Abstract
Neurons in the paraventricular nucleus of the hypothalamus (PVN) integrate peripheral signals and coordinate responses that maintain numerous homeostatic functions. An excess of glucocorticoids during fetal development results in long-lasting consequences tied to disrupted PVN development. The PVN contains a distinct neuronal population and a threefold greater vascular density than the surrounding brain regions that prepubertally is reduced in offspring exposed to excess glucocorticoids in utero. This study expands the examination of sex-specific nonneuronal PVN composition by examining astrocytes, astrocytic endfeet, and pericytes. Blood-brain barrier (BBB) competency and composition were examined along with depressive-like behavior and hypothalamic-pituitary-adrenal function in male and female mice. For PVN vasculature, female offspring of vehicle (veh)-treated mothers had significantly more astrocytes and pericytes than male offspring from the same litters. Female offspring from dexamethasone (dex)-treated mothers had significantly lower levels of astrocytes than female offspring from veh-treated mothers, whereas male offspring from dex-treated mothers had greater levels of pericytes compared with veh-treated male offspring. Using the tail-suspension test, male and female offspring from dex-treated mothers had significantly shorter latencies to immobility, indicating an increase in depression-like behavior, and showed greater plasma corticosterone after restraint stress, which was significantly greater in female offspring from dex-treated mothers even after recovery. Therefore, in addition to long-term sex differences in cellular components of the BBB in the PVN that were differentially regulated by fetal glucocorticoid exposure, there were behavioral differences observed into early adulthood in a sex-specific manner.
Keywords: astrocytes, glucocorticoids, HPA-axis, paraventricular nucleus of the hypothalamus, pericytes
Sex-specific differences are seen in blood-brain barrier components in the paraventricular nucleus of the hypothalamic in offspring of dexamethasone-treated pregnant female mice.
During normal fetal development, there is a continuous rise in endogenous glucocorticoids necessary for initiating gene networks critical for organ maturation. Early and excess exposure to glucocorticoids during fetal development (e.g., via prenatal stress or administration of synthetic glucocorticoids to mothers at risk for preterm delivery) affects gene regulation and can have long-lasting physiological and behavioral consequences. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is important in the association of adult diseases and disorders stemming from disrupted fetal development. For example, children prenatally exposed to the synthetic glucocorticoid dexamethasone (dex) display disrupted HPA axis output in childhood [1]. Furthermore, when examining both male and female children exposed during fetal development to glucocorticoids who were born at term (eliminating premature birth as a variable), there was a female-specific increase in plasma cortisol after a mild psychosocial stressor [2]. Additionally, glucocorticoids and the HPA axis have been linked to the development of major depressive disorder [3]. These studies demonstrate that fetal glucocorticoid expose disrupts the HPA axis, more frequently in female than in male individuals, but the mechanisms are not understood.
In response to physical or emotional stress, neuroendocrine cells within the paraventricular nucleus of the hypothalamus (PVN) release corticotropin-releasing hormone/factor and arginine vasopressin, which stimulates cells within the anterior pituitary to secrete adrenocorticotropin hormone. Circulating adrenocorticotropin hormone acts on specific steroidogenic cells within the adrenal gland to stimulate the release of glucocorticoids [4, 5] and is terminated by glucocorticoid activation of the glucocorticoid receptor at the level of the PVN and pituitary [4–6]. The PVN is a sexually dimorphic brain region that integrates peripheral signals to regulate many important functions that include initiating flight-or-fight responses and maintaining homeostasis, vasomotor tone, and energy balance [7–10]. In addition to its dense neuronal population, the PVN has a unique vasculature, which contains a notably higher blood vessel density than the surrounding brain regions [11] that develops postnatally in rats and mice [12–14]. In mice, the highest vascular density is localized to the rostral two-thirds of the PVN [13], which coincides with the majority of neuroendocrine neurons (i.e., corticotropin-releasing hormone/factor and arginine vasopressin) [15]. The roles of this dense vascular bed are unknown and have been studied only in male mice, supporting the need for future studies to include female subjects to determine sex-specific effects. Additionally, exposure to excess glucocorticoids during fetal development can disrupt the development of this unique PVN vasculature as observed by decreased blood vessel density in prepubertal male and female mice [14].
One characteristic that sets brain blood vessels apart from the periphery is a selective barrier referred to as the blood-brain barrier (BBB). The BBB regulates a microenvironment necessary for reliable neuronal signaling by protecting the brain from potentially harmful molecules and regulating access to peripheral signals. The BBB consists of a continuous layer of endothelial cells, which form the walls of blood vessels and which are linked to each other with tight junctions (reviewed in Refs. 16–19). Within the PVN, excess glucocorticoids during fetal development result in an entrance of circulating low-molecular-weight molecules in prepubertal mice [14].
Other key cell types important for BBB competency include astrocytes and pericytes. Astrocytes are glial cells that arise during postnatal development [20] and can detect and modulate neuronal activity and blood vessel function through regulation of endothelial cell junctions and transport (reviewed in Ref. 21). Astrocytic endfeet in close proximity to microvessel walls are specialized and are important for maintaining ion and volume regulation [16]. Pericytes play a role in tight junction formation, structural stability, and angiogenesis [22, 23] and regulate vascular stability [24]. Pericytes have contractile properties that can regulate capillary diameter and blood flow through their finger-like processes that ensheath capillary walls [18, 24]. During postnatal development, pericytes increase their coverage of blood vessels and have more presence and coverage of blood vessels specifically within the PVN compared with other brain regions [14], further supporting the unique vasculature of the PVN. Exposure to excess glucocorticoids during fetal development has resulted in an increase in pericyte coverage in the germinal matrix of rabbits and humans [25] and within the PVN of prepubertal male and female mice [14]. Currently, little is known regarding specific locations within the brain or sex for astrocytes and pericytes and how they are affected by fetal glucocorticoid exposure. Because the PVN regulates numerous critical and homeostatic functions and is known to have a unique vasculature, we examined blood vessel density, BBB competency, astrocytes, pericytes, and HPA axis output in adult male and female mice with and without fetal excess glucocorticoid exposure.
