Abstract
Enantiopure vicinal amino alcohols and derivatives are essential structural motifs in natural products and pharmaceutically active molecules, and serve as main chiral sources in asymmetric synthesis. Currently known asymmetric catalytic protocols for this class of compounds are still rare and often suffer from limited scope of substrates, relatively low regio- or stereoselectivities, thus prompting the development of more effective methodologies. Herein we report a dual catalytic strategy for the convergent enantioselective synthesis of vicinal amino alcohols. The method features a radical-type Zimmerman–Traxler transition state formed from a rare earth metal with a nitrone and an aromatic ketyl radical in the presence of chiral N,N′-dioxide ligands. In addition to high level of enantio- and diastereoselectivities, our synthetic protocol affords advantages of simple operation, mild conditions, high-yielding, and a broad scope of substrates. Furthermore, this protocol has been successfully applied to the concise synthesis of pharmaceutically valuable compounds (e.g., ephedrine and selegiline).
Chiral vicinal amino alcohols are found in many bioactive compounds and may serve as chiral ligands. Here, the authors report a photocatalytic enantioselective cross-coupling of nitrones with aromatic aldehydes with a chiral ligand-coordinated rare earth ion synergistically producing enantiopure vicinal amino alcohols.
Introduction
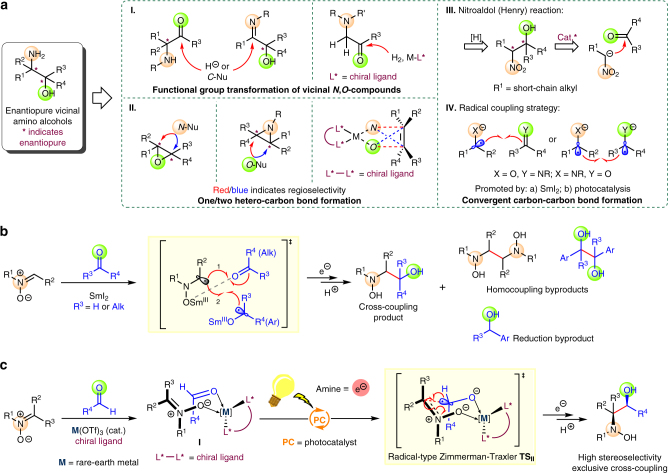
Enantiopure vicinal amino alcohols and their derivatives represent one of the most significant synthetic building blocks and key subunits of pharmaceutically active molecules, chiral auxiliaries and ligands. Synthesis of such compounds has stimulated continuing interest and extensive efforts1–9. Traditional methods for this aim are such addition reactions that mostly require enantiopure substrates or reagents, including functional group transformation of vicinal N,O-compounds1–3, addition of N/O-heteroatoms to substrates4–7, and nitro-group’s derivatization via nucleophilic nitroaldol (Henry) reaction8 (I–III in Fig. 1a). However, these strategies, including their catalytic enantioselective evolutions3,6–8, suffered from either structurally limited substrates/products or relatively low regioselectivity.
Fig. 1.
Retrosynthetic analysis and synthetic protocols of enantiopure vicinal amino alcohols. a The general protocols for synthesis of enantiopure vicinal amino alcohols and their derivatives. b Previous studies by Py and our group on SmI2-mediated cross-coupling of nitrones with aldehydes/ketones may lead to homocoupling and reduction byproducts and are not ideally suitable for developing catalytic enantioselective variant. c Enantioselective reductive cross-coupling reaction of nitrones with aromatic aldehydes via the synergistic catalysis of chiral ligand-coordinated Lewis acid and photocatalyst was described. Through the radical-type Zimmerman–Traxler TSII, vicinal hydroxyamino alcohols could be obtained exclusively with high stereoselectivity. This mild reaction is operationally simple with a wide array of nitrones and aromatic aldehydes
Compared with the above protocols, radical cross-coupling between amine and alcohol moieties represents an inherently efficient and flexible way for construction of vicinal amino alcohols (Fig. 1a-IV). By using SmI2 as reductant and oxophilic coordination center, reductive cross-coupling of imine derivatives9–11 or nitrones12–15 with carbonyl compounds (e.g., aldehydes/ketones) allows for an easy access to these compounds with various structures. However, the use of SmI2 in stoichiometric quantity poses a substantial challenge for enantioselective induction from chiral ligands16, along with unavoidable side reactions such as pinacol-type homocoupling and reduction of substrates (Fig. 1b). Recently, photocatalysis17–22 also provided several schemes on vicinal amino alcohols and their derivatives23–28, including three enantioselective protocols catalyzed by photocatalyst-merged dual catalyst systems with chiral phosphoric acid organocatalyst23 or chiral rhodium Lewis acid24, as well as bifunctional Lewis acid/photoredox catalyst25 of chiral-at-metal iridium complex29. Nevertheless, all of these methods relied heavily on specially designed substrates.
