Abstract
In the last decade, the ‘Cumulative Pressure and Impact Assessment’ (CPIA) approach emerged as a tool to map expected impacts on marine ecosystems. However, CPIA assumes a linear response of ecosystems to increasing level of cumulative pressure weighting sensitivity to different anthropogenic pressures through expert judgement. We applied CPIA to Mediterranean coralligenous outcrops over 1000 km of the Italian coastline. Extensive field surveys were conducted to assess the actual condition of coralligenous assemblages at varying levels of human pressure. As pressure increased, a clear shift from bioconstructors to turf-dominated assemblages was found. The linear model originally assumed for CPIA did not fit the actual relationship between expected cumulative impact versus assemblage degradation. A log-log model, instead, best fitted the data and predicted a different map of cumulative impact in the study area able to appreciate the whole range of impact scenarios. Hence, the relative importance of different drivers in explaining the observed pattern of degradation was not aligned with weights from the expert opinion. Such findings stress the need for more incisive efforts to collect empirical evidence on ecosystem-specific responses to human pressure in order to refine CPIA predictions.
Introduction
Worldwide, marine coastal systems are threatened by increasing human pressures often acting simultaneously1. Ecological research has documented the impact of individual stressors on species, habitats and ecosystems. Studies have shown that sedimentation, nutrient enrichment, pollution, resource exploitation, presence of non-indigenous species, habitat destruction and fragmentation can alter ecosystem functioning at varying scales in time and space, changing the number and composition of species and their relative abundances through direct and indirect effects2,3.
The need for a deeper understanding of the effects of multiple stressors on ecosystems was highlighted about twenty years ago4 and is still considered one of the most challenging questions for ecosystem-based management5. In this framework, environmental impact assessments have attempted to move from considering single-source of impact towards more comprehensive approaches investigating ecological responses to multiple interacting human disturbances6. Mesocosms7, manipulative8 or correlative field experiments9, and modelling10 have been used to quantify the effects of multiple stressors on marine biodiversity. More recently, the recognition that human activities and their potential impacts are spatially explicit has led to the development of the cumulative pressure and impact assessment (CPIA) approach6, which focuses on mapping the distribution of human pressures and expected impact on marine ecosystem. However, despite its application to different environmental contexts worldwide (e.g.1,11,12), the CPIA approach still relies on the assumptions that the effects of pressures are fully additive and that cumulative impacts increase linearly at increasing pressures13. This could be not retained to changing environmental settings, strongly affecting the reliability of the ensuing impact estimates14. A further issue in CPIA concerns the use of scores based on expert judgement to weight the potential effects of anthropogenic pressures on different ecosystems. Although scores from expert opinion could represent useful proxies of ecosystems sensitivity, comparisons with quantitative assessments raised doubts on their appropriateness in weighting the actual effects of human pressures on marine systems15. Indeed, a note of caution on the estimated cumulative impacts from CPIA is intrinsic to the approach6, unless accompanied with careful ground-truthing16, and information on relationships between expected impact and the actual condition of habitats and assemblages.
The Mediterranean Sea is largely affected by multiple stressors leading to a serious loss of marine biodiversity and the degradation of ecosystem functioning17. Estimates of cumulative impacts at basin scale (i.e.,12) highlighted that 60–99% of the territorial waters of EU member states are subject to high impact. Coralligenous outcrops are mainly produced by the accumulation of calcareous algae and invertebrates. They characterize at least the 30% of the coasts of the basin18 and represent the most important Mediterranean marine habitat, in terms of biodiversity and productivity, after Posidonia oceanica meadows19. Several studies addressed the effects of single sources of impact (e.g., waste waters, fishing, sedimentation, thermal anomalies) on this habitat at local scale9,20. However, there is an absence of large-scale quantitative assessments of the effects of different combinations of human pressures, impairing the understanding of the processes leading to structural and functional shifts in this system21.
Here, we applied the CPIA framework on Mediterranean coralligenous outcrops over 1000 km of coast (Apulia, SE Italy), combining, for the first time, detailed information on human pressures and maps of habitat distribution with an extensive field survey to directly assess the state of coralligenous assemblages at varying levels of human pressure. The aim of this study is to understand the relationship between human pressures and this priority habitat at regional scale, addressing two of the most relevant sources of uncertainty in CPIA: (i) the assumption of a linear relationship between the estimated cumulative impact and the actual condition of the investigated assemblages, and (ii) the relation between pressure weights from expert opinion and actual correlations among pressures and assemblage responses. The outcomes are expected to shed light on factors affecting the effectiveness of the CPIA approach to provide reliable estimates of potential effects of multiple anthropogenic pressures, contributing to inform spatial management of cumulative impacts on marine systems.
Results
Cumulative pressure level on coralligenous outcrops
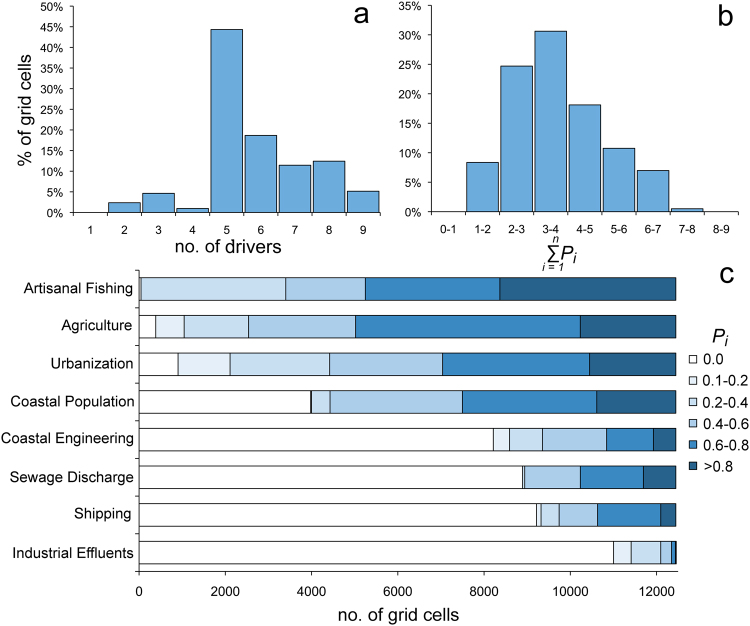
A total of 12,447 cells of the grid were characterized by coralligenous outcrops on the sea bottom within 30 m depth (about 498 km2). No cells were completely unaffected by anthropogenic drivers, and more than 40% of cells resulted exposed at least to 5 of them (Fig. 1a). Less than 10% of cells seemed to receive the potential influence of fewer (<4) drivers, whereas about half of coralligenous outcrops were potentially influenced by a larger number of drivers ≥ 6 (Fig. 1a). However, most cells (>60%) showed relatively low levels of cumulative pressure (i.e., ), whereas the remaining cells (about 30%) exhibited medium levels (4–6) and only ∼8% were exposed to high (>6) levels of cumulative pressure (Fig. 1b). The most frequent drivers, irrespective of their pressure level, were Artisanal Fisheries (>99% of cells), Agriculture (97%), and Urbanization (93%), whereas the least frequent ones were Industrial Effluents (12%), Shipping (26%), Sewage Discharge (29%), and Coastal Engeneering (34%); the most frequent drivers were also those acting in most cells with medium to high levels of pressure (Fig. 1c).
