Abstract
Purpose
Epithelixal ovarian cancer is the fourth cause of cancer death in developed countries with 77% of ovarian cancer cases diagnosed with regional or distant metastasis, with poor survival rates. Docetaxel (DTX) is a well-known anticancer agent, with clinically proven efficacy in several malignancies, including ovarian cancer. However, the adverse effects caused by the active ingredient or currently marketed formulations could even deprive the patient of the advantages of treatment. Therefore, in the current study, polymeric nanoparticles (NPs) equipped with aptamer molecules as targeting agents were proposed to minimize the adverse effects and enhance the antitumor efficacy through directing the drug cargo toward its site of action.
Materials and methods
Electrospraying technique was implemented to fabricate poly (butylene adipate-co-butylene terephthalate) (Ecoflex®) NPs loaded with DTX (DTX-NPs). Afterward, aptamer molecules were added to the DTX-NPs, which bound via covalent bonds (Apt-DTX-NPs). The particle size, size distribution, zeta potential, entrapment efficiency, and release profile of the NPs were characterized. Using MTT assay and flow-cytometry analysis, the in vitro cytotoxicity and cellular uptake of the NPs were compared to those of the free drug. Following intravenous administration of Taxotere®, DTX-NPs, and Apt-DTX-NPs (at an equivalent dose of 5 mg/kg of DTX), pharmacokinetic parameters and antitumor efficacy were compared in female Balb/c and HER-2-overexpressing tumor-bearing B6 athymic mice, respectively.
Results
The obtained results demonstrated significantly enhanced in vitro cytotoxicity and cellular uptake of Apt-DTX-NPs in a HER-2-overexpressing cell line, comparing to DTX-NPs and the free drug. The results of in vivo studies indicated significant increment in pharmacokinetic parameters including the area under the plasma concentration–time curve, mean residence time, and elimination half-life. Significant increment in antitumor efficacy was also observed, probably due to the targeted delivery of DTX to the tumor site and enhanced cellular uptake as evaluated in the aforementioned tests.
Conclusion
Hence, the proposed drug delivery system could be considered as an appropriate potential substitute for currently marketed DTX formulations.
Keywords: Ecoflex, nanoparticles, aptamer, electrospraying, pharmacokinetic, ovarian cancer, docetaxel
Introduction
Epithelial ovarian cancer is the fourth cause of cancer death in developed countries. Approximately 77% of women with ovarian cancer are diagnosed with regional or distant metastasis, with poor survival rates. These patients usually undergo aggressive treatment including multiple surgeries, radiotherapy, and chemotherapy.1,2 Moreover, high probability of disease recurrence after surgery or adjuvant therapy results in an immense distress on patients, which affects their quality of life.3
Docetaxel (DTX) is a well-known member of taxane family, with clinically proven efficacy in several types of malignancies, including ovarian cancer.4 However, several drawbacks including poor aqueous solubility, low oral bioavailability, and various notable adverse effects have negatively affected the clinical application of DTX. The adverse effects, including hypersensitivity reactions, fluid retention, neuro and musculoskeletal toxicity, and neutropenia, are caused either by the active pharmaceutical ingredient (API) or the components of the solvent system needed to solubilize the hydrophobic DTX, such as Tween 80 or ethanol implicated in currently marketed formulations such as Taxotere® (Sanofi-Aventis Pharma Inc., Paris, France) or Duopafei® (Qilu Pharmaceutical Co., Ltd., Jinan, China).4
Numerous efforts have been dedicated to develop a delivery system for DTX, which attenuate the API and formulation-related adverse effects, while maintaining its therapeutic efficacy. Polymeric nanoparticles (NPs), liposomes, micelles, solid lipid NPs, and nanostructured lipid carriers are some of the studied delivery systems with reported suitable characteristics, such as improved drug solubility and membrane transport.5–11
Among the mentioned delivery systems, polymeric NPs could serve as an excellent vehicle, offering the opportunity of protecting the active cargo against enzymatic and hydrolytic degradation. On the other hand, in order to minimize the API-related adverse effects that are caused by biodistribution of the cytotoxic agent throughout the body, the delivery system could be designed by the addition of targeting ligands which specifically bind to a predetermined receptor.12
It has been demonstrated that HER-2 receptor gene is amplified in a remarkable number of ovarian cancer cases.13 HER-2 is one of the transmembrane epidermal growth factor receptors. It is suggested that the overexpression of this receptor in ovarian cancer is associated with more aggressive disease, with poor prognosis and lower survival rate. Moreover, residual cancer is observed in all HER-2-overexpressing cases of ovarian cancer, in comparison to 45% of cases with normal HER-2 expression.14–16
There are several monoclonal antibodies developed against the extracellular domain of HER-2 receptor, which could be used as targeting ligands in delivery systems targeting HER-2-positive tumors. However, expensive and work-intensive production, as well as poor stability and limited shelf-life are notable drawbacks of monoclonal antibodies. For this reason, a great deal of interest was drawn toward aptamer as an alternative targeting ligand. It is chemically synthesized using simpler and more cost-effective procedures, with higher stability and possibility of performing favorable modifications.17–21 Aptamers are oligonucleotides that are able to bind to their targets with specificity and affinity comparable to monoclonal antibodies, because of the specific three-dimensional structures that they form according to their sequences.21
In accordance with the aforementioned issues, a targeted polymeric nanoparticulate system composed of poly(butylene adipate-co-butylene terephthalate) (Ecoflex®; BASF Company, Ludwigshafen, Germany) and HER-2 specific aptamer was investigated in the current study, and its properties such as in vitro cytotoxicity against a HER-2-positive ovarian cancer, pharmacokinetic profile, and in vivo antitumor efficacy in tumor-bearing nude mice were evaluated.
Ecoflex® is a biodegradable and biocompatible polymer with a complete and relatively fast degradation profile and excellent flexibility. It was initially designed by BASF Company (Ludwigshafen, Germany) and has been used worldwide for packaging applications since 1998. However, Xinfu Pharmaceutical Co. Ltd, Hangzhou, China, has recently paid great interest to this polymer for pharmaceutical applications, particularly for the production of biomedical nanocomposites.22 Considering the aforementioned advantages of this polymer, it was implicated to construct an anticancer drug delivery system in the current study. Due to the limitations imposed by the physicochemical properties of Ecoflex®, there are limited studies on fabrication of NPs by this polymer. Electrospray technique was used to fabricate the NPs in the current study to overcome these limitations. To our knowledge, there is no report on the production of NPs of Ecoflex® by electrospraying method and conjugation to aptamer molecules as the targeting moiety.