We recently envisioned that an efficient and flexible strategy for enantiopure vicinal amino alcohols might be realized by aptly combining merits of the aforementioned SmI2-mediated12–15 and photocatalytic23–28 protocols from nitrones and aldehydes. That is, a photocatalytic protocol featuring with intermolecular single-electron-transfer (SET) can be used to reduce selectively the substrate of higher electron affinity (i.e., higher reduction potential)26,30 and an oxophilic Lewis acid co-catalyst (e.g., rare earth metal cation) can be introduced to bind simultaneously both substrates31,32, and, more importantly, an appropriate chiral ligand to form precursor complex I and to induce desired enantioselectivity likely through a radical-type Zimmerman–Traxler transition state TSII33,34 in the subsequent cross-coupling. Herein we report a synergistic catalysis of chiral N,N′-dioxides (Feng’s ligands)35,36 coordinated rare earth ion and Ru-photocatalyst for enantioselective radical convergent synthesis of enantiopure vicinal hydroxyamino alcohols from nitrones and aromatic aldehydes, together with a mechanism deciphering catalytic cycle and stereoselectivity of this reaction.
Results
Optimization of the reaction conditions
Our investigation into this dual catalysis protocol began by studying the model reaction of nitrone 1a with 4-fluorobenzaldehyde under various conditions (Table 1). Initial experiments revealed the synergistic catalytic effect of Lewis acid and photocatalyst, which led to desired vicinal hydroxyamino alcohol (±)− 2a-1. Among several chiral ligand classes examined (see Supplementary Table 1), Feng’s chiral N,N′-dioxides (such as L1-a and L1-b), which are well-known privileged chiral catalysts37, provided enantioselective results for this reductive coupling reaction (see Supplementary Tables 2 and 3), despite that this class of ligands has not proved to be effective for asymmetric radical reaction before35,36. Meanwhile, we noted that chiral N,N′-dioxides were potentially reduced by this dual-catalyst system owing to the oxidation ability of tertiary amine oxides (see Supplementary Note 1 for details). Fortunately, by optimization of Lewis acids we found that rare earth ions coordinated N,N′-dioxides were stable enough under our conditions to give optically enriched product. PyBOX ligands, which are originally utilized by Yoon and colleagues38 for photocatalyst/chiral Lewis acid dual-activation catalysis, also provided enantioselectivity but only with low ee (such as L2-a and L2-b). Other optimization studies involving chiral ligands, Lewis acids, co-reductants, temperature and solvents were performed. Finally, by using a 65W compact fluorescent lamp (CFL) as the light source, the coupling of nitrone 1a with 4-fluorobenzaldehyde was carried out in the presence of Ru(bpy)3(PF6)2 (2.0 mol%), Sc(OTf)3 (15 mol%), L1-b (18 mol%), and N,N,N′,N′-tetraethylethylenediamine (TEEDA, 4.0 eq) in 1,2-dichloroethane (DCE) at 0 °C for 48 h to offer the desired product 2a-1 in good yield (93% combined yield) with high diastereoselectivity and enantioselectivity (1/12 dr and 92% ee).
Table 1.
Photocatalytic enantioselective synthesis of vicinal hydroxyamino alcohol 2a-1
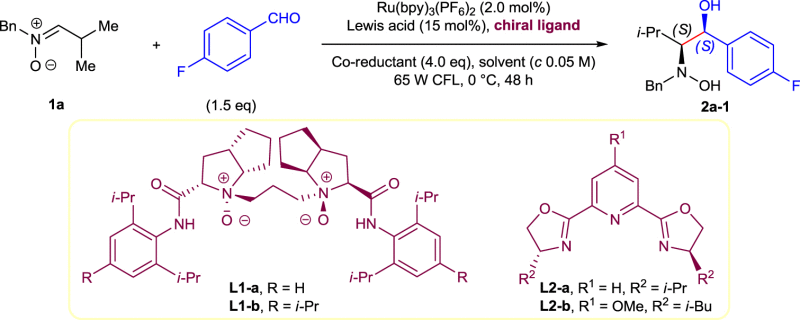
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Lewis acid | Chiral ligand (mmol%) | Co-reductant | Solvent | Yielda (%) | drb | eec (%) |
| 1 | Sc(OTf)3 | L1-a (18) | TEEDA | DCM | 81 | 1/10 | 89 |
| 2 | Sc(OTf)3 | L1-b (18) | TEEDA | DCM | 73 | 1/11 | 92 |
| 3 | Sc(OTf)3 | L1-b (18) | TEEDA | DCE | 93 | 1/12 | 92 |
| 4 | La(OTf)3 | L2-a (30) | DIPEA | CH3CN | 73 | 1/5.7 | − 16 |
| 5 | La(OTf)3 | L2-b (30) | DIPEA | CH3CN | 83 | 1/6.9 | − 24 |
a The reactions were performed on 0.3 mmol scale of nitrone 1a, yields were determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard
b dr values were detected from 1H NMR analysis of crude products (δH 5.53, 4.87 in CDCl3)
c ee values were detected from chiral HPLC analysis of the major diastereo isomer