Figure 1.
(a) % of grid cells per number of drivers. (b) % of grid cells exposed to different levels of cumulative pressure (, where Pi is the value of the pressure associated to the driver i). (c) Pressure of single drivers per number of grid cells (Acidification was excluded due to the fact that it acts uniformly over the region).
State of the assemblages and relationships with anthropogenic pressures
The two axes of the PCoA ordination plot explained the 99% of the total variation in assemblage structure (see Figure S9 in Supplementary material) based on four main morpho-functional components of coralligenous outcrops, namely Calcified Algae, Invertebrates, Erect Macroalgae, Turf Algae (see Appendix S2). The 84% of total variation among sites was explained by the PCoA axis 1, which showed a clear shift from sites with assemblages characterized by the dominance of turf-forming algae (left side) to sites where typical bioconstructors of coralligenous outcrops (i.e., encrusting and erect calcified algae and invertebrates) were dominant (right side) (Figure S9). For each sampled site, the level of degradation derived from PCoA axis 1 was reported in Fig. 2. Sites 1–4, which were located within MPAs and in most cases far from the coast in areas featured by low levels of human pressure, showed a low level of degradation; the majority of sites exhibited medium or medium-high degradation, whereas sites 17, 22, 23, exhibited high to very high level of human pressure of these areas (Fig. 2).
Figure 2.
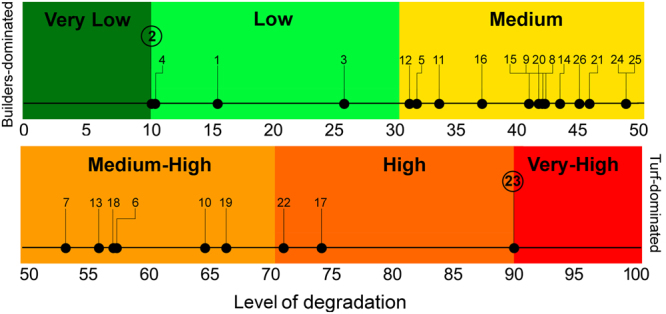
Condition of assemblages from PCoA analysis in the 26 investigated sites (see Fig. S9). Values of sampled sites along axis 1 of PCoA from the best (Site 2) and the worse (Site 23) recorded conditions were rescaled to vary between 10 and 90. Positions of Site 2 and 23 were assumed to mark the limit between very low-low and between high-very high degradation, respectively. Thresholds from very low to very high were set analogously to Halpern et al.6 and corresponded to a gradient of increasing degradation of coralligenous outcrops from a condition in which calcified algae and invertebrate builders were dominant towards a turf-dominated condition.
Plot of the residuals of actual data on coralligeous outcrops against the expected conditions calculated following the linear relationship identified by Halpern et al.6, indicated that such linear model did not explain adequately the relationship between Ic and the true condition of coralligenous assemblages (Figure S10 in Supplementary material). The Wald-Wolfowitz runs test returned a probability of P = 0.029 (number of runs = 8), indicating that the observed pattern in the residuals is non-random, and therefore the specific linear relationship (Ic = 0.1762 × [level of system degradation] − 0.3381) found by Halpern et al.6 is unlikely to be robust also for coralligenous outcrops (Fig. 3). Runs test indicated that all models (linear, logarithmic, log-linear, and log-log) used to fit actual data on the relationship between Ic versus assemblage conditions were not unlikely (Table 1). Regression analysis and AICc indicated that the best fit of data was achieved by using a log-log model (Table 1, Fig. 3). Thresholds in Ic strongly varied among different models, although all models led to increase the limits for categories that define less impacted conditions (Table 1).
Figure 3.
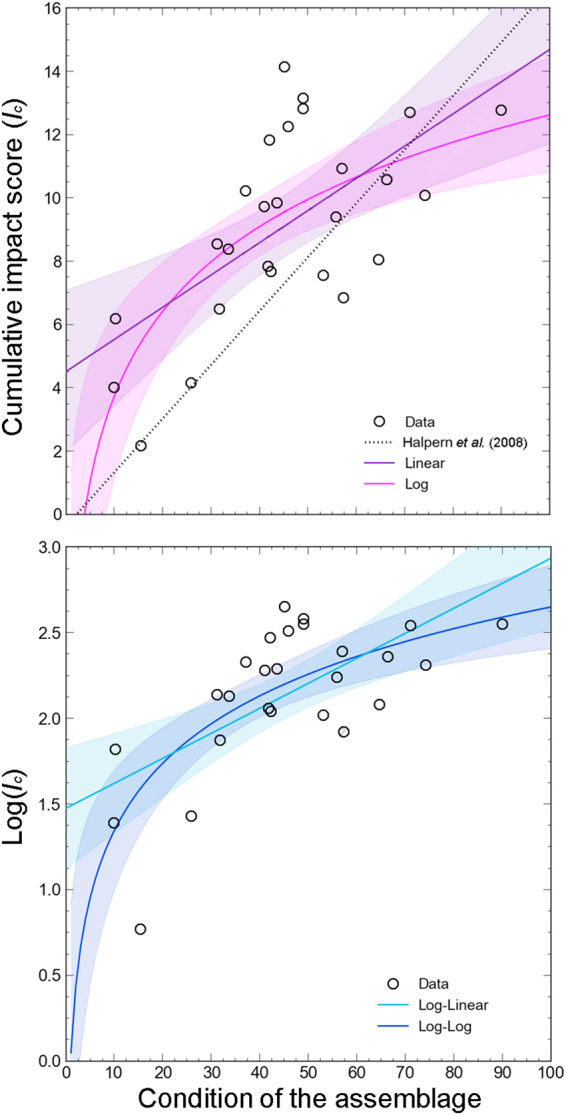
Linear/natural logarithmic (upper plot) and log-linear/log-log (lower plot) models of cumulative impact score (Ic) against the condition of coralligenous assemblages. The linear relationships provided by Halpern et al.6 was also showed (upper plot). Shaded areas around regression lines represented the 95%CI.
Table 1.