Materials and methods
Materials
DTX was provided by Cipla (Mumbai, India) and Eco-flex® (MW =100,000 g/mol) by BASF Company. Pluronic F-127, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), rhodamine B (RhB), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), potassium ferricyanide, and potassium ferrocyanide were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Polyethylene glycol (PEG, MW =6,000), acetonitrile, dichloromethane, N,N-dimethyl formamide, and N-hydrox-ysuccinimide (NHS) were obtained from Merck (Merck KGaA, Darmstadt, Germany). RPMI medium, fetal bovine serum (FBS), and trypsin/EDTA were supplied by Biosera Europe (ZI du Bousquet, France). Penicillin/streptomycin was obtained from Thermo Fisher Scientific (Waltham, MA, USA) and diazepam was purchased from Sigma-Aldrich Co. DNA aptamer with base sequence of (5′-AGCCGCGAGGGGAGGGATAGGGTAGGGCGCGGCT-3′) was custom- synthetized by Gen Fanavaran Co (Tehran, Iran). SKOV-3 and MDA-MB-468 cell lines were purchased from the Iranian Biological Resource Center (Tehran, Iran). Female Balb/c mice (5–6 weeks old, 25±2 g) were obtained from Laboratory Animal Center of Faculty of Pharmacy and Pharmaceutical Science, Isfahan University of Medical Science, Iran. Athymic female nude B6 mice were obtained from Pasteur Institute (Amol, Iran).
All the animals were pathogen free and had free access to food and water. Animal experiments were performed in accordance with the guidelines proposed in the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health and approved by the Research Committee of Isfahan University of Medical Science (ethical code: IR.MUI.REC.1394.3.284).
Preparation of aptamer-targeted DTX-loaded Ecoflex® NPs
Preparation and optimization of DTX-loaded NPs (DTX-NPs) was carried out via an electrospraying technique previously reported by Varshosaz et al.23 Briefly, a solution containing 70 mg of DTX and 210 mg of Ecoflex® and PEG 6000 (in a 95:5 ratio) in 42 mL of dichloromethane:dimethyl formamide mixture (2.7:1 ratio) constituted the organic phase. This organic solution was dispersed in an aqueous phase containing 1% (w/v) of Pluronic F-127 in deionized water. Using electrospraying technique at a voltage of 20 kV, 12 cm distance between electrodes, and feeding rate of 1 mL/h, the droplets of organic phase were atomized and dried in the electrical field before they reached the aqueous phase. Thus, an aqueous suspension of polymeric NPs loaded with DTX was obtained.
Following a pretreatment step where EDC and NHS were reacted with DTX-NPs, HER-2-specific aptamer with an amine modification was added to the suspension to allow for attachment to the NPs via a covalent link between the –COOH and –NH2 functional groups present on the NPs and aptamer molecules, respectively. The amount of conjugated aptamer was equal to 2 wt% of the polymer concentration in the reaction.24 The resulted aptamer-targeted DTX-NPs (Apt-DTX-NPs) were lyophilized using a freeze dryer (Martin Christ Ltd, Osterode, Germany) and maintained in a solid form until further use.
Particle size, particle size distribution, and zeta potential of NPs
The particle size, particle size distribution, and zeta potential of the obtained Apt-DTX-NPs were characterized using a laser light scattering particle size analyzer (Malvern Instruments, Malvern, UK). Aqueous suspensions of the NPs in deionized water were used as samples and each measurement was performed in triplicate.
DTX entrapment efficiency measurement
Predetermined amount of lyophilized powder of Apt-DTX-NP samples was dispersed in an aqueous medium containing 0.5% (w/v) Tween® 80 (Sigma-Aldrich Co., St Louis, MO, USA) to dissolve DTX. After centrifugation at 14,000 rpm for 15 minutes (Eppendorf Centrifuge 5430; Hamburg, Germany), the free drug was separated from the NPs by filtration through Amicon® ultracentrifuge filter (cutoff 10,000 Da) (EMD Millipore, Billerica, MA, USA). The amount of free drug was determined by high-pressure liquid chromatography (HPLC), and the entrapment efficiency was calculated using the following equation:
| (1) |
DTX release profiles
In vitro release of drug from the Apt-DTX-NPs was studied in an aqueous medium containing potassium hydrogen phosphate (pH 7.4, 0.01 M) and 0.5% (w/v) Tween® 80. Briefly, a dialysis bag (cutoff 12,000 Da) containing an aqueous suspension of Apt-DTX-NPs was placed inside an adequate amount of release medium to reach a final concentration of 1.3 μg/mL DTX, which equals to 15% of saturated solubility (9.8±0.3 μg/mL) of DTX in the release medium to maintain the sink condition. Drug release from the Apt-DTX-NPs took place under gentle stirring at 37°C±0.5°C for 30 hours; meanwhile, 100 μL samples were withdrawn and replaced with fresh medium at predetermined time intervals. The DTX concentration in samples was analyzed by HPLC after omitting the Apt-DTX-NPs, using Amicon® ultracentrifugal filters.
HPLC analysis method
The apparatus was an HPLC system (Waters Associates, Milford, MA, USA) equipped with a UV detector at the wavelength of 230 nm and a reverse phase C-18 column (Spherisorb® 5 μm ODS2 4.6×250 mm; Waters Associates). A mixture of acetonitrile and water (65:35, v/v) constituted the mobile phase and was used at the flow rate set at 1 mL/min.25 Using the line equation of the standard curve plotted in the range of 0.25–20 ng/mL, peak area of DTX in each sample was analyzed, considering inter-day and intra-day standard deviations.
In vitro cytotoxicity assay
SKOV-3 and MDA-468 were, respectively, the HER-2-positive and HER-2-negative cell lines.26,27 The presence or lack of the HER-2 receptor on these cells was confirmed by immunohistochemical method.