* bpy, 2,2’-bipyridyl; CFL, compact fluorescent lamp; DCE, 1,2-dichloroethane; DCM, dichloromethane; i-Bu, isobutyl; i-Pr, isopropyl;
* TEEDA, N,N,N’,N’-tetraethylethylenediamine; DIPEA, N,N-diisopropylethylamine
Enantioselective reductive cross-coupling of nitrones with aldehydes
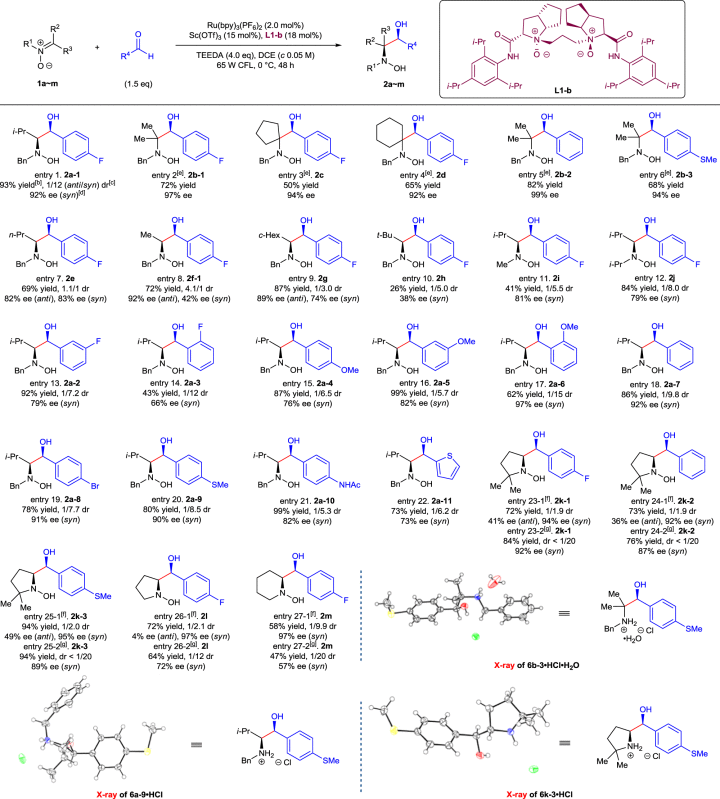
With these optimized conditions in hand, we next examined the scope of nitrones. As illustrated in Table 2, symmetrical ketonitrones 1b to 1d gave the desired vicinal hydroxyamino alcohols with single chiral centers, respectively, in moderate to good yields with excellent enantioselectivity (Table 2, entries 2–6). The absolute configuration of 2b-3 was determined to be (S) by derivatization39 and single-crystal X-ray diffraction analysis (see Supplementary Fig. 2). Notably, as for these symmetrical ketonitrones, dichloromethane (DCM) was better than DCE. Next, we turned our attention to the scope of aldonitrones. Compared with nitrone 1a, more or less steric hindrance of substituents R3 (R2 = H) of nitrones diminished the reaction yields and stereoselectivity (Table 2, entry 1 vs. entries 7–10). The influences of N-alkyl substituent groups of nitrones were also investigated and N-benzyl nitrone 1a provided a better result than N-methyl nitrone 1i and N-isopropyl nitrone 1j (Table 2, entry 1 vs. entries 11 and 12).
Table 2.
Enantioselective reductive cross-coupling of nitrones with aldehydesa
|
a General method: Ru(bpy)3(PF6)2 (2.0 mol%), Sc(OTf)3 (15 mol%), L1-b (18 mol%), DCE (c 0.05 M), 65 W CFL, 0 °C, 48 h
b Isolated yield
c dr values (anti/syn) were detected from 1H NMR or chiral HPLC analysis of crude products
d ee values were detected from chiral HPLC analysis
e DCM was used as solvent
f Modified method 1: Ru(bpy)3(PF6)2 (2.0 mol%), Sc(OTf)3 (15 mol%), L1-a (18 mol%), DIPEA (4.0 eq), DCM (c 0.05 M), 65 W CFL, −5 °C, 48 h
g Modified method 2: Ru(bpy)3(PF6)2 (2.0 mol%), La(OTf)3 (15 mol%), L2-b (30 mol%), TEEDA (4.0 eq), CH3CN (c 0.05 M), 65 W CFL, −10 °C, 72 h
We also explored the scope of aldehydes and found aromatic aldehydes to be excellent partners with nitrone 1a; aliphatic aldehydes, which were compatible with previous SmI2-mediated conditions12, are unavailable for this reaction. As for para-substituted aromatic aldehydes, the more electron-deficient one gave the higher stereoselectivity (Table 2, entries 1, 15, 18 to 21), whereas ortho- and meta-substituted aromatic aldehydes were opposite to this (Table 2, entry 14 vs. entry 17, entry 13 vs. entry 16). Moreover, a series of functional groups including thioether, secondary amide and thiophene were well tolerated in this reaction (Table 2, entries 20–22).
Furthermore, by using L1-a as chiral ligand and N,N-diisopropylethylamine (DIPEA) instead of TEEDA (see Methods, modified method 1), cyclic nitrone (2k to 2m) also can be cross-coupled with aromatic aldehydes in moderate to excellent yields with high enantioselectivity but low diastereoselectivity (Table 2, entries 23–27; 58–94% combined yields, 92–97% ee, 1/1.9–1/9.9 dr). It is noteworthy that, by using a complex of La(OTf)3 with PyBOX ligand L2-b as the chiral Lewis acid (see Methods, modified method 2), reductive cross-coupling of nitrone 1k with aromatic aldehydes can also offer the desired products in good-to-excellent yields with high stereoselectivity (Table 2, entries 23–25; 76–94% combined yield, 87–92% ee, dr <1/20). Nevertheless, this modified condition was not quite compatible with nitrones 1l and 1m. The absolute configuration of vicinal hydroxyamino alcohols 2a-9 and 2k-3 were both determined to be (S,S) by single-crystal X-ray diffraction analysis (see Supplementary Fig. 2). Notably, aromatic ketones can also cross-couple with nitrones smoothly under racemic photocatalytic conditions to produce the desired vicinal hydroxyamino alcohols. However, following the general method or modified methods mentioned in Table 2, only traces of desired products were observed. In addition, aromatic nitrones, such as N-benzylbenzylidene amine oxide (1o, see Supplementary Fig. 3), always provided complex results in this reaction.