Results of regression analysis of cumulative impact score (Ic) against the state of coralligenous outcrops using different models.
| Regression analysis | ||||||
|---|---|---|---|---|---|---|
| Model | R 2 | SE | Significance level | AICc | Run test | |
| P (%) | no. of runs | |||||
| Linear | 0.40 | 2.41 | *** | 45.90 | 57.93 | 15 |
| Log | 0.47 | 2.28 | *** | 42.93 | 59.19 | 15 |
| Log-Linear | 0.42 | 0.33 | *** | −57.02 | 57.93 | 15 |
| Log-Log | 0.51 | 0.31 | *** | −61.50 | 43.23 | 14 |
| Ranks of cumulative impact score | ||||||
| Thresholds | ||||||
| Degradation level | Halpern et al. 6 | Linear | Log | Log-Linear | Log-log | |
| 10 (Low) | 1.40 | 5.55 | 3.78 | 5.07 | 3.86 | |
| 30 (Medium) | 4.95 | 7.58 | 8.00 | 6.79 | 7.19 | |
| 50 (Medium-High) | 8.47 | 9.62 | 9.97 | 9.10 | 9.59 | |
| 70 (High) | 12.00 | 11.66 | 11.26 | 12.18 | 11.60 | |
| 90 (Very high) | 15.52 | 13.69 | 12.23 | 16.31 | 13.37 | |
Results of AICc and run tests were also reported. SE = standard error of regression. ***P < 0.001. For each model, the corresponding thresholds for ranks of cumulative impact score were provided. Thresholds from Halpern et al.6 were also reported.
Distance-based multivariate multiple regression (DISTLM) showed that the 53.2%, of total variability among sites was explained considering the full set of drivers. Marginal tests on single drivers showed that correlation was significant only for Coastal Population, Shipping, Sewage Discharge, and Coastal Engineering, which in turn, also mostly contributed to the explained variation in coralligenous assemblages among sites (Table 2). Weights assigned to drivers based on expert opinion only partially matched the contribution to explain the observed patterns in coralligenous assemblages. Drivers with high weight (i.e., considered as having a great impact) from expert opinion had a weak, not significant, relationship with the actual condition of the assemblages, except for Coastal Population (Table 2). In contrast, drivers with lower weights (<2) showed a significant and relatively high correlation with multivariate patterns of assemblages (Table 2).
Table 2.
Results of marginal tests and contribution of each driver to explain the multivariate pattern of variation along the gradient of degradation of coralligenous outcrops from sampled sites.
| Driver | Pseudo-F | P | Explained variation | wi from Halpern et al.36 |
|---|---|---|---|---|
| Coastal Population | 11.85 | 0.001 | 0.33 | 2.5 |
| Artisanal Fisheries | 0.52 | 0.553 | 0.02 | 2.3 |
| Urbanization | 1.91 | 0.176 | 0.07 | 2.2 |
| Agriculture | 2.37 | 0.120 | 0.09 | 2.2 |
| Sewage Discharge | 6.12 | 0.017 | 0.20 | 2.1 |
| Coastal Engineering | 3.39 | 0.050 | 0.12 | 1.9 |
| Industrial Effluents | 1.55 | 0.213 | 0.06 | 1.6 |
| Shipping | 9.56 | 0.002 | 0.28 | 1.4 |
Significant results were given in bold. For each driver, weights (wi) based on expert opinion from Halpern et al.36 were also provided.
Regional map of expected cumulative impact
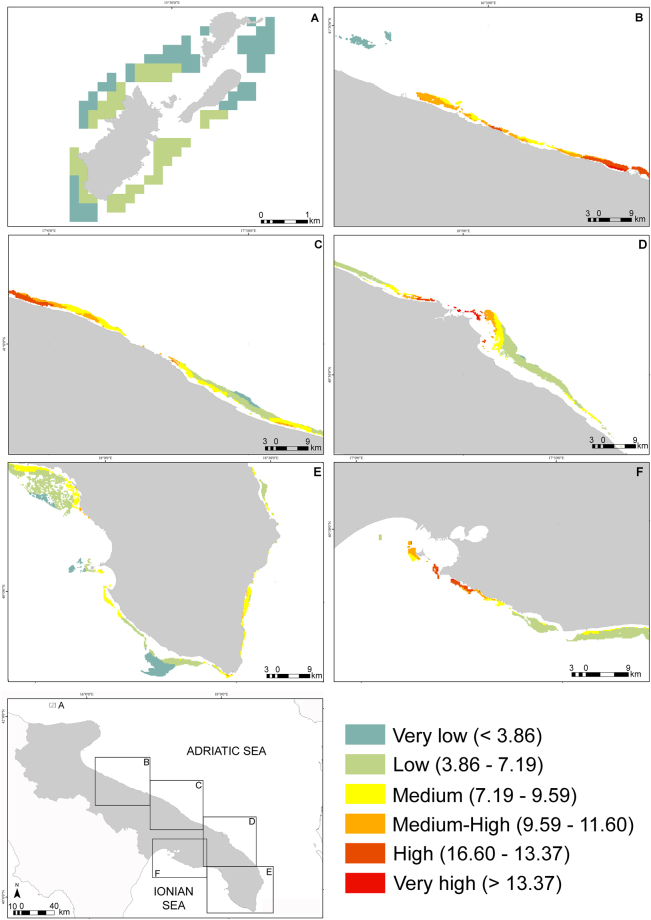
The regional map of cumulative impact of coralligenous outcrops was finally produced based on the new thresholds calculated from the log-log relationship between Ic and the condition of coralligenous outcrops (Fig. 4). The highest and widespread expected cumulative impact was recorded in sectors B and C, which are among the most populated and urbanized stretches of coast in the region. Spots of high or very high cumulative impact, corresponding to large coastal urban settlements, characterized sectors D and F. Sector E (a poorly urbanized stretch of coast along a huge terrestrial reserve) and A (a protected archipelago 12 nautic miles off the coast) appeared as the less impacted areas.
Figure 4.
Regional map (WGS84) of cumulative impact score (Ic) to coralligenous assemblages calculated following the best fitting model (log-log). The whole extent of coralligenous outcrops within 30 m depth at a regional scale was split into six sectors (a–f) to help displaying the spatial distribution of Ic. All coloured polygons in the map represent the areas characterized by the presence of coralligenous outcrops, whereas different colours indicated different levels of the expected cumulative impact on the outcrops. Limits of Ic defining different ranks of expected impact, from very low to very high, were reported in brackets. Maps were created using the ArcGIS® 10.1 software by ESRI (Environmental Systems Resource Institute, http://www.esri.com).
Maps of regional cumulative impact on coralligenous outcrops changed substantially whether using thresholds from Halpern et al.6 or the new thresholds obtained from the best fitting (log-log) model based on actual data (Fig. 5). The classic ranking led to assign all cells to 4 categories, i.e. low, medium, medium-high, high, with cumulative impact classified as medium in about half of cells. The new ranking, instead, identified also a small portion of very highly impacted cells and about 10% of cells with very low impact; most of cells were classified as subjected to a low impact.