The cells were maintained in RPMI 1640 culture medium supplemented with 10% FBS and 1% penicillin (100 IU/mL)-streptomicin (100 μg/mL) under 98% relative humidity and 5% CO2 incubation conditions. Cell suspensions containing 6×104 cells/mL of each cell line were seeded in 96-well cell culture plates and incubated for 24 hours under the aforementioned conditions to ensure the attachment of cells. Afterward, the cells were treated with different samples: Apt-DTX-NPs, non-targeted DTX-NPs, free DTX, and control samples comprising blank aptamer-targeted NPs (Apt-NPs), blank non-targeted NPs (Blank-NPs), and dimethyl sulfoxide (DMSO) at the concentration similar to that used to solubilize free DTX. Each sample was prepared with different DTX concentration (10–100 ng/mL). Following a 24-hour incubation period, 20 μL of MTT solution (5 mg/mL) was added to each well and incubation was continued for further 3 hours. Finally, the supernatant was replaced with DMSO to dissolve formazan crystals produce by live cells, and the absorbance of each well was measured using ELISA plate reader (Biotek Instruments, Winooski, VT, USA) at 570 nm.
The cytotoxicity assay was performed in triplicate and the cell survival in each treatment group was calculated by the following equation, and the differences were statically evaluated by ANOVA test followed by least-square difference post hoc test.
| (2) |
Cellular uptake
Cellular uptake of the targeted NPs by the HER-2-positive cells was quantitatively compared to non-targeted NPs using RhB as a fluorescent probe, which was entrapped instead of DTX in the NPs.28 In order to confirm that the fluorescence detected in flow-cytometry studies was due to the uptake of Apt-RhB-NPs into the cells, free RhB was removed using dialysis bag (cutoff 12,000 Da) after production of RhB-loaded NPs. Moreover, the release profile of RhB from NPs was studied to make sure that RhB release from Apt-RhB-NPs was negligible in the first 2 hours.
The cells were seeded in six-well plates and after 24 hours of initial incubation, treatment samples were added and incubated further for 2 hours. Afterward, cells were collected using trypsin-EDTA mixture (0.25%–0.01%), washed twice with PBS, and centrifuged at 1,800 rpm to remove any non-internalized particles. Finally, the cells were resuspended in PBS for flow-cytometry measurement using FACS Calibur (Becton Dickinson, Franklin Lakes, NJ, USA).
Pharmacokinetic studies
Pharmacokinetic studies were performed on female Balb/c mice (5–6 weeks old, 25±2 g) which were classified into three groups according to the treatment sample received: Taxotere®, DTX-NPs, and Apt-DTX-NPs (with 24 animals in each group). The samples were intravenously injected via tail vein at an equivalent dose of 5 mg/kg of DTX versus body weight. At each predetermined time point of 5, 15, 30, 60, 90, 120, 240, and 600 minutes following the administration, three animals of each group were sacrificed and whole plasma samples were collected for further analysis.
In order to quantify the concentration of DTX in each sample, 20 μL of methanolic solution of diazepam (20 mg/mL as internal standard) and 4 mL of diethyl ether were added to 200 μL of plasma sample. After 2 minutes of shaking, the upper organic layer was transferred to another test tube and the solvent was dried out under gentle nitrogen steam. The residue was reconstituted in 200 μL of mobile phase, and 70 μL aliquots were injected into HPLC column with the aforementioned analytical system. The peak area ratio of DTX to internal standard was measured for each sample and analyzed using the line equation of plasma calibration curve plotted in the range of 0.1–16 ng/mL. The pharmacokinetic parameters including the area under the plasma concentration–time curve from zero to infinity, the apparent volume of distribution (Vd), systemic plasma clearance, distribution half-life, elimination half-life (t1/2β), and mean residence time (MRT) were calculated.
ANOVA test was used to statistically analyze the obtained data with p-value <0.05 considered as statistically significant. The statistical analyses were performed using IBM SPSS, version 19.
Tumor induction in athymic nude mice and in vivo antitumor efficacy
Aliquots of 200 μL of cell suspension containing 5×106 cells in PBS was subcutaneously injected into right flank of female athymic B6 nude mice (7–8 weeks, 18±2 g). Tumor dimensions were measured using a vernier caliper, and tumor volume was calculated using the following equation:29
| (3) |
where L and W are the longest and shortest diameters of tumor, respectively.
The tumor size was monitored until it reached the size of range 50–100 mm3. Then, the animals were classified into four groups (n=3 in each group). Taxotere®, non-targeted DTX-NPs, Apt-DTX-NPs, and normal saline (negative control) were administered intravenously via tail vein with as equivalent dose of 5 mg/kg of DTX versus body weight, in three doses every 3 days. The tumor volumes and body weight of each animal were monitored every 3 days, and animals were sacrificed after 24 days, and the tumor mass was surgically removed and weighed before being fixed in formalin and sent for pathological investigations.
The antitumor activity of each treatment was evaluated on the basis of the tumor inhibition rate (TIR [%]) calculated by the following equation:30
| (4) |
Immunohistochemistry findings
In order to ensure that the induced tumor was a HER-2-overexpressing tumor, immunohistochemical assay was performed both on the cell suspension and tumor samples obtained from the sacrificed mice.
Briefly, formalin-fixed and paraffin-embedded samples were used to obtain 4 μm sections, which were pretreated by the following steps of deparaffinization, rehydration through immersion in xylene, gradation in ethanol, and rinsing with water. Then, the slides were incubated for 30 minutes in 1% H2O2 and methanol medium in order to block the peroxidase activity. Afterward, in the antigen retrieval step, sections were immersed in citrate solution (pH 6.0) and microwaved for 5 minutes, and the sections were maintained in the same solution for another 15 minutes before being washed with distilled water. An anti-HER-2/neu polyclonal antibody (Dako, Glostrup, Denmark) was diluted 1:2,000 and a secondary biotinylated antibody was used with EnVision Detection System (Dako), with diaminobenzidine (Dako) as the chromogen. In the next step, the immunostained slides were observed independently by two pathologists under an Olympus lens microscope (Olympus Corporation, Tokyo, Japan) equipped with a 40× objective lens (field of vision diameter: 0.45 mm). Scoring was performed on a 0 to 3+ scale.31,32
Statistical analysis
All values were presented as mean and standard deviation (mean ± SD), and the significance of differences was investigated using ANOVA or unpaired Student’s t-test at p<0.05.