1. Materials and Methods
A. Animals
Mice from an inbred FVB/N background were maintained in plastic cages with aspen bedding (autoclaved Sani-chips; Harlan Teklad, Madison, WI) in the Painter Building of Laboratory Animal Resources at Colorado State University. Food (Harlan Teklad, Madison, WI) with filtered tap water and an environmental enrichment of paper napkins was provided ad libitum in a 14/10 hour light/dark cycle, similar to previous studies [13, 14, 26, 27].
Mice were mated overnight, and the day of a visible plug was designated as embryonic day (E)0. Pregnant dams were injected subcutaneously with the glucocorticoid receptor agonist dex (0.1 mg/kg; Sigma-Aldrich, St. Louis, MO) or phosphate-buffered saline (PBS) as vehicle (veh) once daily from E11 to E17 [14, 28], which corresponds with neurogenesis within the developing hypothalamus [20]. The day of birth was designated postnatal day (P)0. Mice were weaned and ear punched for identification on P19 and then left relatively undisturbed until P50 when they were subjected to behavioral tests followed by tissue collection. To avoid litter effects, the data presented originated from four dex-treated and three veh-treated litters.
B. Ethics Statement
All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approval of the Colorado State University Institutional Animal Care and Use Committee.
C. Behavior Testing
All mice were handled for 2 weeks before the start of testing and for at least 2 days before each behavioral test. To minimize potential hormone-dependent variability, female mice were tested only during estrus, which was identified by cornified epithelial cells in the vaginal cytology assessed using vaginal smears. Behavior testing began in early adulthood (after P50, when female mice were cycling), and all mice were exposed to the same behavior test within a 10-day window to ensure female mice were in estrus and tested with experimental male mice all before P75. Testing occurred during the same 4-hour window between 11:00 am to 3:00 pm in the light phase. The order of tests was: open field test, tail suspension test (TST), and the restraint stress paradigm, with a 4-day rest period between behavior tests. For the TST, mice were suspended by their tail to a horizontal rod with adhesive tape at a height of 40 cm for 6 minutes [27], and time to first bout of immobility was determined as an indication of depression-like and helpless-like despair behavior [29, 30]. Animals that climbed their tail were excluded from the analysis. During each testing day, the order of animal testing (i.e., sex and treatment) was randomized, and at least one male from each treatment (veh and dex) was tested on the same day with female mice in estrus. All testing apparatuses were washed once with 10% ethanol solution and once with water and were dried between animals. The same investigator conducted all tests, was careful to be free of any fragrances, and remained in an outer anteroom while the animal was left alone in the testing room. Behavior was video recorded using a Sony HD Handicam and analyzed by an individual blinded to treatment and sex using the Stopwatch+ program (Center for Behavioral Neuroscience, Atlanta, GA).
To determine if offspring from dex-treated mothers showed altered HPA axis response to stress in adulthood, corticosterone levels were assayed from serum samples before and after restraint stress [27]. Blood was collected into heparinized tubes from the tail at the initiation restraint stress (time 0; completed within 3 minutes of initial handling), after 30 minutes of restraint (time 30), and 60 minutes after release from restraint (time 90) just before perfusion fixation. Animals were anesthetized with ketamine (80 mg/kg) and xylazine (8 mg/kg) 5 minutes before (85 minutes after restraint) the final blood collection and transcardially perfused at 4 mL/min with heparanized PBS (pH 7.4) containing fluorescein isothiocyanate (FITC) (MW 389.4; Thermo Fisher Scientific, Waltham, MA) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed, post fixed overnight, and stored in 0.1 M phosphate buffer at 4°C. Samples were centrifuged at 1300 relative centrifugal force for 10 minutes, and serum was transferred to a new tube and stored at −80°C until use.
D. Immunohistochemistry
Tissue was processed as previously described [13, 14, 26]. Brains were embedded in 5% agarose and cut coronally into 50-μm-thick sections using a vibrating microtome (VT1000S; Leica Biosystems, Buffalo Grove, IL). Free-floating serial sections were collected in 0.05 M PBS (pH 7.4). Unreacted aldehydes were neutralized in 0.1 M glycine for 30 minutes followed by 0.5% sodium borohydride for 15 minutes. Sections were washed in PBS and incubated in a blocking solution [5% normal goat serum (NGS), 0.5% Triton X-100 (Tx), and 1% hydrogen peroxide in PBS] for at least 30 minutes. Sections were then incubated in primary antisera directed against Desmin (1:200, RRID: AB_2335684; M0760, Dako, Santa Clara, CA) or GFAP (1:250, RRID: AB_10013382; Z0334, Dako) in 1% bovine serum albumin and 0.5% Tx. Desmin was selected as a specific pericyte marker because antibodies against PDGFβ produced immunoreactivity in additional cell types within the mouse brain. For desmin, sections were processed for antigen retrieval [13, 14]. In place of the standard processing steps before antisera application, sections immunolabeled for Desmin were washed in room temperature PBS for 15 minutes followed by a 1-hour wash in sodium citrate (0.05 M, pH 8.6) and placed in sodium citrate buffer preheated to 80°C for 30 minutes. They were allowed to slowly come back to room temperature (∼30 to 35 minutes) and were returned to PBS for an additional 15 minutes of washes. All sections were incubated over two nights at 4°C in primary antisera. Sections were then washed at room temperature with 1% NGS and 0.02% Tx in PBS followed by incubation with the appropriate secondary antibodies for 2 hours using either Cy3-conjugated anti-rabbit (1:200, RRID: AB_2307443; Jackson ImmunoResearch, West Grove, PA) or Cy3-conjugated anti-mouse (1:200, RRID: AB_2340811; Jackson ImmunoResearch) antibodies in PBS containing 1% NGS and 0.32% Tx.
E. Radioimmunoassay
Restraint stress was initiated by placing mice in a 50-mL plastic conical tube. Blood was collected via the tail vain at the start of restraint stress, after 30 minutes of restraint stress, and after 60 minutes of recovery. Plasma corticosterone concentrations were measured by radioimmunoassay. Plasma samples were diluted 1:25 in 0.01 M PBS, and corticosterone-binding globulin was denatured by incubating the samples at 65°C for 1 hour. Samples and standards were incubated overnight at 4°C in the presence of antiserum (MP Biomedicals, Solon, OH) and H3 corticosterone (Perkin-Elmer, Boston, MA) in 0.1% gelatin dissolved in 0.01 M PBS. Unbound corticosterone was removed by adding dextran-coated charcoal, which was then separated by centrifugation. Bound corticosterone was decanted into new vials, mixed with scintillation fluid, and counted using a Multipurpose Scintillation Counter (LS6500; Beckman Coulter, Brea, CA). Plasma corticosterone concentrations were determined by comparison with a standard curve (5 to 700 ng/mL). The intra-assay limits of detection ranged from 2.24 to 85.1 µg/dL and were determined by an internal control that was measured at regular intervals throughout the assay.