Mechanistic investigations
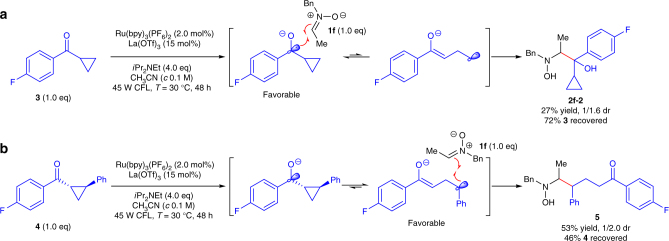
A series of experiments were carried out to get a deep insight into this reaction (see Supplementary Discussion). Control experiments showed no product was formed in the absence of photocatalyst, Lewis acid, amine reductant or light source, thus established that the reaction is synergistically catalyzed by Lewis acid and photocatalyst through a light-driven reductive process. Moreover, a radical mechanism is consistent with the phenomenon that photoreaction was entirely inhibited when 1 equivalent TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) was added to the reaction mixture. We postulated that this photocatalytic reaction, unlike the previous SmI2-mediated reactions, is initiated by the visible light excited SET reduction of aldehydes to ketyl radicals. To verify our hypothesis, radical clock reactions of nitrone 1f under the racemic photocatalytic condition were carried out (Fig. 2). Considering the ring opening of α-cyclopropylbenzyl radical is not a thermodynamic feasible process40 (e.g., with 4-fluorophenyl ketone 3, vicinal hydroxyamino alcohol 2f-2 was obtained in 27% yield as a 1/1.6 (minor/major) mixture of inseparable diastereomers, along with 72% of recovered ketone 3), we designed and synthesized cyclopropyl-containing ketone 4 as a radical clock precursor41. Cyclopropyl opening of ketone 4 followed by cross-coupling with nitrone 1f provided δ-hydroxyamino ketone 5 in 53% yield as a 1/2.0 (minor/major) mixture of diastereomers, along with 46% of recovered ketone 4. Thus, the radical clock reactions proved that our photocatalytic reaction of nitrones with aldehydes is initiated by the SET reduction of aldehydes.
Fig. 2.
Radical clock reactions. a The normal cross-coupling was observed without ring opening product from ketone 3. b The radical clock generated from well-designed radical clock precursor 4 was rearranged and added to nitrone 1f
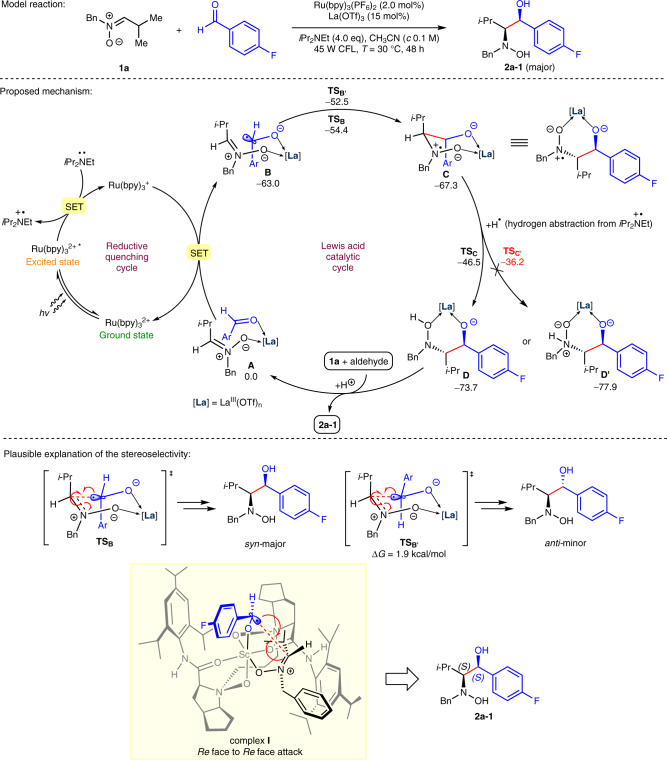
The hypothesis was supported by the cyclic voltammetry studies and density functional theory (DFT) calculations (see Supplementary Fig. 4~6 and 8~11). The coordination of 4-fluorobenzaldehyde with Sc(OTf)3 in CH3CN resulted in a significant reduction in the aldehyde’s half-wave potential which shifted from − 1.86 V to − 0.62 V [vs. saturated calomel electrode (SCE)]. With the fact that nitrone has a higher affinity with Lewis acid (see Supplementary Fig. 7, the reactants’ solubility studies), a complex A of nitrone, aldehyde and Lewis acid was supposed to be a plausible starting point of the reaction, which leads to the proposed mechanism shown in Fig. 3. Photoexcitation and reductive quenching of Ru(bpy)32+ by DIPEA affords [iPr2(Et)N·]+ and Ru(bpy)3+ (E1/2II/I = − 1.33 V vs. SCE in MeCN42,43), which is sufficient to reduce complex A by intermolecular SET (onset potential Eop > −0.5 V vs. SCE) and generate the radical complex B. Indeed, DFT calculations confirmed that the electron affinity of A is much higher (~ 63.0 kcal mol−1 in free energy) than that of nitrone 1a (~ 23.4 kcal mol−1) and 4-fluorobenzaldehyde (~ 45.1 kcal mol−1) in solvent, and the as-generated cross-coupling precursor B has spin density localized predominantly on the aldehyde moiety. Subsequently, N-radical intermediate C (or C′ of anti-configuration) is formed through an analogous 6-endo-trig radical annulation and the transition state TSB leading to a syn-configuration is predicted to be by 1.9 kcal mol−1 favored over the anti-configuration transition state TSB′. C upon hydrogen abstraction from [iPr2(Et)N·]+ affords regioselectively intermediate D (via TSC) other than D′ (via TSC′). Finally, protonation of D gives the desired vicinal hydroxyamino alcohol 2a-1 as a major diastereomer. Moreover, DFT calculations also showed that the formation of cross-coupling precursor B is overwhelmingly favored over the formation of homocoupling precursors, accounting well for the reaction specificity towards cross-coupling rather than homocoupling. On the basis of this mechanism, the diastereoselectivity of vicinal hydroxyamino alcohols, such as 2a-1, can be illustrated through comparing the energy of six-member ring transition state TSB with that of TSB′. The enantioselectivity is revealed by chiral scandium complex I36 involving a Re-to-Re-facial attack of the ketyl radical to nitrone 1a.