Figure 5.
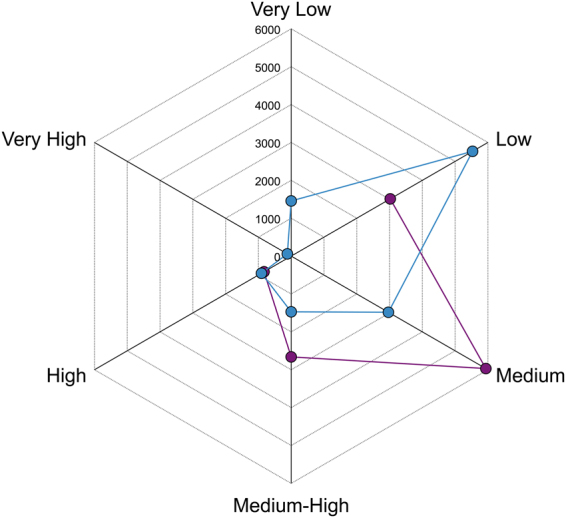
Spider plot of the number of grid cells assigned to the different classes of Ic following thresholds based on the linear model provided by Halpern et al.6, and the new thresholds based on the log-log model from actual data on coralligenous outcrops (respectively reported in violet and blue). Each of the six axes referred to each class of Ic.
Discussion
The use of refined data on the distribution and intensity of human pressures coupled with a habitat-specific calibration of thresholds in impact scores provided a more realistic picture of the severity of cumulative impact on coralligeous outcrops. The use of thresholds from Halpern et al.6 led most of coralligenous in the region to be classified as medium-impacted, with no areas of very low or very high impact. In contrast, the use of thresholds from the best fitting model based on actual data allowed us to discriminate the whole range of impact classes and, although depicting a general condition of low impact similarly to other cumulative impact assessments on the same habitat in the Mediterranean Sea (e.g.22), highlighted also the presence of very highly impacted cells deserving management priority. Interestingly, all sites included within MPAs exhibited low degradation, emphasising the critical role of protection in preventing human-driven shifts towards undesirable ecological conditions23 and increasing the resilience of assemblages24. This is a very strong argument supporting the use of MPAs as reference sites in large scale monitoring programs, such as those involved in the Marine Strategy Framework Directive (MSFD25), since the knowledge of baselines is lacking for most of marine assemblages and habitats and sound reference conditions represents a fundamental step in any quality status assessment26.
Our findings show that, irrespective of the relevant geographic extent (about 1000 kms) of the study area, distinct patterns of coralligenous degradation were associated to different levels of human pressure over and beyond spatial variability and changes in environmental conditions. At increasing level of pressures, a clear shift from assemblages featured by bioconstructors to assemblages dominated by turf-forming algae was detected. A complex suite of interactions emerged from our analyses on multiple pressures, indicating a critical role of coastal population, shipping, sewage discharge, and coastal engineering in driving the observed patterns of degradation. In most cases, coralligenous outcrops were subjected to a medium-high number of different drivers. Artisanal fishery and land based pollution (e.g., agriculture and urbanization) were the drivers acting at broad scale across the region, although accounting for a minor contribution to the observed patterns of degradation. Interestingly, sensitivity weights assigned by expert opinion to agriculture and artisanal fisheries were higher than those assigned to drivers strongly correlated with degraded conditions, suggesting that calibrations based on actual data are strictly necessary to adapt general sensitivity weights to regional or local contexts. This, in turn, could provide an objective basis to guide management policies in prioritizing actions on those anthropogenic drivers that could cause major cumulative impacts. In most cases, current knowledge on the relative importance of different drivers for ecosystem shifts and all potential synergies (or antagonism) is still incomplete. We can just assume that the effects of multiple stressors are additive. Stressors can nevertheless interact in complex ways, and non-additive effects have been demonstrated to be common in nature27. As an example, nutrient enrichment is recognized as a global problem associated with a range of human activities, with non-additive interactions with other stressors28 such as, for instance, physical damage from destructive fishing practices (e.g.8). Disentangling the combined effects of multiple stressors is, more than ever, necessary for a successful management of marine ecosystems29, and structured experimental designs with factorial combinations of stressors can provide invaluable insights in this perspective.
Mapping the distribution of human pressures and the ensuing cumulative effects is critical to inform the management of offshore and coastal zones30, as also recognized by recent commitments in Europe (e.g., MSFD) and elsewhere31. In this view, the cumulative impact score proposed by Halpern et al.6 has rapidly developed into a global standard and adapted to a variety of scenarios and scales, providing a general indication of the ecological status of several areas in the ocean11. The main concern is that aggregating the relative contribution of different pressures into a single index may not reflect important interactions that exist among individual sources of disturbance, environmental conditions and ecological responses, which likely represents a major cause of uncertainty for CPIA predictions32,33. A major issue relies on the fact that a given anthropogenic driver may exert multiple types of pressure. Since it is not the driver per se, but rather the associated pressures that ultimately relate to the ecological response, weighting drivers to estimate the expected impact in CPIA could be misleading, unless we assume that each pressure correspond to a specific driver, which is often not the case in the real world. The erroneous interchangeability of the concepts associated to the terms ‘driver’ and ‘pressure’ in the original method further contributed to generate confusion in the application of CPIA, although this ambiguity has been amended in recent reformulation of the approach (see1,34,35).
Congruence of cumulative impact scores is still a central question in CPIA, since the power to discriminate among expected levels of impact can change substantially depending on data resolution, thresholds of impact scores and weights assigned to anthropogenic drivers33. As recently discussed by Korpinen and Andersen13 in a global review on CPIA studies, in most works the effects of different pressures in order to calculate cumulative impact scores were weighted based on expert judgement (e.g.36). However, the understanding of potential effects of human pressures on different ecosystems is far from being exhaustive, thus limiting the possibility of comparisons between empirical evidence and expert opinion12,37. In addition, despite several studies used global weights in regional CPIA assessments (e.g.12,38), others have stressed the importance to calibrate CPIA to the specific region of interest (e.g.15,39,40). We found a substantial mismatch between sensitivity to pressures attributed by experts and correlations of pressures with the actual conditions of coralligenous outcrops. It could be argued that, irrespective of associated sensitivity weights, the most widespread and intense pressures were likely to be more correlated to changes in assemblage condition than those with lower intensity and reduced spatial coverage. Therefore, the observed mismatch could not be univocally interpreted as a departure of weight scores from the true habitat sensitivity to different pressures. In our case, however, most of pressures with limited spatial extension and intensity (i.e., Sewage discharge, Coastal Engineering, Shipping) were among the main drivers of the observed patterns in assemblage condition despite their relatively low weight scores (see Fig. 1 and Table 2), suggesting that, over and beyond pressure intensity and distribution, the development of context-specific weights might be more appropriate than the use of global weights for regional CPIA assessements14.