F. Analysis
FITC, immunoreactive (ir)-desmin–positive pericytes, and ir-GFAP–positive astrocytes images were acquired for the PVN and for two control regions [lateral hypothalamus (LH) and cerebral cortex (CTX)] on a Zeiss 510-Meta laser-scanning confocal microscope (Zeiss, Oberkochen, Germany). FITC was imaged using a 488/543 nm bandpass filter and emission detected using a 505/530 nm bandpass emission filter. Cy3 for Desmin and GFAP was imaged using a 488/543-nm bandpass filter and emission detected using a 585/615-nm bandpass emission filter. Z-stacks were acquired with six optical sections taken every 3 μm obtained at a magnification of ×400 using a ×40 oil immersion objective. The section with the densest vascular network was selected by an investigator blind to treatment group for each PVN region (rostral, mid, caudal) for analysis [13, 14]. To view the vascular network within the brain, Z-stacks were compiled.
Blood vessel density, width, and extravascular leakage were determined as previously described [14]. Images were inverted using Photoshop, light corrected using ImageJ (version 1.43u), and analyzed for length, as a measure of density, using Angiogenesis Tube Formation (Metamorph Software, version 7.7.0.0; Molecular Devices, Inc., Sunnyvale, CA). Extravascular leakage was analyzed using open-source CellProfiler (available from the Broad Institute at www.cellprofiler.org). Blood vessels were identified, and a 10-pixel expansion was mapped from each blood vessel to create a mask to quantify leakage. This value was divided by FITC measured within blood vessels to account for differences in perfusion quality. Total ir-desmin and ir-GFAP were measured for area of immunoreactivity and normalized to blood vessel area using Metamorph software. For astrocytes in proximity to FITC-labeled blood vessels, confocal stacks were merged, and FITC-labeled blood vessels and astrocytes were independently threshold, converted to binary, and multiplied together. Areas that overlapped were quantified (ImageJ, NIH, Bethesda, MD). Statistical significance was determined for immunohistochemistry by analysis of variance as sex (male vs female) × treatment (veh vs dex) × region (rostral vs mid vs caudal) as a repeated measures, and seconds in the TST test and corticosterone in the stress test were analyzed by two-way analysis of variance for sex (male vs female) × treatment (veh vs dex) using SPSS software (SPSS Inc., Chicago, IL). Values are reported as mean ± standard error of the mean, and P < 0.05 was considered significant. Animals were derived from multiple litters. Representative images for figures were normalized for optimal contrast in Adobe Photoshop (version CS for Macintosh, Adobe, San Jose, CA).
All mice underwent the same behavioral tests and tissue collection (cohorts 1 and 2). Results presented for behavioral testing, immunohistochemistry, and BBB permeability are from cohort 1. For corticosterone levels, data were collected from both cohorts, although there were more samples from cohort 2. The female-specific dex-treated increase in corticosterone levels was observed in both cohorts.
2. Results
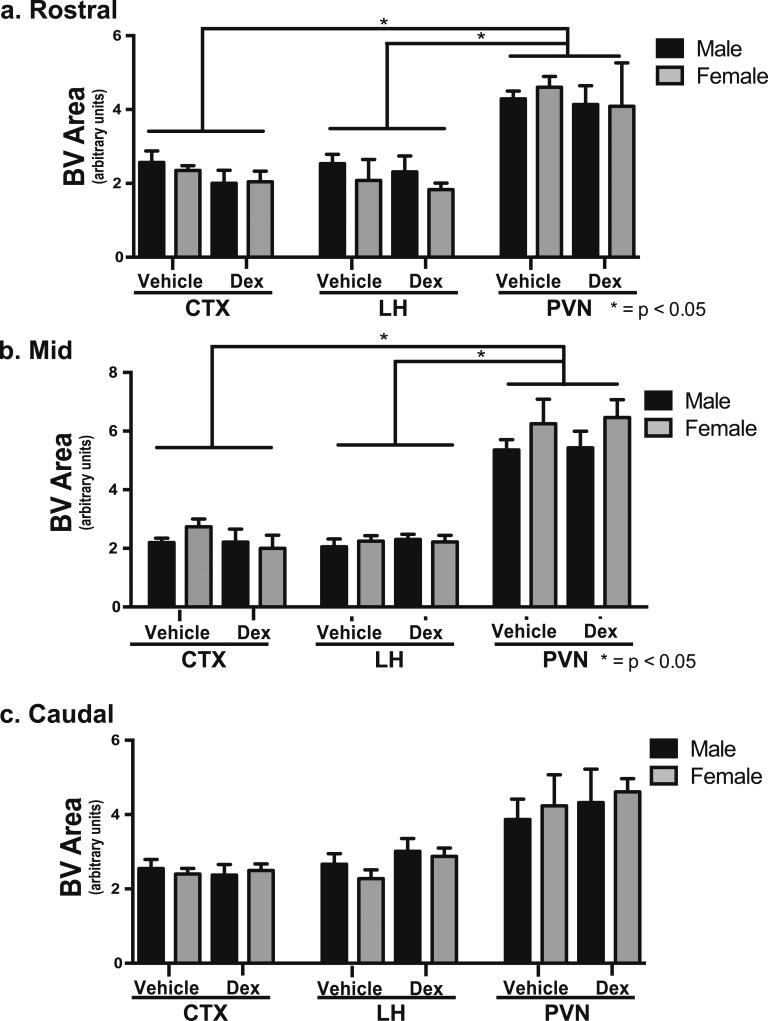
Male and female offspring of veh- and dex-treated mothers were examined for blood vessel density in the CTX, LH, and PVN. There was significantly greater blood vessel density in the rostral (Fig. 1a) and mid (Fig. 1b) regions of the PVN compared with the CTX or LH in male and female offspring of veh- and dex-treated mothers [F(1,13) = 9.02, P < 0.01]. There were no discernible differences observed in blood vessel area in any brain region examined in the offspring of dex-treated mothers in early adulthood [F(1,13) = 0.02, P > 0.50] (Fig. 1). These findings suggest that the decreased vascular density previously observed before puberty in offspring from dex-treated mothers [14] may have resulted from delaying the postnatal angiogenic period within the PVN.