Fig. 3.
Proposed mechanism of this photocatalytic enantioselective reductive cross-coupling reaction. Relative Gibbs free energies (ΔG in kcal mol−1 at 298 K) for key intermediates and transition states were computed at the SMD-B3LYP/DZP-level of theory
Concise synthesis of (+)-ephedrine and (−)-selegiline
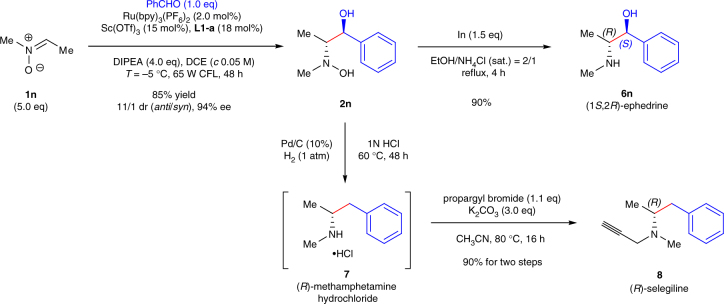
With the dual aim of taking further insight into this reaction and demonstrating the utility of this enantioselective radical protocol, we undertook the synthesis of ephedrine and selegiline (Fig. 4). Following the modified method 1, asymmetric reductive coupling of nitrone 1n with benzaldehyde gave product 2n as the major diastereomer (85% combined yield, 11/1 dr) with 94% ee44. After the indium-mediated reduction39 of 2n, vicinal amino alcohol 6n was obtained in 90% yield. The spectral data of 6n matched those reported of ephedrine [6n•HCl: [α]D20 = +30.6 (c 2.0, H2O); lit.45 for (−)-ephedrine•HCl: [α]D20 = −34.7 (c 5.0, H2O)]. The optical rotation revealed that our synthetic vicinal amino alcohol 6n is (1 S,2 R)-(+)-ephedrine. Although the relative stereochemistry of vicinal hydroxyamino alcohol 2n is different with the results listed in Table 2, this diastereoselectivity is supported by the DFT calculations that revealed the involvement of six-member ring transition state (see Supplementary Fig. 10). Furthermore, dehydroxylation of vicinal hydroxyamino alcohol 2n was carried out in an aqueous solution of HCl under a mild Pd/C-catalyzed hydrogenolysis condition to provide methamphetamine hydrochloride 7. N-propargylation of crude 7 in acetonitrile with K2CO3 afforded (−)-selegiline (8) [[α]D25 = −1.02 (c 1.0, EtOH); lit.46 for 8: [α]D20 = −1.29 (c 6.43, EtOH, >99% ee)] in 90% yield for two steps, which is a medicine used for the treatment of Parkinson’s disease, depression, and senile dementia47.
Fig. 4.
Concise synthesis of (+)-ephedrine 6n and (−)-selegiline 8. A concise two-step synthesis of (1S,2-R)-(+)-ephedrine 6n and an efficient three-step preparation of (R)-(−)-selegiline 8 have been achieved both with 70% overall yield and 94% ee
Discussion
In summary, we demonstrated a photocatalytic enantioselective reductive cross-coupling reaction of nitrones with aromatic aldehydes via the synergistic catalysis of Ru-photocatalyst and chiral N,N′-dioxide ligand-coordinated rare earth ion. In this protocol, chiral Lewis acid represents an indispensable template for assembling to the key intermediate and triggers the asymmetric radical process to afford enantiopure vicinal hydroxyamino alcohols in moderate to excellent yields with high stereoselectivity. Taking advantage of this catalytic mechanism, unavoidable pinacol-type homocoupling side reactions in previous SmI2-mediated system were entirely inhibited. Notably, chiral N,N′-dioxide ligands were used in a radical-mediated system to account for a high level of stereoselectivity. Furthermore, this reaction is operationally simple with a wide array of readily available substrates under mild condition, allowing for the step-economy synthesis of highly valuable enantiopure vicinal amino alcohols (e.g., (1S,2R)-(+)-ephedrine) and amphetamine derivatives (e.g., (R)-(−)-selegiline) rivaling those of industrial biosynthetic procedures48. Based on a deep insight of this reaction, we believed that a foundation has been established for further research and application of these related reactions.