The level of estimation of expected impact from human pressures may severely affect the calculation of the cumulative impact score, which in turn, could mislead management actions. To date, very few studies concerned the validation of CPIA predictions by contrasting actual versus expected conditions of marine systems. Andersen et al.16 found a substantial alignment of expected impacts from CPIA with ecosystem condition, working at basin scale in the Baltic Sea. However, basin-scale CPIA approaches are likely to be adequate for sub-basin or regional management only if available data on ecosystems and threats insisting on the area are accurate enough to discriminate local conditions41. Downscaling expected impact from large to regional or local scale may be even more problematic in particular areas, such as the Maditerranean Sea12, due to the heterogeneity of habitats and distribution of threats15. Also, ground-truthing CPIA predictions may result in relatively weak relationships between expected impacts and actual conditions of ecosystems if the range of pressure level is small41, which could be often the case at a local scale.
Despite the fact that several issues may contribute to the uncertainty of predictions33,35, the CPIA approach is increasingly applied without substantial modifications to its original formulation. This is particularly significant considering the assumption of a linear relationship between pressure level and expected impact32, which is at the core of impact score assignments. We demonstrated that, at least for coralligenous outcrops, the linear model and derived thresholds in Ic provided by Halpern et al.6 failed to explain adequately the relationship between cumulative impacts and the condition of the system, casting doubts on their general application to a variety of ecosystems and geographic contexts. In our case, a non-linear, and specifically a log-log model, described better the relationship between Ic vs. the condition of the system. This pattern of pressure-state relationship appears rather plausible with respect to linearity of response. It reflects the ‘cliff’ paradigm42,43, in which natural ecosystems are viewed as resilient entities, able to absorb anthropogenic disturbance, at least until a certain level. Beyond this level the risk of ecosystem collapse is high, and if occurring, difficult to return to the original condition44. Analogously, our log-log empirical relation between coralligenous response versus increasing expected impact implies that the system may initially resist to pressure so that, a relatively large rise in cumulative pressure could have only limited effects. Once pressure intensity further increases, effects become more and more evident and, above certain levels, the system could rapidly deteriorate even as a consequence of small increments in cumulative pressure. Due to widespread evidence sustaining non-linearity of response in real-world ecosystems (e.g.33,45), generalizations on the pressure-state model to use in CPIA appears unfeasible. Such findings reinforce the idea that the use of weights (wi) allows a calibration of the cumulative impact score (Ic) only in terms of the sensitivity of different systems to different pressures13, emphasising the need to integrate calculation of Ic to account also for ecosystem-specific responses to increasing pressure level.
Maintaining biodiversity and achieving a Good Environmental Status (GES) of marine environments through an ecosystem-based approach to the management of human activities and a sustainable use of marine goods and services is the ultimate aim of the European MSFD44. Hence, a specific requirement of the MSFD is to consider the cumulative synergistic effects of human pressure in the assessment of GES34. Due to the dimension of the challenge, reliable tools for a rapid assessment of the condition of ecosystems and the detection of early warning signals of potential regime shifts are strongly advocated46. Our results confirm the potential of CPIA as a profitable framework to model the expected conditions of marine systems based on the distribution of human pressures, representing a cost-effective approach for marittime spatial planning. What is urgently needed is a more incisive effort to synthetize available information and fill existing gaps in linking human pressures and the response of ecosystems, in order to improve the effectiveness of CPIA in setting priority areas for conservation, mitigation, and restoration strategies. This study, by providing new information on patterns of degradation and sensitivity to pressure of a priority habitat, such as coralligenous outcrops, is a first step in this direction and represents a concrete effort to improve the effectiveness of CPIA that can be extended to other habitats in the Mediterranean and elsewhere.
Methods
Cumulative pressure and impact assessment on coralligenous outcrops
A continuous habitat map (1:25000) based on georeferenced data on the occurrence of coralligenous outcrops up to 30 m depth along the Apulian coasts was obtained from mapping activities (http://www.sit.puglia.it/portal/portale_rete_ecologica/biomap), which combined high-resolution morphobathymetric data (Multibeam echosounder), sismostratigraphic profiles (Chirp sonar and sub-bottom profiler), and acoustic seabed photogrammetry (using Side Scan Sonar) at regional scale.
CPIA on coralligenous outcrops was conducted following the framework of Halpern et al.6. As first, we considered all threats to marine ecosystems from the comprehensive list provided in Halpern et al.36. Fourteen threats, out of a total of 35 in the list, were not considered in the analysis since they were absent from (i) the whole region (Ocean mining, Offshore development), (ii) the areas where the investigated habitat is distributed (Demersal, destructive and non-destructive fishing, Pelagic-high bycatch fishing, Freshwater input), (iii) the depth range characterizing the investigated outcrops (Sea temperature increase, Sea level rise, Ozone/UV, Harmful algal blooms, Hypoxia), or negligible (Atmospheric pollution, Species invasion). Nine additional threats (Benthic Structures, Ecoturism, Diseases, Aquarium fishing, Illegal fishing, Artisanal, non-destructive fishing, Recreational fishing, Sediment input, Nutrient input) were also excluded since no data were available (see Table S1 in Supplementary material for further details). A total of 12 threats were retained and spatial data on related anthropogenic drivers (i.e., Sewage discharge, Industrial Effluents, Agriculture, Urbanization, Coastal Engineering, Coastal Erosion, Coastal Population, Aquaculture, Artisanal Fisheries, Ocean Acidification, Shipping, Commercial Activity) were collected and mapped (Table S1). The intensity of pressures associated to the 12 drivers was quantified in terms of: (1) population equivalent for Sewage discharge, (2) land cover of industrial areas within 300 m from the coast for Industrial Effluents, (3) land cover of agricultural areas within 1 km from the coast for Agriculture, (4) land cover of urban areas within 1 km from the coast for Urbanization, (5) size of coastal structures for Coastal Engineering, (6) landward beach displacement for Coastal Erosion, (7) population size and density for Coastal Population, (8) surface of farmed areas for Aquaculture, (9) number of vessels per size categories for Artisanal Fisheries, (10) aragonite saturation state for Ocean Acidification, (11) total tonnage of shipped goods for Shipping, and (12) number of ship tracks per cell for Commercial Activity. The overlay analysis between the spatial distribution of the investigated habitat and the area of influence of Coastal Erosion, Aquaculture, and Commercial Activity allowed to exclude any potential interference with coralligenous outcrops within 30 m depth. All modelled spatial data layers concerning the origin and intensity of the remaining pressures were integrated and used in the CPIA analysis and maps. A detailed description of threats and related anthropogenic drivers considered in the analysis is provided in the Supplementary material.