Figure 1.
Fetal exposure to Dex did not affect blood vessel (BV) density within mouse PVN in early adulthood. The rostral and mid PVN has significantly more BV area than the CTX or LH. There were no differences due to dex treatment or for sex observed in the rostral, mid, or caudal area of the PVN (n = 6 vehicle male, n = 4 vehicle female, n = 5 dex male, n = 6 dex female).
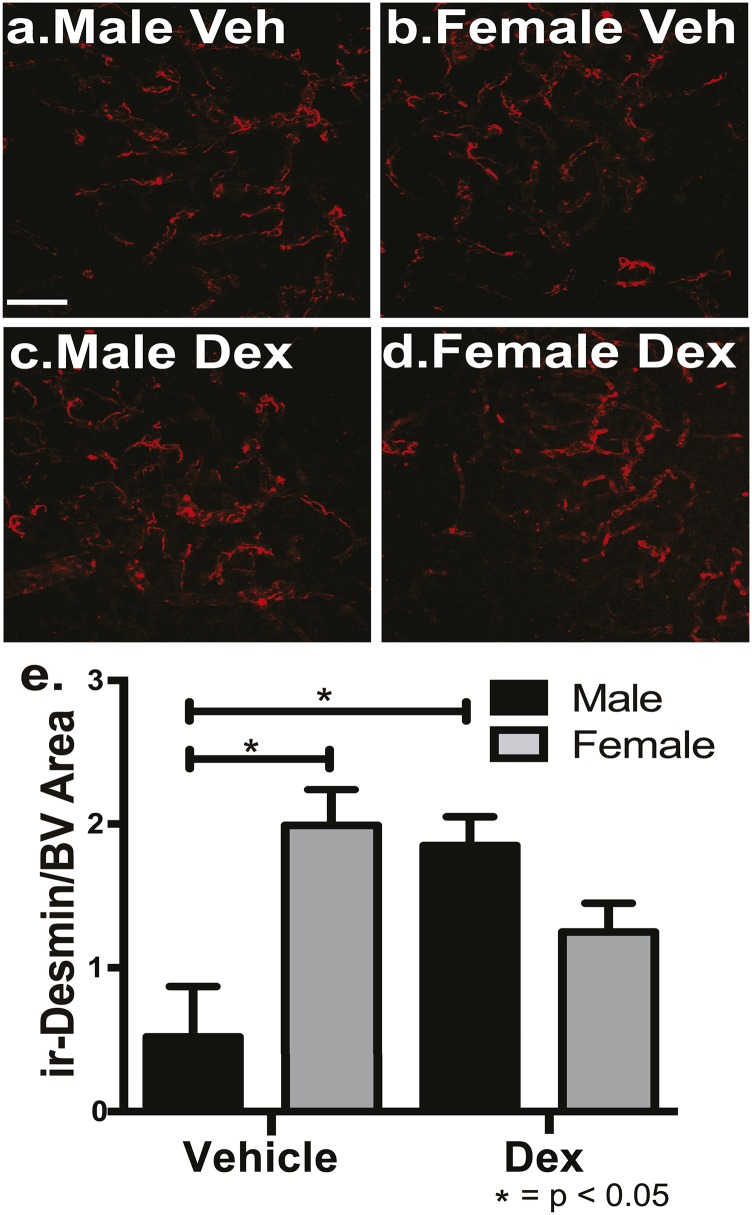
To determine if there are sex-specific differences in BBB components that are differentially affected by glucocorticoid exposure during fetal development, astrocytes and pericytes were examined in early adult male and female offspring from veh- and dex-treated mothers. Pericytes were measured using ir-desmin as a marker in male and female offspring of veh- and dex-treated mothers. There was a significant interaction for sex (male vs females) and treatment [veh vs dex; F(1,19) = 4.91, P < 0.04]. Post hoc analysis showed there was significantly more pericytes in female offspring compared with male offspring from veh-treated mothers within the PVN (P < 0.05) (Fig. 2a, 2b, 2e). For dex treatment, male offspring from dex-treated mothers had significantly greater levels of pericytes coverage compared with male offspring from veh-treated mothers (P < 0.05) (Fig. 2a, 2c, 2e). There was a strong trend for female offspring of dex-treated mothers to have less pericyte coverage compared with female offspring from veh-treated mothers (P < 0.07) (Fig. 2b, 2d, 2e). There were no significant differences observed in the CTX or LH due to sex or treatment of pericyte coverage (P = 0.50). Thus, there are sex-specific, dex-dependent, and region-specific differences in pericyte coverage.
Figure 2.
Female mice have significantly more ir-desmin–positive pericytes compared with male mice within the mouse PVN, and fetal exposure to dex increased desmin–positive pericytes coverage only in the male PVN in early adulthood. There were significantly more pericytes in veh-treated female mice (b, e) compared with veh-treated male mice (a, e) (P < 0.05) within the entire PVN. There was a significant increase in total pericytes in dex-treated male mice (c, e) compared with veh-treated male mice (a, e) (P < 0.05; n = 6 vehicle male, n = 4 vehicle female, n = 5 dex male, n = 6 dex female). Scale bar, 50 µm.
