Graphical abstract
Abbreviations: AlP, aluminium phosphide; Lam, moringa oleifera extract; CAT, catalase; GR, glutathione reductase; SOD, superoxid dismutase; MDA, malondialdehyde (product of lipid peroxidation); ROS, reactive oxidative stress
Keywords: Toxicity, Aluminium phosphide, Cardiac toxicity, Oxidative stress, Moringa oleifera extract
Highlights
-
•
Aluminium phosphide (AlP) affects many organs especially heart.
-
•
Antioxidant activities of Moringa was investigated after acute AlP intoxication.
-
•
Moringa preventive effects were determined histopathologically.
-
•
Moringa significantly re-equilibrates antioxidant parameters back near to their normal values.
Abstract
Background
Moringa oleifera extract (Lam) has many antioxidant and protective properties. Objective: to investigate the antioxidant activities of Lam in counteracting the high oxidative stress caused by acute sub-lethal aluminium phosphide (AlP) intoxication in rat heart. These activities will be detected by histopathological examination and some oxidative stress biomarkers.
Methods
a single sub-lethal dose of Alp (2 mg/kg body weight) was administered orally, and Lam was given orally at a dose (100 mg/kg body weight) one hour after receiving AlP to rats.
Results
aluminium phosphide caused significant cardiac histopathological changes with a significant increase in malondialdehyde (MDA); lipid peroxidation marker; and a significant depletion of antioxidant enzymes (catalase and glutathione reductase). However, treatment with Lam protected efficiently the cardiac tissue of intoxicated rats by increasing antioxidants levels with slight decreasing in MDA production compared to untreated group.
Conclusions
This study suggested that Moringa oleifera extract could possibly restore the altered cardiac histopathology and some antioxidant power in AlP intoxicated rats, and it could even be used as adjuvant therapy against AlP-induced cardiotoxicity.
1. Introduction
Aluminium phosphide (AlP) is one of agrochemical pesticides that is used to increase agriculture production [1]. Furthermore, it extensively misused as suicidal poison due to low cost availability. In Egypt, AlP is emerging as a common self-poisoning agent [2].
AlP multisystem toxic involvement has been connected with phosphine gas and oxidative stress [3]. Phosphine gas induces oxidative stress through inhibition of enzymatic antioxidants e.g. catalase (CAT), glutathione, glutathione reductase (GR) and superoxide dismutase (SOD) [4]. Inhibition of SOD, CAT and GR will produce superoxide radicals and reduce nitric oxide (NO) bioavailability. The reduced NO level increases neutrophil adherence to coronary vessels with subsequent vasoconstriction. On the other hand, excess superoxide radicals react with NO enhancing lipid oxidation [5,6].These alterations will lead to cellular injury and apoptosis through peroxidation of membrane lipids and disruption of membrane permeability [7,8].
Cardiac tissue is more vulnerable to AlP induced oxidative stress than other human tissues, as it has an elevated oxidative metabolic activity and an increased polyunsaturated fatty acids content [9] [[10], [11], [12]]. To the extent that seventy percent of AlP related deaths were attributable to cardiovascular complication [13]. Impairment of cardiac functions could be detected by several echocardiographic techniques and indices [9].
Moringa oleifera (Lam) is an umbrella shaped tree, and is known as ‘the miracle tree’ due to its health benefit effect [14]. Lam has many natural antioxidant compounds e.g. flavonoids, ascorbic acid, carotenoids, and phenolics. Moringa; as phenolic containing compound, has cardio-protective effect and prevents oxidative myocardial cell damage through enhancing oxidative stress defence enzymes, preventing lipid membrane peroxidation [15,16], and inhibiting the disruption of mitochondrial membrane [17].
Given the evidence that Lam may have a role in managing of AlP acute toxicity, we investigated antioxidant activities of Lam in counteracting the high oxidative stress induced by acute AlP intoxication in rat heart.
2. Materials and methods
In this study, the Lam antioxidant activities was detected histopathologically and biochemically through detection of malondialdehyde (MDA) concentration, (SOD), (CAT) and (GR) activities in rat heart. The study was ethically approved by the Institutional Animal Care and Use Committee (IACUC), Cairo University with number (CU/III/S/41/17).
2.1. Chemicals
Tablet form of aluminium phosphide (3 gm) was purchased from Sandhya Industries Pvt. Ltd., Gujarat, India. While Moringa extract (Lam) was purchased from Egyptian National Research Center (1 gm/mL aqueous preparations).
2.2. Animals and experimental design
Twenty-four male Wister rats weighting 100–135 g were used in the study. Animals were housed six cages (four rats/cage), kept under standard laboratory conditions; temperature was 25 ± 2 °C with 40% humidity and allowed free access on commercial diet and tap water provided ad libitum.
Rats were divided into three groups with eight animals each. Group I (control) was served as untreated rats and received 0.9% saline solution orally through gastric tube. Group II (AlP intoxicated rats) was given oral single sub-lethal dose of AlP (2 mg/Kg body weight) through gastric tube [18]. Group III (Lam treated group) was given oral single sub-lethal dose of AlP (2 mg/Kg body weight) and oral single dose of Lam (100 mg/Kg body weight) [4] one hour after receiving AlP dose. All groups were observed for 8 h then all rats were sacrificed under pentobarbital anaesthesia by decapitation.
2.3. Histopathological examination of heart tissue
Full thickness heart samples from each group were fixed in 10% neutral buffered formalin. The fixed specimens were then trimmed, washed and dehydrated in ascending grades of alcohol, cleared in xylene, embedded in paraffin, sectioned at 4–6U thickness and stained by hematoxylin and eosin dye for photo microscopic examination according to Bancroft et al.[19].