Methods
General
For 1H and 13C NMR spectra of compounds in this manuscript see Supplementary Methods. For details of the synthetic procedures and tables including detail experimental, see Supplementary Methods.
General procedure
An oven-dried 25 ml Schlenk tube equipped with a magnetic stir bar was added nitrone (0.30 mmol, 1.0 eq), L1-b (42.4 mg, 0.054 mmol, 18 mol%), Ru(bpy)3(PF6)2 (5.2 mg, 0.006 mmol, 2.0 mol%), and Sc(OTf)3 (22.1 mg, 0.045 mmol, 15 mol%) in the glove box. When the tube was sealed and removed from the glove box, DCE (or DCM) (6.0 ml) was added, followed by the aldehyde (0.45 mmol, 1.5 eq), and TEEDA (0.26 ml, 1.2 mmol, 4.0 eq). The tube was placed approximately 10 cm away from a 65 W CFL. After being stirred at 0 °C under an argon atmosphere for 48 h, the reaction mixture was filtered through a thin pad of silica gel (100–200 mesh), washed with EtOAc, and concentrated under reduced pressure. The residue was purified by flash chromatography to afford desired vicinal hydroxyamino alcohols 2a to 2j.
Modified method 1
An oven-dried 25 ml Schlenk tube equipped with a magnetic stir bar was added nitrone (0.30 mmol, 1.0 eq), L1-a (37.9 mg, 0.054 mmol, 18 mol%), Ru(bpy)3(PF6)2 (5.2 mg, 0.006 mmol, 2.0 mol%), and Sc(OTf)3 (22.1 mg, 0.045 mmol, 15 mol%) in the glove box. When the tube was sealed and removed from the glove box, DCM (6.0 ml) was added, followed by the aldehyde (0.45 mmol, 1.5 eq), and DIPEA (0.21 ml, 1.2 mmol, 4.0 eq). The tube was placed approximately 10 cm away from a 65 W CFL and the reaction mixture was stirred at − 5 °C under an argon atmosphere for 48 h to afford desired products 2k to 2m.
Modified method 2
An oven-dried 25 ml Schlenk tube equipped with a magnetic stir bar was added nitrone (0.30 mmol, 1.0 eq), L2-b (32.4 mg, 0.09 mmol, 30 mol%), Ru(bpy)3(PF6)2 (5.2 mg, 0.006 mmol, 2.0 mol%), and La(OTf)3 (26.4 mg, 0.045 mmol, 15 mol%) in the glove box. When the tube was sealed and removed from the glove box, CH3CN (6.0 ml) was added, followed by the aldehyde (0.45 mmol, 1.5 eq) and TEEDA (0.26 ml, 1.2 mmol, 4.0 eq). The tube was placed approximately 10 cm away from a 65 W CFL and the reaction mixture was stirred at − 10 °C under an argon atmosphere for 72 h to afford desired products 2k to 2m.
Data availability
The crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC) as CCDC 1537335 (6a-9•HCl), 1537337 (6b-3•HCl), and 1537338 (6k-3•HCl), and can be obtained free of charge from www.ccdc.cam.ac.uk/structures. Any further relevant data are available from the authors upon reasonable request.
Electronic supplementary material
Acknowledgements
We are grateful to the NSF of China (21472157, 21672175, 21332007, 21273177, and 91545105), the Fundamental Research Funds for the Central Universities (No. 20720160048), the NFFTBS (No. J1310024), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) for financial support. We also thank Dr. Xiao-Yu Cao of XMU for kind and helpful discussions on this paper, and Mr. Zi-Ang Nan at iChEM for recrystallization for X-ray quality crystals, X-ray crystallographic analysis with refinement, and valuable discussions.
Author contributions
C.-X.Y. discovered the reaction. C.-X.Y., H.-H.L., and J.-T.C. performed the experiments and analyzed the data. Y.-P.R. and T.-T.Z. performed the chiral HPLC analysis. X.L. and Y.Y.M. performed computational studies on the mechanism. X.Z. and C.-X.Y. designed the project and wrote the manuscript. Feng’s chiral ligand was suggested by P.-Q.H. X.Z., X.L., and P.-Q.H. directed the project and polished the manuscript. All of the authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-017-02698-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiao Zheng, Email: zxiao@xmu.edu.cn.
Xin Lu, Email: xinlu@xmu.edu.cn.
Pei-Qiang Huang, Email: pqhuang@xmu.edu.cn.