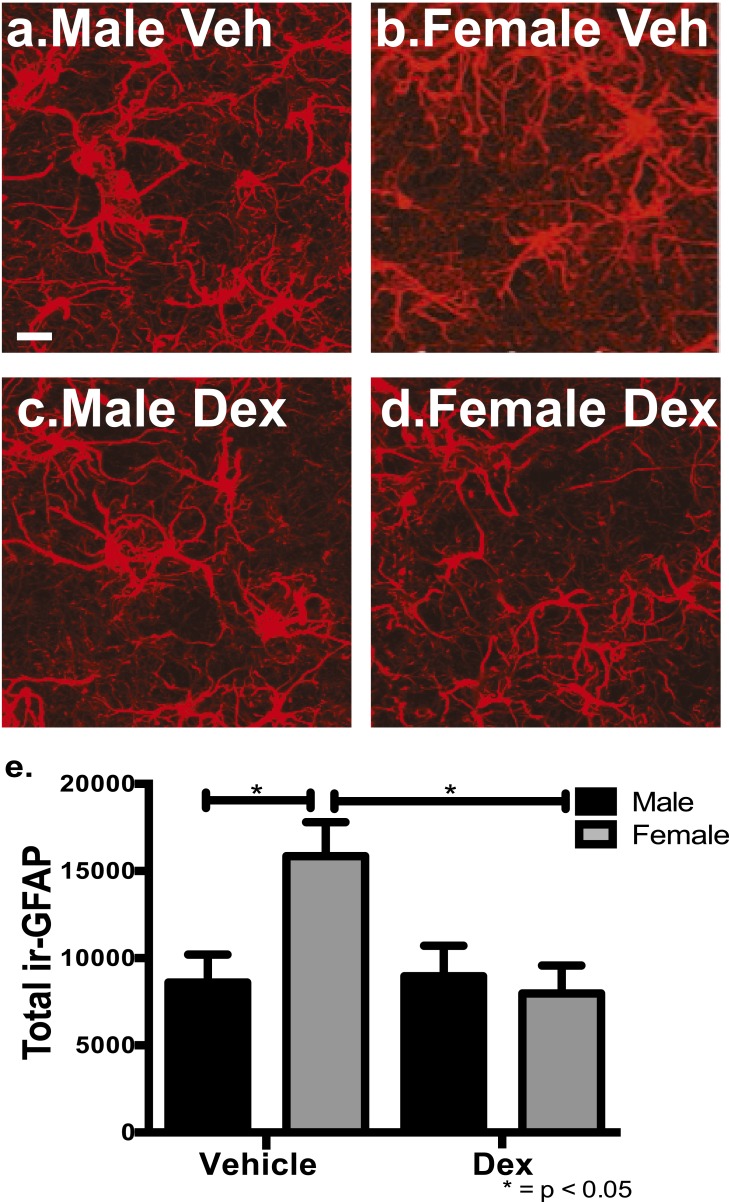
Astrocytes were examined using ir-GFAP as a marker in male and female offspring of veh- and dex-treated mothers in early adulthood. For total astrocytes, there was a significant difference for treatment [dex vs veh; F(1,17) = 4.72, P < 0.04] and for treatment × sex [male vs female; F(1,17) = 5.67, P < 0.03]. Post hoc analysis showed there were significantly more astrocytes in female offspring of veh-treated mothers compared with littermate male offspring within the PVN (P < 0.05) (Fig 3a, 3b, 3e). When mothers were treated with dex, there were significantly fewer astrocytes within the entire PVN in the female offspring (Fig. 3d, 3e) compared with female offspring of veh-treated mothers (P < 0.05) (Fig. 3b, 3e). There were no significant differences observed in the CTX or LH due to sex or treatment of total astrocytes. These results demonstrate that there are sex-specific differences in astrocytes in the PVN between male and female mice, and excess glucocorticoids during fetal development decreased that presence in young adult female mice.
Figure 3.
Female mice have significantly more total ir-GFAP astrocytes compared with male mice within the mouse PVN, and fetal exposure to dex decreased astrocytes only in the female PVN in early adulthood. There were significantly more total astrocytes in veh-treated female mice (b) compared with veh-treated male mice (a) (P < 0.05). There was a significant decrease in total astrocytes in the mid region of the PVN in dex-treated female mice (d, e) compared with veh-treated female mice (b, e) (P < 0.05; n = 6 vehicle male, n = 4 vehicle female, n = 5 dex male, n = 6 dex female). Scale bar, 30 µm.
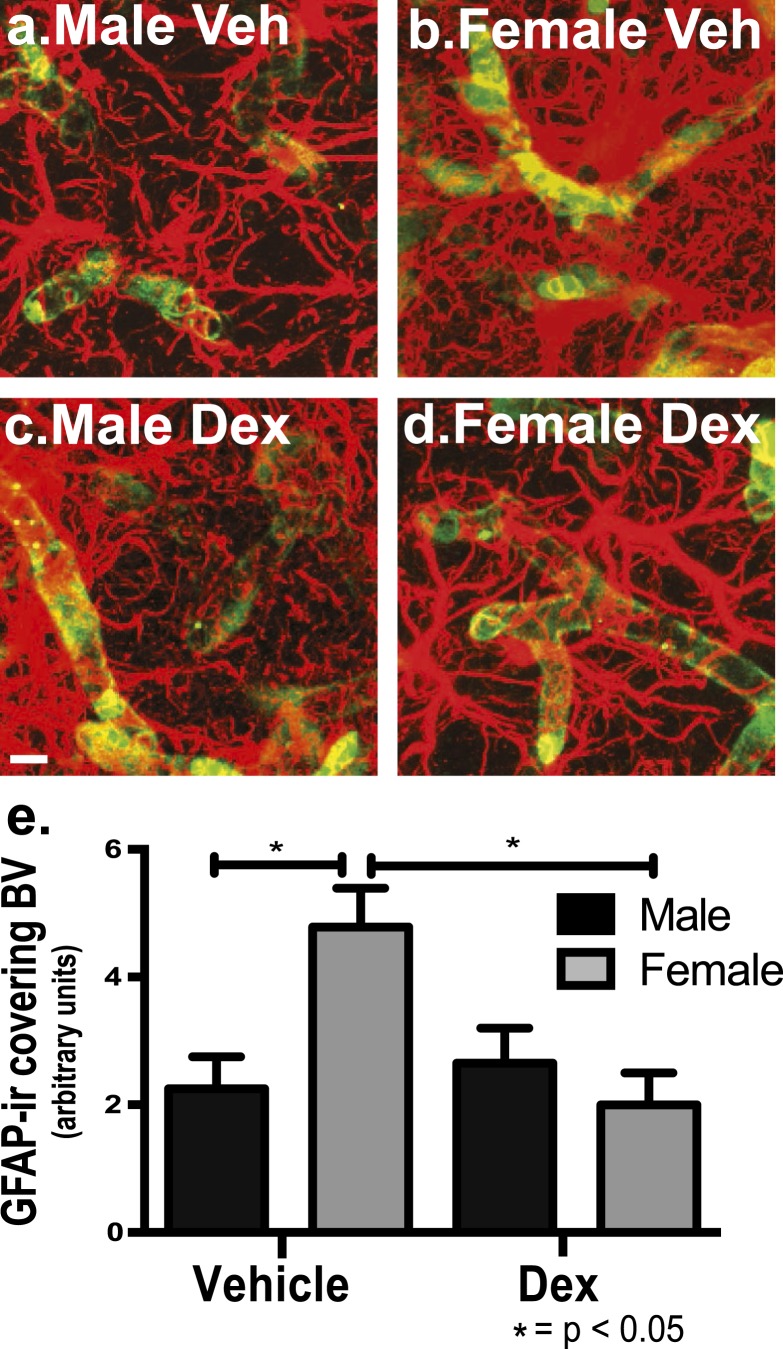
Because there was a difference in total astrocyte presence, the next step was to examine astrocytic endfeet coverage. There were sex differences that were differentially affected by excess fetal glucocorticoid exposure for putative astrocytic end-feet coverage of blood vessels within the mid, most dense vascular region of the PVN in early adulthood. Astrocytes were quantified when there was optical overlap with blood vessels as a measure of astrocyte proximity to blood vessels within the entire PVN. For total astrocytes optically overlapping blood vessels, there was a significant difference for treatment [dex vs veh; F(1,19) = 4.09, P < 0.05] and for treatment × sex [male vs female; F(1,19) = 6.41, P < 0.01]. Post hoc analysis showed there were significantly more ir-GFAP–positive astrocytes in female offspring compared with male offspring of veh-treated mothers in the mid region of the PVN (P < 0.05) (Fig. 4a, 4e). For dex-treated offspring, there was a significant decrease in female offspring of dex-treated mothers for astrocytes covering blood vessels within the mid region of the PVN (Fig. 4d, 4e) compared with female offspring of veh-treated mothers (P < 0.05) (Fig. 4b, 4e).