Results
Characterization of Apt-DTX-NPs
The particle size, particle size distribution, zeta potential, entrapment efficiency, and release efficiency of Apt-DTX-NPs and DTX-NPs are summarized in Figure 1. The non-targeted DTX-NPs demonstrated significantly lower values of particle size and zeta potential (202.4±8.3 nm [polydispesity index {PDI}: 0.29±0.01] and −17.6±0.4 mV, respectively), in comparison to Apt-DTX-NPs (274.7±46.1 nm [PDI: 0.44±0.02] and −9.9±0.19 mV, respectively).
Figure 1.
(A) Physicochemical characteristics of the HER-2-specific aptamer-targeted Ecoflex® nanoparticles loaded with DTX in comparison to non-targeted Ecoflex nanoparticles loaded with DTX. (B) Particle size and zeta potential diagrams of non-targeted Ecoflex nanoparticles loaded with DTX. (C) Particle size and zeta potential diagrams of aptamer-targeted Ecoflex nanoparticles loaded with DTX.
Abbreviations: DTX, docetaxel; PDI, polydispersity index; Apt-DTX-NPs, aptamer-conjugated nanoparticles loaded with docetaxel; RE30, drug release efficiency in 30 hours.
Drug entrapment efficiency in Apt-DTX-NPs was 75.3%±1.5%, which was slightly lower (p>0.05) than the non-targeted DTX-NPs (82.0%±8.5%). Release efficiency appeared to be constant by addition of aptamer molecules, which was found to be 47.1% in 30 hours for both formulations.
Immunohistochemistry studies
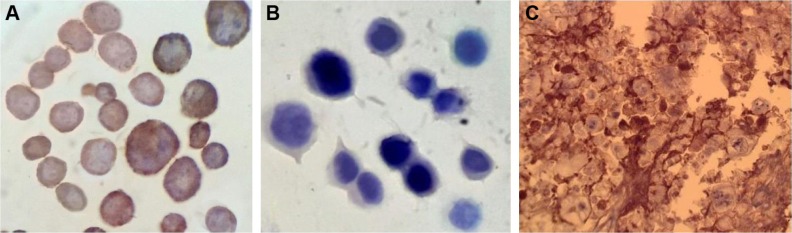
SKOV-3 and MDA-MB-468 cell suspensions were evaluated in order to investigate the presence of HER-2 receptors on these cells. The resulted micrographs represented stained cell membrane in SKOV-3 cells, while the antibody was not attached to the cell membrane of MDA-MB-468 cells, indicating lack of HER-2 receptors on these cells (Figure 2A and B). Besides, immunohistochemistry (IHC) assay on the tumor tissue excised from the tumor-bearing mice confirmed the presence of HER-2 receptor (3+ score) on cell membrane, defined as strong complete membrane staining in more than 30% of tumor cells (Figure 2C).
Figure 2.
Micrographs obtained after immunohistochemistry test on cell suspensions of (A) SKOV-3 cells (HER-2-positive) and (B) MDA-MB-468 cells (HER-2-negative) to compare the presence of HER-2 receptors; and (C) micrographs of SKOV-3 tumor tissue, indicating the presence of HER-2 receptors on cell membrane (considered as 3+ score), defined as strong complete membrane staining in more than 30% of tumor cells.
In vitro cytotoxicity assay
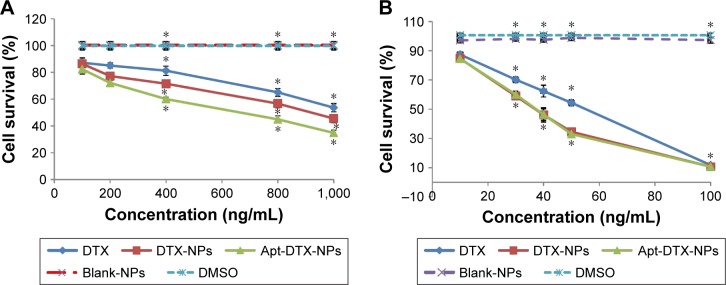
In order to investigate the in vitro cytotoxicity of Apt-DTX-NPs in comparison to non-targeted DTX-NPs and free DTX, MTT assay was performed on SKOV-3 and MDA-MB-468 cells (HER-2-positive and HER-2-negative cells, respectively). Targeted and non-targeted blank NPs, which did not contain DTX, and DMSO, in the same concentration as used for solubilization of free DTX, were used as controls. The results are illustrated in Figure 3.
Figure 3.
Viability percent after 24 hours incubation of (A) SKOV-3 cells treated with DTX-NPs, Apt-DTX-NPs, and free drug with different concentrations in the range of 100–1,000 ng/mL, and (B) MDA-MB-468 cells treated with DTX-NPs, Apt-DTX-NPs, and free drug with different concentrations in the range of 10–100 ng/mL, and compared to control samples including blank NPs (NPs which were not loaded with DTX) and DMSO (in the maximum concentration used in treatment groups).
Note: Significant differences are marked as *p<0.05.
Abbreviations: DTX, docetaxel; DTX-NPs, non-targeted nanoparticles loaded with docetaxel; Apt-DTX-NPs, aptamer-conjugated nanoparticles loaded with docetaxel; Blank NPs, nanoparticles not loaded with DTX; DMSO, dimethyl sulfoxide.
According to the obtained results, treatment with DTX-NPs at concentrations equal to 30–50 ng/mL of DTX in MDA-MB-468 cell line and 400–1,000 ng/mL of DTX in SKOV-3 cell line significantly decreased the cell survival in comparison to the free drug. Moreover, the reduction of cell survival in HER-2-positive cell line, SKOV-3, was significantly higher (p<0.05) when treated with Apt-DTX-NPs when compared to that with the non-targeted DTX-NPs, while in the case of HER-2-negative cell line, MDA-MB-468, no significant difference was observed between these two treatments (p>0.05).