All layers on anthropogenic pressures and coralligenous distribution were mapped through ArcGIS® 10.1 software by ESRI (Environmental Systems Resource Institute, http://www.esri.com/software/arcgis) on a 200 × 200 m (0.04 km2) pixel grid. For each driver, the value of the associated pressure assigned to the cell was that of the raster pixel falling at its center. Negative exponential models were used to estimate the distance decay from 100% until 0% in the intensity of pressures on a 200 m-distance raster matrix. A further theoretical decrease in the intensity of pressures equal to 10% each 10-m depth increment was applied to account for reduction of potential effects of pressures due to bathymetry22. Full details on drivers, associated pressures, and data treatments, along with distribution maps at regional scale, are provided in the Supplementary material. All values were normalized applying a log(X+1) transformation and rescaled in the range 0 to 112.
According to Halpern et al.6 and subsequent formulations1,34, the cumulative impact score on coralligenous outcrops (Ic) was calculated following the equation (1):
| 1 |
where n is the number of drivers within the examined grid cell, Pi is the normalized value of the pressure associated to the anthropogenic driver i, Ec denoted the presence/absence of coralligenous outcrops in the grid cell, and wi is the weighting coefficient that represents the impact weight score for the anthropogenic driver i on the focus ecosystem. Weight scores referred to those listed for “Rocky Reefs” in Halpern et al.36, since coralligenous outcrops can be ascribed to this ecosystem category, which include sublittoral hard bottoms up to 30–60 m depth12.
Finally, a regional map of estimated cumulative impact on coralligenous outcrops was obtained by assigning the corresponding expected cumulative impact to each cell of the habitat map. The same thresholds as in Halpern et al.6 and Micheli et al.12 were used to designate ecologically meaningful categories of impact scores (Ic): very high (>15.52); high (12–15.52); medium-high (8.47–12); medium (4.95–8.47); low (1.4–4.95); and very low impact (<1.4).
Field survey on coralligenous outcrops
Two extensive surveys along the Apulia coast were carried out in order to investigate the extent to which the estimated cumulative impact scores were aligned to the actual condition of coralligenous assemblages. Surveys were conducted in 2012 and 2013 (June-July) across a set of 26 sites. Sites were randomly selected to be representative of coralligenous outcrops from the whole region and of the range of cumulative pressures at a regional scale (Table S2 in Supplementary material), including Marine Protected Areas (MPAs), and areas featured by low, moderate and high levels of human activities. In each site, coralligenous outcrops at 20–25 m depth were photographically sampled along three 25 m-long transects (tens of meters far each other). For each transect, 8 sampling units (each one covering a surface of approximately 0.2 m2) were randomly selected and, for each sampling unit, a composite of 6 adjacent photographic samples (23 × 15 cm) were collected using a high-resolution digital camera. A total of 3744 photographic samples were analysed in order to estimate % cover of sessile taxa. The 64 taxa found were then grouped into four categories representing the main morpho-functional components of coralligenous outcrops (see Table S3 in Supplementary material): Calcified Algae (principal builders and typical component of the hard structure of coralligenous outcrops, including algae with high calcification of the thallus, e.g. encrusting corallines), Erect Macroalgae (contributing to bioconstruction or to three-dimensional complexity of outcrops, including algae with low calcification, e.g. Flabellia petiolata, Halimeda tuna), Turf Algae (opportunistic, filamentous, or turf-forming algae with low or absent calcification, e.g. Gelidium spp.), Invertebrates (typical component of outcrops, second main builders of bioconstructions after calcified algae, including sponges, bryozoans, calcareous tube worms, madreporarians and others). Since the structure of coralligenous assemblages mostly encompasses calcified algae and invertebrates19, whereas the dominance of turf-forming algae is a recognized indicator of disturbed conditions (e.g.47), shifts in dominance of these main components, from calcified algae and invertebrates towards turf-dominated assemblages, can be interpreted as a transition from healthy to degraded conditions of the outcrops.
Statistical analyses
Thresholds of impact scores, which are generally used to classify the estimated cumulative impact in the CPIA approach, were provided in Halpern et al.6 and obtained by fitting a linear regression of the Ic versus the state of degradation of the system. This linear relationship was based on 16 points from different ecosystems (mostly coral reefs and associated habitats), and thresholds in Ic calculated to correspond to <10% (very low), 10–30% (low), 30–50% (medium), 50–70% (medium-high), 70–90% (high), and >90% (very high) percentage of ecosystem degradation. The level of degradation was based on a multivariate gradient analysis on main reef guilds, classifying ecosystems from pristine to ecologically extinct conditions48. Analogously, we quantified the condition of coralligenous outcrops based on their main structural components, assessing whether the linear relationship and thresholds in Ic derived by Halpern et al.6 applied also to our case.
First, a Principal Coordinates Analysis (PCoA) of site centroids based on Bray-Curtis similarity was carried out to visualize patterns of variation in assemblage structure among sites. The contributions of the four main components of assemblage structure to similarity patterns were visualized as correlation vectors on the ordination plot. Most of variation (>80%) in assemblage structure among sites was explained by the PCoA axis 1. Since PCoA values of sites along the axis strongly correlate with the condition of coralligenous assemblages from the best (dominance of calcified algae and animal builders) to the worse (dominance of turf-forming algae) structure (see Results), they were assumed to correspond to a gradient of increasing degradation of coralligenous outcrops. PCoA values of sites, from the lowest (best recorded condition) to the highest (worse recorded condition), along the axis 1 were rescaled to vary from 10 to 90, assuming the lowest and the highest value as the limit between very low-low and high-very high condition of degradation respectively. Then, for each of the 26 surveyed sites, values of Ic were plotted against the corresponding condition of assemblages obtained from the PCoA. Residuals of actual data points with respect to the expected Ic values from the linear model provided by Halpern et al.6 were analysed, and the Wald-Wolfowitz runs test49 was performed to check the correctness of the model. Runs test returns the probability that the observed pattern in the residuals is unlikely (P < 0.05) or not unlikely (P > 0.05), and therefore, that the equation used to fit data is unlike to be correct or could be correct (i.e., the residuals are randomly distributed around the fitted curve) respectively.
Other potential relationships between Ic and the condition of assemblages were explored by fitting linear [y = ax + b], logarithm [y = aln(x) + b], log-linear [ln(y) = ax + b], and log-log [ln(y) = aln(x) + b] models to data points. Corrected Akaike Information Criterion (AICc)50 was used to identify the model best fitting data, and runs tests were also performed to check patterns in the residuals. We anticipate that the log-log model performed better than any other in fitting actual data. Therefore, we re-calculated analogous thresholds in Ic as done by Halpern et al.6 following the log-log model. Finally, we produced a regional map of Ic of coralligenous outcrops based on the new thresholds and compared the results with those obtained using classic thresholds.