Figure 4.
Female mice have significantly more ir-GFAP astrocytes surrounding blood vessels compared with male mice within the mouse PVN, and fetal exposure to dex decreased astrocytes surrounding blood vessels only in the female PVN in early adulthood. There were significantly more astrocytes in proximity to blood vessels in veh-treated female mice (b, e) compared with veh-treated male mice (a, e) (P < 0.05). There was a significant decrease in astrocytes surrounding blood vessels in dex-treated female mice (d, e) compared with veh-treated female mice (b, e) (P < 0.05; n = 6 vehicle male, n = 4 vehicle female, n = 5 dex male, n = 6 dex female). For presentation purposes, the images are saturated to better bring out the overlap in the compartments. Scale bar, 50 µm.
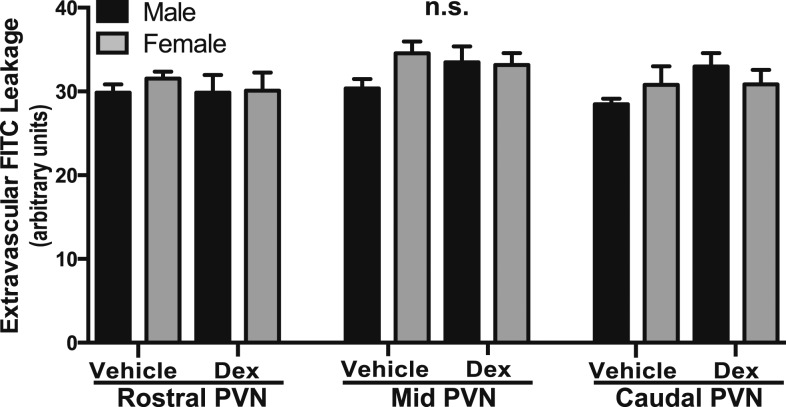
The loss of BBB competency previously observed in the mid PVN before puberty in dex-treated offspring [14] was not observed in the older mice of this study. The presence of extravascular FITC dye leakage was quantified. Despite the alterations in BBB components, there were no differences in dye leakage observed due to sex or treatment in the CTX (Fig. 5a), LH (Fig. 5b), or PVN (Fig. 5c) in early adulthood.
Figure 5.
Fetal exposure to dex did not affect BBB competency in the mouse PVN in early adulthood. There were no statistically significant differences observed in the rostral, mid, or caudal regions of the PVN or in the lateral hypothalamus or cortex due to sex or treatment (n = 6 vehicle male, n = 4 vehicle female, n = 5 dex male, n = 6 dex female).
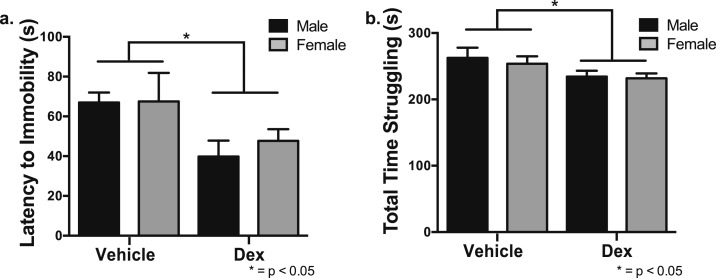
Excess glucocorticoids during fetal development resulted in an increase in helplessness- and depression-like behaviors compared with veh-treated mice in adulthood. Male and female offspring from veh- and dex-treated mothers were examined for depression-like behavior using the TST and HPA axis activation after 30 minutes of restraint stress. For the TST, there was a significant decrease in the time until the first bout of immobility in male and female offspring from dex-treated mothers compared with veh-treated mothers [F(1,20) = 8.45, P < 0.01] (Fig. 6a). Dex-treated offspring also spent significantly less time struggling compared with veh-treated offspring [F(1,22) = 4.25, P < 0.05] (Fig. 6b).
Figure 6.
Dex-treated offspring displayed an increase in depression-like behavior using the TST. (a) Results show both male and female offspring of dex-treated pregnant mice relative to vehicle treated had a significantly decreased latency to their first display of immobility (P < 0.05). (b) Dex-treated mice also spent significantly less time struggling compared with veh-treated mice (P < 0.05; n = 8 vehicle male, n = 6 vehicle female, n = 6 dex male, n = 6 dex female).