Cellular uptake assay
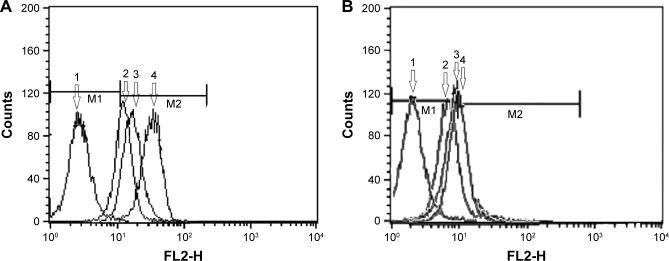
Cellular uptake of aptamer-targeted NPs loaded with RhB in SKOV-3 (HER-2-overexpressing) cells was compared with the uptake of non-targeted RhB-NPs and the free RhB. In order to quantify the amount of sample taken up by the cells in each treatment, flow-cytometry assay was performed and the results are summarized in Figure 4. The autofluorescence of the non-treated cells is represented as M1 portion of the diagrams, and any additional intensity of fluorescence caused by uptake of probe is represented as M2 portion of the diagrams. The intensity of fluorescence of the cells, which was regarded as an index for cellular uptake of free RhB, was enhanced bŷ30.1% in SKOV-3 cells and ~39.8% in MDA-MB-468 cells, when RhB was entrapped in the polymeric NPs. Further increment of the cellular uptake occurred when the aptamer molecules were added onto the delivery system causing almost a 100% increase in fluorescence intensity in SKOV-3 cells, in comparison to a 25.4% increment in MDA-MB-468 cells.
Figure 4.
Flow-cytomertry diagrams indicating the intensity of fluorescence in SKOV-3 (A) and MDA-MB-468 (B) cells, following 2 hours incubation with 1) blank NPs (NPs which were not loaded with RhB), 2) free RhB, 3) RhB-NPs (non-targeted nanoparticles loaded with RhB), and 4) Apt-RhB-NPs (aptamer-conjugated nanoparticles loaded with RhB).
Note: M1 portion represents autofluorescence of the nontreated cells and M2 portion represents any additional intensity of fluorescence caused by uptake of probe.
Abbreviations: FL, fluorescence; RhB, Rhodamine B.
Pharmacokinetic studies
Following intravenous administration of Taxotere®, DTX-NPs, and Apt-DTX-NPs into healthy Balb/c mice, plasma samples were taken at predetermined time intervals. The mean plasma concentration versus time profiles of DTX after each treatment is illustrated in Figure 5, and the eventuated pharmacokinetic parameters are summarized in Table 1. A two-compartment open model could describe the plasma concentration–time profiles of the administered formulations, and pharmacokinetic parameters were calculated on the bases of this model (Table 1).
Figure 5.
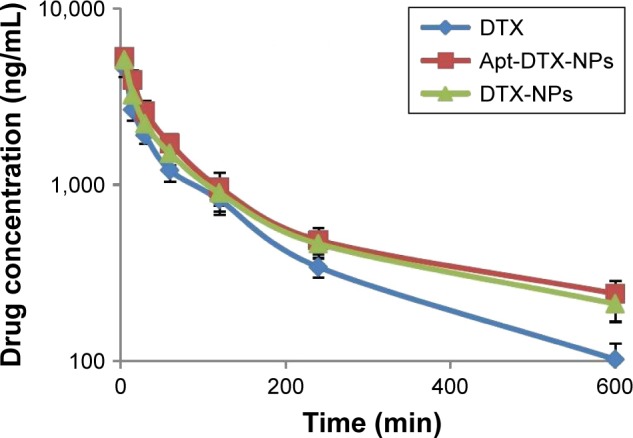
Mean plasma concentration–time profile of docetaxel (DTX) in Balb/c mice following intravenous administration of Taxotere®, DTX-NPs (non-targeted nanoparticles loaded with docetaxel), and Apt-DTX-NPs (aptamer-conjugated nanoparticles loaded with docetaxel). The results are indicated as mean ± SD (n=3).
Table 1.
Pharmacokinetic parameters of Taxotere® and Apt-DTX-NPs (results are presented as mean ± SD; n=3)
| Formulations | AUC0–∞ (µg⋅h/mL) | CL (L/kg/h) | MRT (hours) | Vd (L/kg) | T1/2β (hours) | T1/2α (hours) |
|---|---|---|---|---|---|---|
| Taxotere® | 4.51±0.39* | 1.11±0.10* | 3.95±0.07* | 4.38±0.30 | 2.70±0.05* | 0.63±0.03 |
| Apt-DTX-NPs | 6.97±0.84 | 0.72±0.09 | 6.19±0.17 | 4.44±0.41 | 4.30±0.12 | 0.67±0.03 |
| DTX-NPs | 6.34±1.02 | 0.78±0.12 | 5.91±0.42 | 4.66±1.08 | 4.10±0.29 | 0.64±0.03 |
Note:
p<0.05.
Abbreviations: Apt-DTX-NPs, aptamer-conjugated nanoparticles loaded with docetaxel; AUC, area under the curve; CL, clearance; DTX-NPs, non-targeted nanoparticles loaded with docetaxel; MRT, mean residence time; Vd, volume of distribution; T1/2β, elimination half-life; T1/2α, biodistribution half-life.
The elimination half-life of Taxotere® (2.70±0.05 hours) was significantly lower than Apt-DTX-NPs (4.30±0.12 hours) and DTX-NPs (4.10±0.29 hours). The MRT values also showed a significant increment in DTX-NPs (5.91±0.42 hours) and Apt-DTX-NPs (6.19±0.17 hours) compared to Taxotere® (3.95±0.07 hours), which is also in accordance with reduced total body clearance value of Apt-DTX-NPs (0.72±0.09 L/kg) and DTX-NPs (0.78±0.12 L/kg), in comparison to Taxotere® (1.11±0.10 L/kg). These results indicate that the proposed delivery system would be able to prolong the circulation of DTX. However, the apparent volume of distribution (Vd) was not significantly different in these administered formulations (p>0.05).
In vivo antitumor efficacy
The tumor developed approximately 20 days following the subcutaneous injection of SKOV-3 cells, and the antitumor efficacy of Taxotere®, DTX-NPs, and Apt-DTX-NPs was investigated in mice bearing SKOV-3 human ovarian carcinoma xenograft, when the tumor volume reached 50–100 mm3. In order to evaluate the safety and efficacy of different treatments, tumor volume and animal body weight were monitored every 3 days.