The relationships between the assemblage structure of sites and the full set of anthropogenic drivers was investigated using a non-parametric multivariate regression (DISTLM, distance-based multivariate multiple regression based on a linear model51). Prior to analysis, all explanatory variables (i.e., the variables measuring the pressure associated to anthropogenic drivers) were transformed using log(X+1) and rescaled between 0 and 1. A marginal test was employed to check the individual relationship of each driver with multivariate assemblage data and select those driver having a significant correlation with the observed patterns in coralligenous assemblages. Analyses were based on Bray-Curtis similarities and tests done using 999 permutations. All statistical analyses were carried out using R52.
Data availability
Web links to all sources of pressure data and habitat maps used in the study are reported in the main text or in the Supplementary Information file. The dataset on coralligenous assemblages analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
Research funded by the project BIOMAP - BIOcostruzioni MArine in Puglia (Regione Puglia - Programma P.O FESR 2007/2013 - ASSE IV. Linea 4.4 - http://biomapping.it/index). The European Union’s Horizon 2020 research for the project MERCES (Grant agreement No. 689518, http://www.merces-project.eu) and the EU Interreg MED AMAre Project (http://msp-platform.eu/projects/amare-actions-marine-protected-areas) are also acknowledged. We also thank Peter Mackelworth for the carefull revision of English language.
Author Contributions
S.F. conceived the idea and supervised the work; G.G., G.F., S.F. and S.B. conducted field works and collected the data; S.B. and G.F. set methodologies and analysed the data; S.B., S.F. and G.G. led the writing of the manuscript, with improvements provided by G.F. and A.T. All authors contributed critically to the drafts and gave final approval for publication.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
S. Bevilacqua and G. Guarnieri contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20297-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. Bevilacqua, Email: stanislao.bevilacqua@unisalento.it
S. Fraschetti, Email: simona.fraschetti@unisalento.it
References
- 1.Halpern BS, et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 2015;6:7615. doi: 10.1038/ncomms8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 3.Claudet J, Fraschetti S. Human-driven impacts on marine habitats: a regional meta-analysis in the Mediterranean Sea. Biol. Conserv. 2010;143:2195–2206. doi: 10.1016/j.biocon.2010.06.004. [DOI] [Google Scholar]
- 4.Breitburg D, Seitzinger S, Sanders J. The effects of multiple stressors on freshwater and marine ecosystems - Preface. Limnol. Oceanogr. 1999;44:737–738. doi: 10.4319/lo.1999.44.3_part_2.0837. [DOI] [Google Scholar]
- 5.Rosenberg AA, McLeod KL. Implementing ecosystem-based approaches to management for the conservation of ecosystem services. Marine Ecology Progress Series. 2005;300:270–274. doi: 10.3354/meps300270. [DOI] [Google Scholar]
- 6.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 7.Jokiel PL, et al. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs. 2008;27:473–483. doi: 10.1007/s00338-008-0380-9. [DOI] [Google Scholar]
- 8.Guarnieri G, Bevilacqua S, Vignes F, Fraschetti S. Grazer removal and nutrient enrichment as recovery enhancers for overexploited rocky subtidal habitats. Oecologia. 2014;175:959–970. doi: 10.1007/s00442-014-2944-4. [DOI] [PubMed] [Google Scholar]
- 9.Piazzi L, Gennaro P, Balata D. Effects of nutrient enrichment on macroalgal coralligenous assemblages. Mar. Pollut. Bull. 2011;62:1830–1835. doi: 10.1016/j.marpolbul.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Stelzenmüller V, Lee J, South A, Rogers SI. Quantifying cumulative impacts of human pressures on the marine environment. A geospatial modelling framework. Mar. Ecol. Prog. Ser. 2010;398:19–32. doi: 10.3354/meps08345. [DOI] [Google Scholar]
- 11.Ban NC, Alidina HM, Ardron JA. Cumulative impact mapping: Advances, relevance and limitations to marine management and conservation, using Canada’s Pacific waters as a case study. Mar. Policy. 2010;34:876–886. doi: 10.1016/j.marpol.2010.01.010. [DOI] [Google Scholar]
- 12.Micheli F, et al. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLOS ONE. 2013;8:e79889. doi: 10.1371/journal.pone.0079889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korpinen S, Andersen JH. A Global Review of Cumulative Pressure and Impact Assessments in Marine Environments. Front. Mar. Sci. 2016;3:153. doi: 10.3389/fmars.2016.00153. [DOI] [Google Scholar]
- 14.Halpern BS, Fujita R. Assumptions, challenges, and future directions in cumulative impact analysis. Ecosphere. 2013;4:1–11. doi: 10.1890/ES13-00181.1. [DOI] [Google Scholar]
- 15.Guarnieri G, et al. The Challenge of Planning Conservation Strategies in Threatened Seascapes: Understanding the Role of Fine Scale Assessments of Community Response to Cumulative Human Pressures. PLOS ONE. 2016;11:e0149253. doi: 10.1371/journal.pone.0149253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen JH, Halpern BS, Korpinen S, Murray C, Reker J. Baltic Sea biodiversity status vs. cumulative human pressures. Estuar. Coast. Shelf Sci. 2015;161:88–92. doi: 10.1016/j.ecss.2015.05.002. [DOI] [Google Scholar]
- 17.Coll M, et al. The Mediterranean Sea under siege: spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 2012;21:465–480. doi: 10.1111/j.1466-8238.2011.00697.x. [DOI] [Google Scholar]
- 18.Martin CS, et al. Coralligenous and maërl habitats: predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci. Rep. 2014;4:5073. doi: 10.1038/srep05073. [DOI] [Google Scholar]
- 19.Ballesteros E. Mediterranean coralligenous assemblages: a synthesis of present klowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006;44:123–195. [Google Scholar]
- 20.Tamburello L, Benedetti-Cecchi L, Ghedini G, Alestra T, Bulleri F. Variation in the structure of subtidal landscapes in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 2012;457:29–41. doi: 10.3354/meps09703. [DOI] [Google Scholar]
- 21.Deter J, Descamp P, Ballesta L, Boissery P, Holon F. A preliminary study toward an index based on coralligenous assemblages for the ecological status assessment of Mediterranean French coastal waters. Ecol. Indic. 2012;20:345–352. doi: 10.1016/j.ecolind.2012.03.001. [DOI] [Google Scholar]
- 22.Holon F, et al. Fine-Scale Cartography of Human Impacts along French Mediterranean Coasts: A Relevant Map for the Management of Marine Ecosystems. PLOS ONE. 2015;10:e0135473. doi: 10.1371/journal.pone.0135473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraschetti S, Guarnieri G, Bevilacqua S, Terlizzi A, Boero F. Protection Enhances Community and Habitat Stability: Evidence from a Mediterranean Marine Protected Area. PLOS ONE. 2013;8:e81838. doi: 10.1371/journal.pone.0081838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevilacqua S, Terlizzi A, Fraschetti S, Russo GF, Boero F. Mitigating human disturbance: can protection influence trajectories of recovery in benthic assemblages? J. Anim. Ecol. 2006;75:908–920. doi: 10.1111/j.1365-2656.2006.01108.x. [DOI] [PubMed] [Google Scholar]
- 25.European Parliament and Council. Marine Strategy Framework Directive, 2008/56/EC. Off. J. Eur. Union L164/19 (2008).