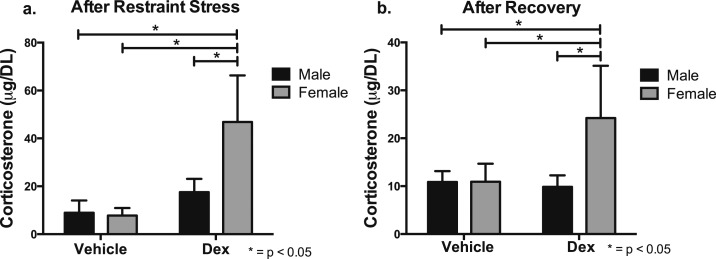
There were increases in HPA axis output in young adult female offspring of dex-treated mothers. Serum corticosterone levels were measured prior to restraint stress, after 30 minutes of restraint stress, and after 1 hour of recovery. Initially, there were no differences in corticosterone levels observed at baseline for sex or treatment (time 0). However, after 30 minutes of restraint stress, there was a significant increase in corticosterone levels in all mice and a heightened response observed in dex-treated female mice compared with veh-treated female, veh-treated male, and dex-treated male offspring [F(3, 41) = 18.75, P < 0.0001] (Fig. 7a). After being returned to their home cage for a recovery period of 60 minutes, there were significantly higher corticosterone levels in dex-treated female offspring compared with veh-treated female, veh-treated male, and dex-treated male offspring [F(3, 41) = 21.76, P < 0.0001] (Fig. 7b).
Figure 7.
Dex-treated female offspring display an increase in HPA axis output after restraint stress and recovery. After 30 minutes of restraint stress, all mice had significantly elevated levels of corticosterone compared with baseline. (a) After 30 minutes of restraint stress, dex-treated female offspring had increased plasma corticosterone relative to veh-treated female, veh-treated male, and dex-treated female offspring. *P < 0.05. (b) This effect was maintained out to 60 minutes recovery specifically in dex-treated female offspring. *P < 0.05 (n = 4 vehicle male, n = 4 vehicle female, n = 4 dex male, n = 5 dex female).
3. Discussion
The PVN has been known for some time to have a denser vascular network than the surrounding tissue [11–13, 30, 31]. Only recently has evidence of plasticity in this vascular network started to emerge [14, 32, 33]. The current study expands on the characteristics of the unique vasculature within the PVN by examining sex differences in BBB components and the long-term consequences of maternal dex treatment of their offspring reared into adulthood. There were differences in astrocytes in the PVN between male and female offspring, and excess glucocorticoids during fetal development decreased that presence in young adult female mice. At the same time, these animals exhibited an increase in helplessness- and depression-like behaviors compared with veh-treated mice in adulthood as well as a heightened HPA response to short-term stress.
The PVN plays critical roles in maintaining homeostasis and stress responses. Disruption in BBB components in offspring from dex-treated mothers may affect BBB function under specific physiological challenges, resulting in altered neuronal signaling. For example, spontaneously hypertensive rats have a breakdown of the BBB within the PVN, causing a feed-forward loop that increases blood pressure [33]. Previously, it was shown that fetal glucocorticoid exposure decreased the BBB competency within the prepubertal PVN in male and female mice [14]. In the current study, BBB integrity was examined during early adulthood to determine if the loss of BBB competency is maintained or is adaptive and able to recover. Unexpectedly, there was no significant difference in BBB competency based on sex or maternal treatment within the PVN, CTX, or LH, suggesting a recovery of basal BBB integrity between prepubertal development and adulthood. Although there was permeability previously during a critical period, it would be detrimental to an organism to have a prolonged loss of BBB integrity, especially in a brain region like the PVN.
There is little information on the development and maintenance of neurovascular units in a sex-specific manner throughout multiple brain regions, including the PVN. In the current study, astrocytes and pericytes were examined in male and female mice within the PVN and compared with the LH and CTX during early adulthood. Results demonstrated that female compared with male offspring of veh-treated mothers had more pericytes and astrocytes in the PVN that was not observed in brain regions such as the CTX or LH.
This study reveals a sex-specific difference in BBB components within the PVN. Determining a role for this difference deserves further investigation. For dex treatment, within the PVN male offspring had more pericyte coverage, and female offspring had fewer total astrocytes and astrocytes in proximity to blood vessels. Previously, prepubertal male and female offspring had an increase in pericyte coverage within the PVN after fetal exposure to excess glucocorticoids [14]. Because pericytes stabilize the vasculature [25] and can regulate capillary diameter through constricting vascular walls [34, 35], differences due to fetal glucocorticoid excess may affect blood flow within the PVN in male mice in early adulthood (this decrease may be due to additional coverage). This has been shown to modulate metabolic exchange between capillaries and the parenchyma [36]. Within the PVN, changes in capillary diameter have been proposed to increase blood flow in lactating female rats [32], although in vivo measurements would be difficult to obtain due to the depth within the central region of the brain. For astrocytes, female offspring of dex-treated mothers had a decrease in total astrocytes and astrocytes in proximity to blood vessels. It has been shown that astrocytic endfeet are an important component for a functional BBB and can regulate water influx and efflux through aquaporin 4 [37–39]. In addition, certain endothelial transporters, such as phospho-glycoprotein or the glucose transporter GLUT-1, are present in perivascular glial end-feet, and alterations could reduce BBB regulation of glucose and other metabolites (reviewed in Ref. 39). Under normal conditions, there is little extravascular FITC leakage (e.g., Fig. 2); however, there is a possibility that the loss of astrocytes in proximity to blood vessels in male offspring or the increase in pericyte coverage in female offspring of dex-treated mothers may affect BBB function and integrity within the PVN under particular physiological challenges. These findings suggest changes in the astrocyte population within the PVN may affect neuronal function directly though their important role in neuronal signaling and/or indirectly though disrupting peripheral signaling reaching the neuroendocrine neurons through changes in neurovascular units. Therefore, in addition to focusing on neuronal changes for potential mechanisms of long-lasting physiological and behavioral consequences of fetal glucocorticoid exposure, there is a need to examine sex differences in vascular characteristics and nonneuronal cells. Recently, the dex transcriptome in embryonic hypothalamic-derived neural stem cells was examined, and significant differences in gene expression between male and female subjects were found [40]. These studies and others are needed to provide insight into how excess glucocorticoids during fetal development can affect the hypothalamic development in multiple cells types in a sex-dependent manner.