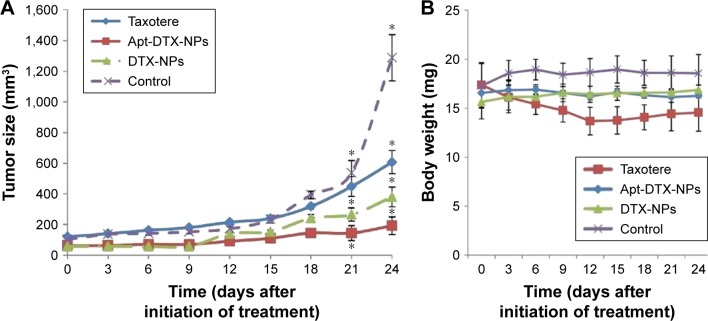
As illustrated in Figure 6A, the tumor showed an excessive growth in the negative control group, treated with normal saline, and reached 1,289±151 mm3 in 24 days, while the tumor growth rate was inhibited remarkably in animals treated with Taxotere®, DTX-NPs, or Apt-DTX-NPs. Also, there was a significant difference between the efficacy of Apt-DTX-NPs and other treatments; in that, the maximum tumor volume was observed in the group treated with targeted nanoparticulate delivery system (192±57 mm3), which was significantly less than those treated with Taxotere® (607±75 mm3) and DTX-NPs (385±65 mm3).
Figure 6.
Tumor inhibition effect of different treatments by (A) changes in tumor volume and (B) changes in body weight of mice after treatment with normal saline (control group), Taxotere®, Apt-DTX-NPs (aptamer-conjugated nanoparticles loaded with docetaxel), and DTX-NPs (non-targeted nanoparticles loaded with docetaxel). The results are indicated as mean ± SD (n=3) and significant differences are marked as *p<0.05.
Figure 6B represents the changes in body weight of the animals during the 24-day study period in different groups. In the negative control group, in which normal saline was administered to the animals, the body weight showed an increasing trend, up to 8% increment during 24 days. While in Taxotere® treated group, body weight of animals showed a significant initial decrease, up to 21.7%, which was accompanied by a notable loss of activity in animals. In this group, 3 days after cessation of the injections, body weight remained almost constant up to the end of study. In case of groups treated with targeted and non-targeted DTX-NPs, the body weight did not show any significant change.
Twenty four days following the initiation of the treatment, animals were sacrificed, and the tumor mass of each animal was excised and weighted. Each tumor mass was weighted and the TIR values were calculated. In the group treated with Apt-DTX-NPs, the TIR value was 84%, which was the highest among the studied groups, while this value was 76% and 67% for DTX-NPs and Taxotere®, respectively.
Discussion
Due to low water solubility of DTX, high concentration of polysorbate 80 has been used in the currently marketed formulations. Owing to the hypersensitivity reactions caused by polysorbate 80 and its incompatibility with common intravenous administration procedures, efforts have been dedicated to eliminate the use of this ingredient and find a suitable substitute DTX delivery system. Considering the biocompatibility, biodegradability, mechanical strength, and ease of processing, polymeric NPs could provide such a system.33 In comparison to the polymeric NPs reported in previous studies, which utilized Ecoflex® as a biodegradable and biocompatible polymer, electrospraying technique appeared to create smaller NPs with high entrapment efficiency for DTX. For instance, Keum et al prepared polylactide-co-glycolide (PLGA) NPs loaded with DTX by an emulsification and solvent evaporation method using different types and concentrations of surfactants and organic solvents, and reported particle size, zeta potential, and entrapment efficiencies ranging from ~250 to 1,000 nm, −3 to −20 mV, and 25% to 85%, respectively.33 Also, in another study by Sanna et al,34 polymeric NPs of poly(lactic acid), PLGA, and PLGA-poly(caprolactone) were loaded with DTX and the obtained encapsulation efficiencies were in range of 35%–47%.
Application of electrospraying technique for fabrication of polymeric NPs in the current study could explain the lower particle size of DTX-NPs compared to the previously mentioned studies even after the addition of the aptamer molecules, which led to increment of particle size (Figure 1); however, this value is still lower than the particle size reported for non-targeted NPs. This result could be due to the advantage of high-voltage field which caused the droplets of organic phase to be broken into NPs immediately after they were pumped out of the syringe and were dried out before they reached the aqueous phase.35 Therefore, electrospraying method seems to provide an appropriate choice for fabrication of Ecoflex® polymeric NPs with suitable characteristics.
Attachment of aptamer molecules as targeting ligands to the DTX-NPs was via formation of amide bonds between the –COOH functional groups of Ecoflex® and the –NH2 functional group which was added as a modification to the aptamer molecule. The covalent attachment of the targeting ligand to the NPs is considered as an advantage over physical binding proposed by Sun et al,36 due to the possibility of detachment faced in physical binding.
The physicochemical characteristics of the DTX-NPs were influenced by attachment of aptamer molecules (Figure 1). Increment of particle size and particle size distribution occurred as a result of addition of aptamer molecules to the NPs surface, and a significant decrease in the absolute zeta potential value could be explained by a decrease in the surface charge caused by COO− groups when they are involved in amide reaction with amine groups of aptamer molecules. Drug entrapment efficiency and release efficiency did not show any significant changes between the groups (Figure 1).
The presence of HER-2 receptor on SKOV-3 tumor tissue induced in mice was confirmed by IHC assay performed on excised tumor tissue with staining in more than 30% of tumor cells, which could be considered as 3+ score (Figure 2). In the case of cell suspensions, due to lack of solid tissue and the resulted imprecise sections, IHC test could not result in well-defined membrane staining; however, clear difference was observed between HER-2-negative and HER-2-positive cell micrographs (Figure 2).
The results obtained from MTT assay demonstrated higher cytotoxicity of DTX-NPs compared to the free drug in both cell lines, and also higher cytotoxicity of Apt-DTX-NPs compared to non-targeted delivery system in HER-2-positive cells (Figure 3). This could be interpreted with regard to the enhancement of cellular uptake of DTX when it was entrapped in the proposed delivery system, as was demonstrated with flow-cytometry assay (Figure 4). On the other hand, lack of any significant reduction in the cell survival after treatment in control groups confirmed the lack of cytotoxicity of the proposed delivery system in both cell lines.