- 26.Borja Á, Dauer DM, Grémare A. The importance of setting targets and reference conditions in assessing marine ecosystem quality. Ecol. Indic. 2012;12:1–7. doi: 10.1016/j.ecolind.2011.06.018. [DOI] [Google Scholar]
- 27.Darling ES, Côté IM. Quantifying the evidence for ecological synergies. Ecol. Lett. 2008;11:1278–1286. doi: 10.1111/j.1461-0248.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- 28.Strain E, Thomson RJ, Micheli F, Mancuso FP, Airoldi L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob. Change Biol. 2014;20:3300–3312. doi: 10.1111/gcb.12619. [DOI] [PubMed] [Google Scholar]
- 29.Crain CM, Kroeker K, Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008;11:1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 30.Depellegrin D, et al. Multi-objective spatial tools to inform maritime spatial planning in the Adriatic Sea. Sci. Total Environ. 2017;609:1627–1639. doi: 10.1016/j.scitotenv.2017.07.264. [DOI] [PubMed] [Google Scholar]
- 31.Judd AD, Backhaus T, Goodsir F. An effective set of principles for practical implementation of marine cumulative effects assessment. Environ. Sci. Policy. 2015;54:254–262. doi: 10.1016/j.envsci.2015.07.008. [DOI] [Google Scholar]
- 32.Gissi E, et al. Addressing uncertainty in modelling cumulative impacts within maritime spatial planning in the Adriatic and Ionian region. PLOS ONE. 2017;12:e0180501. doi: 10.1371/journal.pone.0180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stock A, Micheli F. Effects of model assumptions and data quality on spatial cumulative human impact assessments. Glob. Ecol. Biogeogr. 2016;25:1321–1332. doi: 10.1111/geb.12493. [DOI] [Google Scholar]
- 34.Borja Á, et al. Overview of integrative assessment of marine systems: the Ecosystem Approach in practice. Front. Mar. Sci. 2016;3:20. [Google Scholar]
- 35.Stelzenmüller V, et al. A risk-based approach to cumulative effect assessments for marine management. Sci. Total Environ. 2017;612:1132–1140. doi: 10.1016/j.scitotenv.2017.08.289. [DOI] [PubMed] [Google Scholar]
- 36.Halpern BS, Selkoe KA, Micheli F, Kappel CV. Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv. Biol. 2007;21:1301–1315. doi: 10.1111/j.1523-1739.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 37.Teck SJ, et al. Using expert judgment to estimate marine ecosystem vulnerability in the California Current. Ecol. Appl. 2010;20:1402–16. doi: 10.1890/09-1173.1. [DOI] [PubMed] [Google Scholar]
- 38.Afflerbach JC, Yocum D, Halpern BS. Cumulative human impacts in the Bering Strait Region. Ecosyst. Health Sustain. 2017;3:1379888. doi: 10.1080/20964129.2017.1379888. [DOI] [Google Scholar]
- 39.Korpinen S, Meski L, Andersen JH, Laamanen M. Human pressures and their potential impact on the Baltic Sea ecosystem. Ecol. Indic. 2012;15:105–114. doi: 10.1016/j.ecolind.2011.09.023. [DOI] [Google Scholar]
- 40.Knights AM, et al. A step-wise process of decision-making under uncertainty when implementing environmental policy. Environ. Science Policy. 2014;39:56–64. doi: 10.1016/j.envsci.2014.02.010. [DOI] [Google Scholar]
- 41.Clark D, Goodwin E, Sinner J, Ellis J, Singh G. Validation and limitations of a cumulative impact model for an estuary. Ocean Coast. Manage. 2016;120:88–98. doi: 10.1016/j.ocecoaman.2015.11.013. [DOI] [Google Scholar]
- 42.Elliott M, Burdon D, Hemingway KL, Apitz SE. Estuarine, coastal and marine ecosystem restoration: confusing management and science – a revision of concepts. Estuar. Coast. Shelf Sci. 2007;74:349–366. doi: 10.1016/j.ecss.2007.05.034. [DOI] [Google Scholar]
- 43.Tett P, et al. Defining and detecting undesirable disturbance in the context of marine eutrophication. Mar. Pollut. Bull. 2007;55:282–297. doi: 10.1016/j.marpolbul.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Tett P, et al. Framework for understanding marine ecosystem health. Mar. Ecol. Prog. Ser. 2013;494:1–27. doi: 10.3354/meps10539. [DOI] [Google Scholar]
- 45.Hunsicker ME, et al. Characterizing driver–response relationships in marine pelagic ecosystems for improved ocean management. Ecol. Appl. 2016;26:651–663. doi: 10.1890/14-2200. [DOI] [PubMed] [Google Scholar]
- 46.Borja Á, Elliott M. Marine monitoring during an economic crisis: the cure is worse than the disease. Mar. Pollut. Bull. 2013;68:1–3. doi: 10.1016/j.marpolbul.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 47.Montefalcone M, Morri C, Bianchi CN, Bavestrello G, Piazzi L. The two facets of species sensitivity: Stress and disturbance on coralligenous assemblages in space and time. Mar. Pollut. Bull. 2017;117:229–238. doi: 10.1016/j.marpolbul.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 48.Pandolfi JM, et al. Global Trajectories of the Long-Term Decline of Coral Reef Ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 49.Wald A, Wolfowitz J. On a test whether two samples are from the same population. Ann. Math. Stat. 1940;11:147–162. doi: 10.1214/aoms/1177731909. [DOI] [Google Scholar]
- 50.Akaike H. A new look at the statistical model identification. IEEE T. Automat. Contr. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 51.McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [DOI] [Google Scholar]
- 52.R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Web links to all sources of pressure data and habitat maps used in the study are reported in the main text or in the Supplementary Information file. The dataset on coralligenous assemblages analysed during the current study are available from the corresponding author on reasonable request.