INTRODUCTION
Clinicians frequently order random blood glucose (RBG) in routine laboratory panels. Although RBG values ≥200 mg/dL with hyperglycemic symptoms are diagnostic of diabetes, interpreting RBG results below this threshold is challenging, because the impact of food and calorie-containing drinks on glucose in non-fasting individuals is unclear. As a result, clinicians often ignore RBG values1—even though they are strongly associated with undiagnosed diabetes and prediabetes and can identify those at risk of dysglycemia.2 , 3 To improve interpretation of RBG values in non-fasting individuals without self-reported dysglycemia, we stratified participants in the National Health and Nutrition Examination Survey (NHANES) by hemoglobin A1C (HbA1c) and characterized the relationship between RBG and time since last caloric intake.
METHODS
We analyzed merged data from the 2007–2012 NHANES—a stratified survey representative of the non-institutionalized US population. Non-pregnant adults aged ≥ 18 years without self-reported diabetes or prediabetes and with both RBG and HbA1c were eligible. NHANES participants were randomly assigned to fast. Fasting participants self-reporting nothing to eat or drink except water within 9 h of laboratory testing were excluded. Serum glucose results from non-fasting participants were considered RBG values. Criterion-standard glycemic status [normoglycemia (HbA1c < 5.7%); undiagnosed dysglycemia (HbA1c ≥ 5.7%)] was determined by HbA1c.
We calculated time since last caloric intake as the difference in time between laboratory testing and last-reported intake of food or non-water beverage. We used linear regression and the postestimation margins command (Stata/SE 13.1; StataCorp LP, College Station, TX) to predict mean RBG values using time since last caloric intake and glycemic status. All analyses incorporated NHANES sampling weights. We present unadjusted analyses to reflect the real-world interpretation of RBG values in clinical practice. The study was deemed exempt by the UT Southwestern Institutional Review Board.
RESULTS
A total of 7161 participants met the study criteria, of whom 3.9% had undiagnosed diabetes and 31% had undiagnosed dysglycemia. The mean (SD) time since last caloric intake was 2.3 (1.8) hours, and 85% reported caloric intake within 4 h of testing. Relative to those with normoglycemia, those with undiagnosed dysglycemia were older, had higher BMI, RBG, and HbA1C values, and a greater burden of cardiovascular risk factors (Table 1).
Table 1.
Characteristics of NHANES Participants Without Diagnosed Diabetes and Prediabetes According to Gold-Standard Glycemic Status
| All patients (N = 7161) |
Normal, A1C < 5.7% (N = 4945) |
Undiagnosed dysglycemia, A1C ≥ 5.7% (N = 2216) |
P-value† | |
|---|---|---|---|---|
| Age, mean (SD), years | 44.7 (17.0) | 41.2 (16.7) | 55.1 (14.0) | <0.001 |
| Female, % | 3306 (50.8) | 2526 (51.4) | 1050 (49.1) | 0.13 |
| Race/ethnicity | ||||
| White, % | 3306 (69.7) | 2399 (71.6) | 907 (63.7) | <0.001 |
| Black, % | 1403 (10.2) | 850 (8.7) | 553 (14.5) | <0.001 |
| Mexican American, % | 1097 (7.9) | 754 (7.9) | 343 (8.0) | 0.96 |
| Hispanic, % | 739 (5.4) | 508 (5.4) | 231 (5.4) | 0.97 |
| Asian/other race, % | 616 (6.8) | 434 (6.2) | 182 (8.4) | 0.01 |
| BMI, mean (SD), kg/m2* | 28.0 (6.3) | 27.3 (6.1) | 30.2 (6.4) | <0.001 |
| Hypertension, % | 1961 (23.7) | 1037 (18.8) | 924 (38.4) | <0.001 |
| Hyperlipidemia, % | 1856 (26.3) | 1012 (21.6) | 844 (40.0) | <0.001 |
| Heart disease, % | 452 (4.7) | 202 (2.8) | 250 (10.4) | <0.001 |
| Family history of diabetes, % | 2406 (31.7) | 1518 (29.0) | 888 (39.7) | <0.001 |
| Random glucose, mean (SD), mg/dL | 92.6 (22.4) | 89.1 (16.2) | 102.9 (30.4) | <0.001 |
| Hemoglobin A1c, mean (SD), % | 5.4 (0.6) | 5.2 (0.3) | 6.0 (0.6) | <0.001 |
| Fasting time, mean (SD), hours | 2.3 (1.8) | 2.2 (1.9) | 2.4 (1.7) | <0.001 |
*N = 7073 due to missing data in NHANES
† P-value for comparison of normal versus dysglycemia
BMI, body mass index
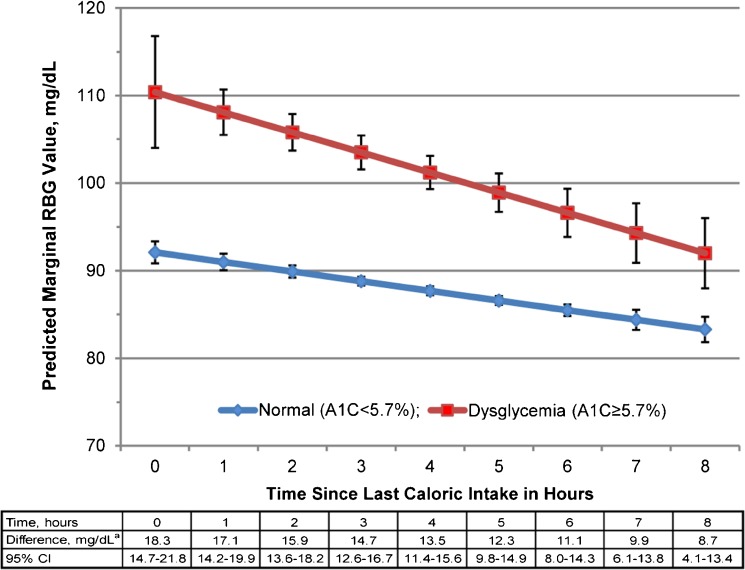
In the first 8 h after caloric intake, those with undiagnosed dysglycemia had significantly higher RBG values than those with normoglycemia. Within 4 h of caloric intake, those with undiagnosed dysglycemia had RGB values that were ≥100 mg/dL and were 14–18 mg/dL higher than those with normoglycemia (Fig. 1). Those with normoglycemia had RBG values <100 mg/dL at all time points. By 6 h, even those with undiagnosed dysglycemia had RBG values <100 mg/dL.