Achieved pharmacokinetic data (Figure 5, Table 1) indicated prolonged residence time of DTX-NPs and HER-2-targeted Apt-DTX-NPs in the body considering a significant increment of T1/2β and MRT values, compared to Taxotere®. These results could be attributed to the presence of NPs for an increased duration of time in the blood circulation and reduced elimination rate. While in animals treated with Taxotere®, the drug would be distributed throughout the animal’s body and will be eliminated more rapidly. Since the pharmacokinetic studies should be performed on healthy animals,37 the specific target of aptamer, which is the HER-2-overexpressing tumor tissue, was not present in the pharmacokinetic test, so the differences between pharmacokinetic parameters of aptamer-conjugated delivery system and nonconjugated system were not significant. However, in HER-2-overexpressing tumor-bearing mice, treatment with Apt-DTX-NPs would result in higher drug concentration at tumor site than treatment with DTX-NPs. This hypothesis could be further investigated by antitumor efficacy results.
The antitumor efficacy was evaluated with regard to tumor volume and TIR value, and its increment following administration of non-targeted DTX-NPs when compared to Taxotere® could be justified by the enhanced permeation and retention effect, which could be proposed as a major advantage of nanoparticulate systems in cancer drug delivery, resulting in passive targeting delivery of the drug cargo. The highest antitumor effect observed in the group treated with Apt-DTX-NPs, among the four studied groups (Figure 6), could be described as a result of combining active and passive targeting of the anticancer drug toward its site of action, thus leading to the presence of higher concentrations of DTX at HER-2-overexpressing tumor site.
Yet, this aptamer-attached Ecoflex® delivery system indicated remarkable antitumor efficacy in comparison to other DTX-loaded NPs reported in previous studies. For instance, DTX-loaded albumin NPs fabricated by Tang et al38 by a novel simple self-assembly method could inhibit tumor size to 524.34 mm3 22 days after injection with DTX at a dose of 7.5 mg/kg, compared to 669.46 mm3 by free DTX and 1,403 mm3 in control group. When the injection dose of DTX-albumin NPs was increased to 30 mg/kg, the tumor volume was inhibited to 233 mm3. While in the current study, lower tumor volume were observed 24 days after injection of DTX-NPs containing 5 mg/kg DTX (Figure 6).
Also, the TIR value obtained after injection of Apt-DTX-NPs with 5 mg/kg DTX concentration was relatively high compared to that reported by previous studies considering the DTX dose and duration of treatment. For instance, Wang et al39 investigated the antitumor efficacy of a lipid-based nanosuspension loaded with DTX. Following intravenous administration of doses equal to 20 mg/kg DTX for 21 days, the obtained TIR value was 84.51%±3.49%, which is almost equal to the value obtained by Apt-DTX-NPs in the current study with one-fourth concentration of the dose (5 mg/kg).
Changes in body weight of the animals during the study period could index the systemic adverse effects of different formulations on the animals.40 In the negative control group, normal saline was administered to the animals; since the animals were deprived of any anticancer treatment, the body weight changes showed an increasing trend (Figure 6), which could be due to increment of the tumor size. While in the animals treated with Taxotere®, body weight showed a significant decrease until the treatment was continued (Figure 6), which indicates the toxicity of the formulation, especially because it was accompanied by a notable loss of activity in animals. Among the animals that were treated with targeted and non-targeted DTX-NPs, the body weight remained almost constant (Figure 6), which could be interpreted as an advantage of the proposed DTX delivery system in comparison to those which cause body weight loss in the animals such as the PLGA/hyaluronic acid NPs reported by Huang et al.41
Conclusion
The aptamer-targeted Ecoflex® NPs, investigated in the current study as a drug delivery system for DTX, was compared with Taxotere® with regard to in vitro/in vivo efficacy and pharmacokinetic characteristics. The obtained results clarified that using this NP system enhanced in vitro cytotoxicity against both HER-2-positive and -negative cell lines, due to an increment in the cellular uptake of DTX. Moreover, addition of HER-2-specific aptamer molecules as targeting ligand caused a further enhancement in the cytotoxicity against HER-2-positive cells. The results of pharmacokinetic studies on these NPs, when compared to Taxotere®, revealed prolonged residence time of DTX in blood circulation, indicating lower nonspecific biodistribution of the cytotoxic drug throughout the body. Antitumor evaluations confirmed significant enhancement of the Apt-DTX-NPs in comparison to Taxotere®. Accordingly, considering the overall in vitro/in vivo properties of the Apt-DTX-NPs as a nontoxic, biodegradable, and biocompatible delivery system, they could propose the opportunity of an effective substitute for the currently marketed formulations of DTX, without the adverse effects associated with the components.
Acknowledgments
The authors appreciate financial support from Isfahan University of Medical Sciences.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 2.Kornblith AB, Thaler HT, Wong G, et al. Quality of life of women with ovarian cancer. Gynecol Oncol. 1995;59:231–242. doi: 10.1006/gyno.1995.0014. [DOI] [PubMed] [Google Scholar]
- 3.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:302–308. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Liu Z, Liu D, Liu C, Juan Z, Zhang N. Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): preparation, pharmacokinetics, tissue distribution and antitumor activity. Int J Pharm. 2011;413:194–201. doi: 10.1016/j.ijpharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Naik S, Patel D, Surti N, Misra A. Preparation of PEGylated liposomes of docetaxel using supercritical fluid technology. J Supercrit Fluids. 2010;54:110–119. [Google Scholar]
- 6.Zhai GX, Wu J, Yu B, Guo C, Yang X, Lee RJ. A transferrin receptor-targeted liposomal formulation for docetaxel. J Nanosci Nanotechnol. 2010;10:5129–5136. doi: 10.1166/jnn.2010.2393. [DOI] [PubMed] [Google Scholar]
- 7.Grosse PY, Bressolle F, Pinguet F. In vitro modulation of doxorubicin and docetaxel antitumoral activity by methyl-beta-cyclodextrin. Eur J Cancer. 1998;34:168–174. doi: 10.1016/s0959-8049(97)00351-1. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128:23–31. doi: 10.1016/j.jconrel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Li R, Qian X, et al. Superior antitumor efficiency of cisplatin-loaded nanoparticles by intratumoral delivery with decreased tumor metabolism rate. Eur J Pharm Biopharm. 2008;70:726–734. doi: 10.1016/j.ejpb.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Chen L, Gu W, et al. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials. 2009;30:226–232. doi: 10.1016/j.biomaterials.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wang D, Zhang J, Pan W. Preparation and pharmacokinetics of docetaxel based on nanostructured lipid carriers. J Pharm Pharmacol. 2009;61:1485–1492. doi: 10.1211/jpp/61.11.0007. [DOI] [PubMed] [Google Scholar]
