders.
Studies receiving ≥6 points were considered high quality; a maximum of 9 points could be awarded.