In addition to vascular characteristics discussed previously, in the current study behavioral and physiological changes were examined in offspring exposed to excess glucocorticoids during fetal development. Male and female dex-treated offspring during the TST displayed less total time spent struggling and a shorter latency to immobility compared with control offspring, suggesting an increase in depression-like behavior. These results were not sex specific and are most likely due to changes in neural circuitry rather than to differences observed in nonneuronal cell types. There was a female-selective increase in HPA axis output in early adulthood, demonstrated by significantly elevated corticosterone levels, similar to what has been shown in female children born at term [2]. These results demonstrate long-lasting behavioral and physiological consequences in dex-treated offspring during early adulthood and demonstrate a female-specific impact on HPA-axis output.
The current study was conducted to examine the unique vasculature within the adult PVN and in response to excess glucocorticoids during fetal development. Results indicate that there are sex differences in astrocytes and pericytes within the PVN present in early adulthood that and exposure to excess glucocorticoids (dex) led to site selective (i.e., within the PVN) and sex-dependent changes. In addition to changes in the cytoarchitecture of the PVN in offspring of dex-treated mothers, there was an increase in depression-like behavior and a female-specific increase in HPA output. Therefore, within the PVN, there are sex-dependent differences and responsiveness in the offspring of dex-treated mothers present in early adulthood.
Acknowledgments
We thank Chad Eitel, Olivia Shoup, and Dr. Jill Goldstein for their helpful comments and technical assistance.
Financial Support: This work was supported by the National Institute of Mental Health, MH082679 (to S.A.T) and the National Institute of Child Health and Human Development, F31HD074496, (to K.A.F.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BBB
- blood-brain barrier
- CTX
- cerebral cortex
- dex
- dexamethasone
- HPA
- hypothalamic-pituitary-adrenal
- ir
- immunoreactive
- LH
- lateral hypothalamus
- NGS
- normal goat serum
- PBS
- phosphate-buffered saline
- PVN
- paraventricular nucleus of the hypothalamus
- TST
- tail-suspension test
- Tx
- Triton X-100
- veh
- vehicle.
References and Notes
- 1.Karemaker R, Kavelaars A, ter Wolbeek M, Tersteeg-Kamperman M, Baerts W, Veen S, Samsom JF, Visser GH, van Bel F, Heijnen CJ. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008;121(4):e870–e878. [DOI] [PubMed] [Google Scholar]
- 2.Alexander N, Rosenlöcher F, Stalder T, Linke J, Distler W, Morgner J, Kirschbaum C. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JM, Handa RJ, Tobet SA. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front Neuroendocrinol. 2014;35(1):140–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrell CS, Gillespie CF, Neigh GN. Energetic stress: The reciprocal relationship between energy availability and the stress response. Physiol Behav. 2016;166:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94(2):169–177. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus: a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12(6):717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35(2):197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill JW. PVN pathways controlling energy homeostasis. Indian J Endocrinol Metab. 2012;16(9, Suppl 3):S627–S636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6(1):269–324. [DOI] [PubMed] [Google Scholar]
- 11.Ambach G, Palkovits M. Blood supply of the rat hypothalamus: II. Nucleus paraventricularis. Acta Morphol Acad Sci Hung. 1974;22(3-4):311–320. [PubMed] [Google Scholar]
- 12.Menéndez A, Alvarez-Uria M. The development of vascularization in the postnatal rat paraventricular nucleus: a morphometric analysis. J Hirnforsch. 1987;28(3):325–329. [PubMed] [Google Scholar]
- 13.Frahm KA, Schow MJ, Tobet SA. The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling. Horm Metab Res. 2012;44(8):619–624. [DOI] [PubMed] [Google Scholar]
- 14.Frahm KA, Tobet SA. Development of the blood-brain barrier within the paraventricular nucleus of the hypothalamus: influence of fetal glucocorticoid excess. Brain Struct Funct. 2015;220(4):2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520(1):6–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. [DOI] [PubMed] [Google Scholar]
- 18.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–360. [DOI] [PubMed] [Google Scholar]
- 19.Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA. Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med. 2013;34(2-3):742–752. [DOI] [PubMed] [Google Scholar]
- 20.Shimagori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72(5):648–672. [DOI] [PubMed] [Google Scholar]
- 22.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. [DOI] [PubMed] [Google Scholar]
- 23.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, Ungvari Z, Ballabh P. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41(8):1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frahm KA, Nash CP, Tobet SA. Endocan immunoreactivity in the mouse brain: method for identifying nonfunctional blood vessels. J Immunol Methods. 2013;398-399:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratton MS, Staros M, Budefeld T, Searcy BT, Nash C, Eitel C, Carbone D, Handa RJ, Majdic G, Tobet SA. Embryonic GABA(B) receptor blockade alters cell migration, adult hypothalamic structure, and anxiety- and depression-like behaviors sex specifically in mice. PLoS One. 2014;9(8):e106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol. 2006;188(3):435–442. [DOI] [PubMed] [Google Scholar]
- 29.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4-5):571–625. [DOI] [PubMed] [Google Scholar]
- 30.Francis BM, Yang J, Hajderi E, Brown ME, Michalski B, McLaurin J, Fahnestock M, Mount HT. Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37(8):1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craigie EH. On the relative vascularity of various parts of the central nervous system of the albino rat. J Comp Neurol. 1920;20:310–319. [Google Scholar]
- 32.Cortés-Sol A, Lara-Garcia M, Alvarado M, Hudson R, Berbel P, Pacheco P. Inner capillary diameter of hypothalamic paraventricular nucleus of female rat increases during lactation. BMC Neurosci. 2013;14(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63(3):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev. 1995;7(3):240–276. [PubMed] [Google Scholar]
- 37.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61(12):1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare Ø, Laake P, Klungland A, Thorén AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci USA. 2011;108(43):17815–17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nico B, Ribatti D. Morphofunctional aspects of the blood-brain barrier. Curr Drug Metab. 2012;13(1):50–60. [DOI] [PubMed] [Google Scholar]
- 40.Frahm KA, Peffer ME, Zhang JY, Luthra S, Chakka AB, Couger MB, Chandran UR, Monaghan AP, DeFranco DB. Research resource: The dexamethasone transcriptome in hypothalamic embryonic neural stem cells. Mol Endocrinol. 2016;30(1):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]