- 12.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9:1–11. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyson FL, Boyer CM, Kaufman R, et al. Expression and amplification of the HER-2/neu (c-erbB-2) protooncogene in epithelial ovarian tumors and cell lines. Am J Obstet Gynecol. 1991;165:640–646. doi: 10.1016/0002-9378(91)90300-g. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Godolphin W, Jones LA, et al. Studies of HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 15.Sandra MS, Baselga S, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berchuck A, Kamel A, Whitaker R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 17.Thiel KW, Giangrande PH. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides. 2009;19:209–222. doi: 10.1089/oli.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy-Nissenbaum E, Radovic-Moreno AF, Wang AZ, Langer R, Farokhzad OC. Nanotechnology and aptamers: applications in drug delivery. Trends Biotechnol. 2008;26:442–449. doi: 10.1016/j.tibtech.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 19.O’Sullivan CK. Aptasensors – the future of biosensing. Anal Bioanal Chem. 2002;372:44–48. doi: 10.1007/s00216-001-1189-3. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MB, Fuller ST, Richardson PM, Doyle SA. An improved method for the in vitro evolution of aptamers and applications in protein detection and purification. Nucleic Acids Res. 2003;31:1–8. doi: 10.1093/nar/gng110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Li L, Zhao H, Mu X. Electrochemical impedance spectroscopy detection of lysozyme based on electrodeposited gold nanoparticles. Talanta. 2011;83:1501–1506. doi: 10.1016/j.talanta.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Varshosaz J, Riahi S, Ghassami E, Jahanian-Najafabadi A. Transferrin-targeted poly(butylene adipate)/terephthalate nanoparticles for targeted delivery of 5-fluorouracil in HT29 colorectal cancer cell line. J Bioact Compat Pol. 2017;32(5):503. [Google Scholar]
- 23.Varshosaz J, Ghassami E, Noorbakhsh A, Jahanian-Najafabadi A, Minayian M, Behzadi R. Poly (butylene adipate-co-butylene terephtha-late) nanoparticles prepared by electrospraying technique for docetaxel delivery in ovarian cancer induced mice. Drug Dev Ind Pharm. doi: 10.1080/03639045.2018.1430819. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 24.Farokhzad OC, Cheng J, Teply BA. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varshosaz J, Taymouri S, Hassanzadeh F, Javanmard SH, Rostami M. Folated synperonic-cholesteryl hemisuccinate polymeric micelles for the targeted delivery of docetaxel in melanoma. Biomed Res Int. 2015;2015:1–17. doi: 10.1155/2015/746093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmlund L, Käck C, Aastrup T, Nicholas IA. Study of the interaction of trastuzumab and SKOV3 epithelial cancer cells using a quartz crystal microbalance sensor. Sensors. 2015;15:5884–5894. doi: 10.3390/s150305884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anido J, Matar P, Albanell J, et al. ZD1839 a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res. 2003;9:1274–1283. [PubMed] [Google Scholar]
- 28.Xu Z, Chen L, Gu W, et al. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials. 2009;30:226–232. doi: 10.1016/j.biomaterials.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Noori Koopaei M, Khoshayand MR, Mostafavi SH, et al. Docetaxel loaded PEG-PLGA nanoparticles: optimized drug loading, in-vitro cytotoxicity and in-vivo antitumor effect. J Pharm Res. 2014;13:819–833. [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Liu D, Wang L, Zhang J, Zhang N. Docetaxel-loaded pleuronic P123 polymeric micelles: in vitro and in vivo evaluation. Int J Mol Sci. 2011;12:1684–1696. doi: 10.3390/ijms12031684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridolfi RL, Jamehdor MR, Arber JM. HER-2/neu testing in breast carcinoma: a combined immunohistochemical and fluorescence in situ hybridization approach. Mod Pathol. 2000;13:866–873. doi: 10.1038/modpathol.3880154. [DOI] [PubMed] [Google Scholar]
- 32.Rajabi P, Bagheri A, Hani M. Intratumoral and peritumoral mast cells in malignant melanoma: an immunohistochemical study. Adv Biomed Res. 2017;6:39. doi: 10.4103/2277-9175.204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keum CG, Noh YW, Baek JS, et al. Practical preparation procedures for docetaxel-loaded nanoparticles using polylactic acid-co-glycolic acid. Int J Nanomedicine. 2011;6:2225–2234. doi: 10.2147/IJN.S24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanna V, Roggio AM, Posadino AM, et al. Novel docetaxel-loaded NPs based on poly(lactide-co-caprolactone) and poly(lactideco-glycolide-co-caprolactone) for prostate cancer treatment: formulation, characterization, and cytotoxicity studies. Nanoscale Res Lett. 2011;6:1–9. doi: 10.1186/1556-276X-6-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaworek A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007;176:18–35. [Google Scholar]
- 36.Sun B, Ranganathana B, Feng S. Multifunctional poly(D,L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by trastuzumab for targeted chemotherapy of breast cancer. Biomaterials. 2008;29:475–486. doi: 10.1016/j.biomaterials.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 37. http://www.ema.europa.eu [homepage on the Internet] Guidelines for the conduct of pharmacokinetic studies in target animal species. London: European Agency for Evaluation of Medicinal Products; [Accessed November 1, 2017]. (EMEA/CVMP/133/99-Final). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004355.pdf. [Google Scholar]
- 38.Tang X, Wang G, Shi R, et al. Enhanced tolerance and antitumor efficacy by docetaxel-loaded albumin nanoparticles. Drug Deliv. 2016;23:2686–2696. doi: 10.3109/10717544.2015.1049720. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Liu Z, Liu D, Liu C, Juan Z, Zhang N. Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): preparation, pharmacokinetics, tissue distribution and antitumor activity. Int J Pharm. 2011;413:194–201. doi: 10.1016/j.ijpharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Lee SW, Yun MH, Jeong SW, et al. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. J Control Release. 2011;155:262–271. doi: 10.1016/j.jconrel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Zhang H, Yu Y, et al. Biodegradable self-assembled nanoparticles of poly(D,L-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel in breast cancer. Biometerials. 2014;35:550–566. doi: 10.1016/j.biomaterials.2013.09.089. [DOI] [PubMed] [Google Scholar]