References
- 1.Reetz MT. Synthesis and diastereoselective reactions of N,N-dibenzylamino aldehydes and related compounds. Chem. Rev. 1999;99:1121–1162. doi: 10.1021/cr980417b. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeier SC. The synthesis of vicinal amino alcohols. Tetrahedron. 2000;56:2561–2576. doi: 10.1016/S0040-4020(00)00149-6. [DOI] [Google Scholar]
- 3.Klingler FD. Asymmetric hydrogenation of prochiral amino ketones to amino alcohols for pharmaceutical use. Acc. Chem. Res. 2007;40:1367–1376. doi: 10.1021/ar700100e. [DOI] [PubMed] [Google Scholar]
- 4.Métro TX, Duthion B, Pardo DG, Cossy J. Rearrangement of β-amino alcohols via aziridiniums: a review. Chem. Soc. Rev. 2010;39:89–102. doi: 10.1039/B806985A. [DOI] [PubMed] [Google Scholar]
- 5.Weng C, Zhang H, Xiong X, Lu X, Zhou Y. Evolution of epoxides to synthesize β-amino alcohols. Asian J. Chem. 2014;26:3761–3768. [Google Scholar]
- 6.Li G, Chang HT, Sharpless KB. Catalytic asymmetric aminohydroxylation (AA) of olefins. Angew. Chem. Int. Ed. Engl. 1996;35:451–454. doi: 10.1002/anie.199604511. [DOI] [Google Scholar]
- 7.Donohoe TJ, Callens CKA, Flores A, Lacy AR, Rathi AH. Recent developments in methodology for the direct oxyamination of olefins. Chem. Eur. J. 2011;17:58–76. doi: 10.1002/chem.201002323. [DOI] [PubMed] [Google Scholar]
- 8.Sasai, H. in Comprehensive Organic Synthesis II (eds. Knochel P. & Molander G. A.). Ch 2.13 (Elsevier, Amsterdam, 2014).
- 9.Burchak ON, Py S. Reductive cross-coupling reactions (RCCR) between C=N and C=O for β-amino alcohol synthesis. Tetrahedron. 2009;65:7333–7356. doi: 10.1016/j.tet.2009.06.003. [DOI] [Google Scholar]
- 10.Zhong YW, et al. A highly efficient and direct approach for synthesis of enantiopure β-amino alcohols by reductive cross-coupling of chiral N-tert-butanesulfinyl imines with aldehydes. J. Am. Chem. Soc. 2005;127:11956–11957. doi: 10.1021/ja054401w. [DOI] [PubMed] [Google Scholar]
- 11.Lin GQ, Xu MH, Zhong YW, Sun XW. An advance on exploring N-tert-butanesulfinyl imines in asymmetric synthesis of chiral amines. Acc. Chem. Res. 2008;41:831–840. doi: 10.1021/ar7002623. [DOI] [PubMed] [Google Scholar]
- 12.Masson G, Py S, Vallée Y. Samarium diiodide-induced reductive cross-coupling of nitrones with aldehydes and ketones. Angew. Chem. Int. Ed. 2002;41:1772–1775. doi: 10.1002/1521-3773(20020517)41:10<1772::AID-ANIE1772>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Burchak ON, Philouze C, Chavant PY, Py S. A direct and versatile access to α,α-disubstituted 2-pyrrolidinylmethanols by SmI2-mediated reductive coupling. Org. Lett. 2008;10:3021–3023. doi: 10.1021/ol800982n. [DOI] [PubMed] [Google Scholar]
- 14.Wu SF, Zheng X, Ruan YP, Huang PQ. A new approach to 3-hydroxyprolinol derivatives by samarium diiodide-mediated reductive coupling of chiral nitrone with carbonyl compounds. Org. Biomol. Chem. 2009;7:2967–2975. doi: 10.1039/b906224f. [DOI] [PubMed] [Google Scholar]
- 15.Wu SF, Ruan YP, Zheng X, Huang PQ. Samarium diiodide-mediated reductive couplings of chiral nitrones with aldehydes/ketones and acyl chlorides. Tetrahedron. 2010;66:1653–1660. doi: 10.1016/j.tet.2010.01.011. [DOI] [Google Scholar]
- 16.Riber D, Hazell R, Skrydstrup T. Studies on the SmI2-promoted pinacol-type cyclization: synthesis of the hexahydroazepine ring of balanol. J. Org. Chem. 2000;65:5382–5390. doi: 10.1021/jo000538n. [DOI] [PubMed] [Google Scholar]
- 17.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]
- 18.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkinson MN, Sahoo B, Li JL, Glorius F. Dual catalysis sees the light: combining photoredox with organo-, acid, and transition-metal catalysis. Chem. Eur. J. 2014;20:3874–3886. doi: 10.1002/chem.201304823. [DOI] [PubMed] [Google Scholar]
- 20.Beatty JW, Stephenson CRJ. Amine functionalization via oxidative photoredox catalysis: methodology development and complex molecule synthesis. Acc. Chem. Res. 2015;48:1474–1484. doi: 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skubi KL, Blum TR, Yoon TP. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JR, Hu XQ, Lu LQ, Xiao WJ. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016;45:2044–2056. doi: 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- 23.Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J. Am. Chem. Soc. 2013;135:17735–17738. doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Harms K, Meggers E. Enantioselective rhodium/ruthenium photoredox catalysis en route to chiral 1,2-aminoalcohols. Chem. Commun. 2016;52:10183–10186. doi: 10.1039/C6CC04397F. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, et al. Asymmetric radical-radical cross-coupling through visible-light-activated iridium catalysis. Angew. Chem. Int. Ed. 2016;55:685–688. doi: 10.1002/anie.201509524. [DOI] [PubMed] [Google Scholar]