Figure 1.
Relationship between random blood glucose and time since last caloric intake according to glycemic status. RBG, random blood glucose; A1C, hemoglobin A1C. *Difference = RBG dysglycemia − RBG normal.
DISCUSSION
In a nationally representative sample of non-fasting, community-dwelling individuals, those with undiagnosed dysglycemia had significantly higher RBG values than those with normoglycemia within 9 h of caloric intake. Rather than ignore RBG values ≥100 mg/dL measured within 4 h of eating or drinking, clinicians should order gold-standard diabetes tests to improve detection of undiagnosed dysglycemia.
Most individuals eat and drink throughout the day and present for clinical care and laboratory testing in a non-fasting state. RBG is elevated within 3 h of caloric intake in individuals without diabetes.4 Our findings demonstrate that these RBG elevations are driven largely by individuals with undiagnosed dysglycemia. Fasting beyond 4 h is unlikely to be clinically meaningful because most individuals, regardless of glycemic state, will be below fasting glucose diagnostic cut-points for dysglycemia. Gold-standard diabetes testing is low-yield in individuals with RBG values <100 mg/dL, because most will have normoglycemia.
Although clinicians may be reluctant to interpret RBG readings at face value because of measurement variation and the impact of age, sex, and BMI on glucose readings,5 asymptomatic RBG values ≥100 mg/dL are a strong indicator of diabetes risk and are associated with undiagnosed dysglycemia.6 Case-identification strategies based on RBG recommend screening for fewer individuals, have higher specificity, and perform better than diabetes screening guidelines, which do not include RBG.3 Given the availability of existing RBG values in electronic health records, ordering gold-standard diabetes tests on patients with unknown glycemic status and RBG values ≥100 mg/dL—regardless of the type or time of last caloric intake—may simplify RBG interpretation and improve detection of dysglycemia.
Acknowledgments
Contributors
M.B. participated in the design, analysis, data interpretation, and drafting of the manuscript. L.X. participated in analysis, data interpretation, and manuscript revision. I.L. participated in the data interpretation and manuscript revision. E.H participated in the design, analysis, data interpretation, and manuscript revision.
M.B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funders
This study was conducted using resources provided by the UT Southwestern Center for Patient-Centered Outcomes Research (AHRQ R24 HS022418).
Dr. Bowen was supported by the NIH/NIDDK (K23 DK104065), the National Center for Advancing Translational Sciences of the NIH (KL2TR001103), and the Dedman Family Endowed Program for Scholars in Clinical Care. Drs. Halm and Xuan were supported in part by AHRQ R24 HS022418.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality. The funding sources had no involvement in study design; data collection, analysis, or interpretation; writing the report; or submission for publication.
Prior Presentations
Preliminary data from this study were presented at the 2015 Society of General Internal Medicine National Meeting, Toronto, Ontario, Canada.
Conflict of Interest
The authors report no potential conflicts of interest relevant to this work.
References
- 1.Ealovega MW, Tabaei BP, Brandle M, Burke R, Herman WH. Opportunistic screening for diabetes in routine clinical practice. Diabetes Care. 2004;27(1):9–12. doi: 10.2337/diacare.27.1.9. [DOI] [PubMed] [Google Scholar]
- 2.Ziemer DC, Kolm P, Foster JK, et al. Random plasma glucose in serendipitous screening for glucose intolerance: screening for impaired glucose tolerance study 2. J Gen Intern Med. 2008;23(5):528–35. doi: 10.1007/s11606-008-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen ME, Xuan L, Lingvay I, Halm EA. Performance of a Random Glucose Case-Finding Strategy to Detect Undiagnosed Diabetes. Am J Prev Med. 2017;52(6):710–6. doi: 10.1016/j.amepre.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moebus S, Gores L, Losch C, Jockel KH. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol. 2011;26(9):719–28. doi: 10.1007/s10654-011-9608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57(6):e1–e47. doi: 10.1373/clinchem.2010.161596. [DOI] [PubMed] [Google Scholar]
- 6.Bowen ME, Xuan L, Lingvay I, Halm EA. Random blood glucose: a robust risk factor for type 2 diabetes. J Clin Endocrinol Metab. 2015;100(4):1503–10. doi: 10.1210/jc.2014-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]