- 26.Fava E, Millet A, Nakajima M, Loescher S, Rueping M. Reductive umpolung of carbonyl derivatives with visible-light photoredox catalysis: direct access to vicinal diamines and amino alcohols via α-amino radicals and ketyl radicals. Angew. Chem. Int. Ed. 2016;55:6776–6779. doi: 10.1002/anie.201511235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hager D, MacMillan DWC. Activation of C−H bonds via the merger of photoredox and organocatalysis: a coupling of benzylic ethers with Schiff bases. J. Am. Chem. Soc. 2014;136:16986–16989. doi: 10.1021/ja5102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musacchio AJ, et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science. 2017;355:727–730. doi: 10.1126/science.aal3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo H, et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature. 2014;515:100–103. doi: 10.1038/nature13892. [DOI] [PubMed] [Google Scholar]
- 30.Lin CW, Hong BC, Chang WC, Lee GH. A new approach to nitrones through cascade reaction of nitro compounds enabled by visible light photoredox catalysis. Org. Lett. 2015;17:2314–2317. doi: 10.1021/acs.orglett.5b00684. [DOI] [PubMed] [Google Scholar]
- 31.Mikami K, Terada M, Matsuzawa H. “Asymmetric” catalysis by lanthanide complexes. Angew. Chem. Int. Ed. 2002;41:3554–3571. doi: 10.1002/1521-3773(20021004)41:19<3554::AID-ANIE3554>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Aspinall HC. Chiral lanthanide complexes: coordination chemistry and applications. Chem. Rev. 2002;102:1807–1850. doi: 10.1021/cr010288q. [DOI] [PubMed] [Google Scholar]
- 33.Lam YH, Grayson MN, Holland MC, Simon A, Houk KN. Theory and modeling of asymmetric catalytic reactions. Acc. Chem. Res. 2016;49:750–762. doi: 10.1021/acs.accounts.6b00006. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman HE, Traxler MD. The stereochemistry of the Ivanov and Reformatsky reactions. I. J. Am. Chem. Soc. 1957;79:1920–1923. doi: 10.1021/ja01565a041. [DOI] [Google Scholar]
- 35.Yu Z, Liu X, Dong Z, Xie M, Feng X. An N,N′-dioxide/In(OTf)3 catalyst for the asymmetric hetero-Diels-Alder reaction between Danishefsky’s dienes and aldehydes: application in the total synthesis of triketide. Angew. Chem. Int. Ed. 2008;47:1308–1311. doi: 10.1002/anie.200704759. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Lin L, Feng X. Chiral N,N′-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 2011;44:574–587. doi: 10.1021/ar200015s. [DOI] [PubMed] [Google Scholar]
- 37.Yoon TP, Jacobsen EN. Privileged chiral catalysts. Science. 2003;299:1691–1693. doi: 10.1126/science.1083622. [DOI] [PubMed] [Google Scholar]
- 38.Espelt LR, McPherson IS, Wiensch EM, Yoon TP. Enantioselective conjugate additions of α-amino radicals via cooperative photoredox and Lewis acid catalysis. J. Am. Chem. Soc. 2015;137:2452–2455. doi: 10.1021/ja512746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cicchi S, Bonanni M, Cardona F, Revuelta J, Goti A. Indium-mediated reduction of hydroxylamines to amines. Org. Lett. 2003;5:1773–1776. doi: 10.1021/ol034434l. [DOI] [PubMed] [Google Scholar]
- 40.Bowry VW, Lusztyk J, Ingold KU. Evidence for reversible ring-opening of the α-cyclopropylbenzyl radical. J. Chem. Soc. Chem. Commun. 1990;26:923–925. doi: 10.1039/C39900000923. [DOI] [Google Scholar]
- 41.Xu J, Samsuri NB, Duong HA. Nickel-catalysed cyclopropanation of electron-deficient alkenes with diiodomethane and diethylzinc. Chem. Commun. 2016;52:3372–3375. doi: 10.1039/C5CC10296K. [DOI] [PubMed] [Google Scholar]
- 42.Juris A, Balzani V, Belser P, von Zelewsky A. Characterization of the excited state properties of some new photosensitizers of the ruthenium (polypyridine) family. Helv. Chim. Acta. 1981;64:2175–2182. doi: 10.1002/hlca.19810640723. [DOI] [Google Scholar]
- 43.Kalyanasundaram K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982;46:159–244. doi: 10.1016/0010-8545(82)85003-0. [DOI] [Google Scholar]
- 44.O’Neil IA, et al. The synthesis and structure of chiral enamine N-oxides. Chem. Commun. 2014;50:7336–7339. doi: 10.1039/c3cc47928e. [DOI] [PubMed] [Google Scholar]
- 45.Raffa RJ, Stern MJ, Malspeis L. Thermometric titration determination of ΔH°, ΔG°, and ΔS° of dissociation of ephedrinium and pseudoephedrinium ions. Anal. Chem. 1968;40:70–77. doi: 10.1021/ac60257a018. [DOI] [Google Scholar]
- 46.Bornholdt J, Felding J, Clausen RP, Kristensen JL. Ring opening of pymisyl-protected aziridines with organocuprates. Chem. Eur. J. 2010;16:12474–12480. doi: 10.1002/chem.201001026. [DOI] [PubMed] [Google Scholar]
- 47.Riederer P, Lachenmayer L, Laux G. Clinical applications of MAO-inhibitors. Curr. Med. Chem. 2004;11:2033–2043. doi: 10.2174/0929867043364775. [DOI] [PubMed] [Google Scholar]
- 48.Panke S, Wubbolts M. Advances in biocatalytic synthesis of pharmaceutical intermediates. Curr. Opin. Chem. Biol. 2005;9:188–194. doi: 10.1016/j.cbpa.2005.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC) as CCDC 1537335 (6a-9•HCl), 1537337 (6b-3•HCl), and 1537338 (6k-3•HCl), and can be obtained free of charge from www.ccdc.cam.ac.uk/structures. Any further relevant data are available from the authors upon reasonable request.