2.4. Assessment of oxidative stress biomarkers in heart tissue
Heart specimens were minced and homogenized (10%) in ice-cold 1.155 KCl-0.01 M sodium and potassium phosphate buffer (pH 7.4) in a Potter–Elvehjem glass homogenizer. The homogenate was centrifuged at 10,000 rpm for 20 min at 4 °C, and the resultant supernatant was separated and analyzed to estimate malondialdehyde (MDA) concentration, superoxide dismutase (SOD), glutathione reductase (GR), and catalase (CAT) activities.
Lipid peroxidation, (MDA) level, in heart homogenates was measured spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using Biodiagnostic kit (Egypt) following Okhawa et al. [20] method.
(CAT) (U/g), (SOD) (U/g) and (GR) (U/g) activities were detected spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using Biodiagnostic kit (Egypt) following Okhawa et al. [20], Aebi [21], Nishikimi et al. [22] and Goldberg and Spooner [23] respectively.
2.5. Statistical analyses
Data were coded and analyzed using the statistical package SPSS version 24. Quantitative variables were presented in mean and standard deviation. Comparisons between groups were done using analysis of variance (ANOVA) with multiple comparisons post hoc test (Chat 2003). P-values less than 0.05 were considered significant.
3. Results
3.1. Histopathological examination of heart tissue
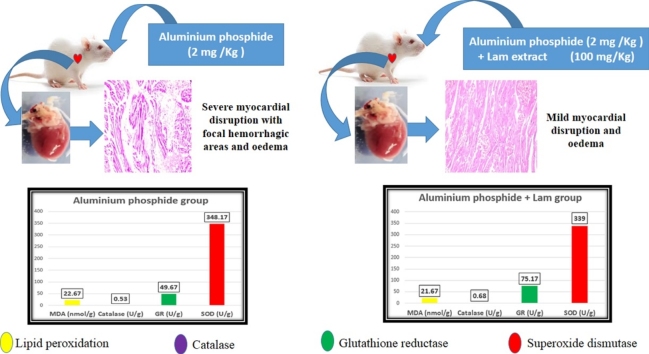
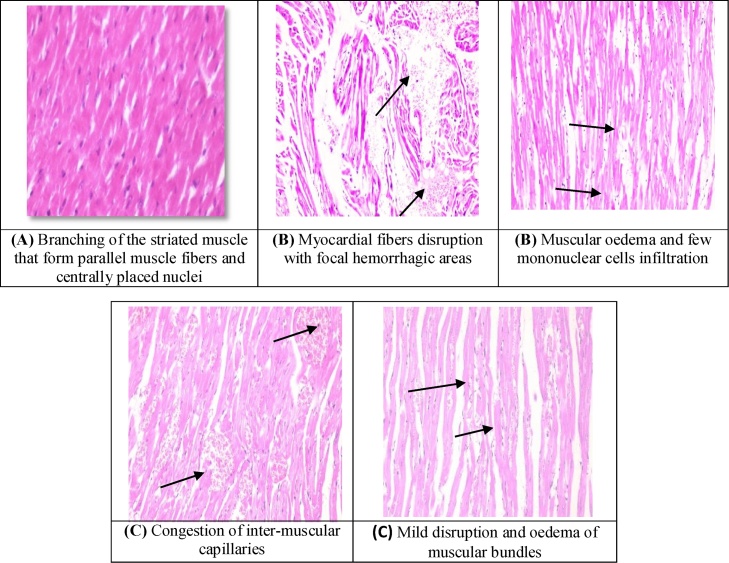
Normal cardiac architecture in the form of parallel muscle fibers, centrally placed nuclei, and intercalated discs was presented in Fig. 1(A). However, AlP intoxicated cardiac muscles showed severe fibers disruption with focal hemorrhagic areas between the muscle bundles, muscular oedema and mononuclear cells infiltration. Muscular bundles necrosis and myocytes swelling were also noticed Fig. 1(B). In addition, Lam treated cardiac muscle revealed congestion of inter-muscular capillaries with mild disruption and oedema of muscular bundles Fig. 1(C).
Fig. 1.
Photomicrograph of heart section in group I (A), group II(B), and group III(C).
3.2. Assessment of oxidative stress biomarkers in heart tissue
The level of malondialdehyde (MDA) was significantly increased in AlP intoxicated and Lam treated groups. The significant highest level of MDA was found in AlP intoxicated group 22.67 ± 3.50 Table 1.
Table 1.
oxidative stress and enzymatic antioxidant parameters among different studied groups.
| Measured parameters | Name | Group I | Group II | Group III |
|---|---|---|---|---|
| Oxidative parameter (nmol/g) | Malondialdehyde | 16.83 ± 1.47 | 22.67 ± 3.50* | 21.67 ± 3.50* |
| Antioxidant parameters (U/g) | Superoxide dismutase | 319.67±0.82 | 348.17 ± 8.01* | 339.00 ± 9.42* |
| Catalase | .78±0.01 | .53±0.07* | .68±0.05*,# | |
| Glutathione reductase | 80.93 ± 9.74 | 49.67 ± 16.22* | 75.17 ± 15.42*,# |
statistically significant compared to group I (P < 0.05).
statistically significant compared to group II (P < 0.05).
While enzymatic antioxidant parameters were significantly decreased in AlP intoxicated and Lam treated groups except GR was significantly increased in Lam treated group. The significant highest levels of CAT and GR were found in Lam treated group 0.68 ± 0.05 and 75.17 ± 15.42 respectively Table 1. Even so, the highest level of SOD was found in AlP intoxicated group 348.17 ± 8.01.
4. Discussion
The induction of oxidative stress by AlP is well documented [24]. Indeed, AlP significantly increased the main products of lipid peroxidation, MDA, and decreased the activity of CAT and GR, while SOD activity was increased. The explanation for this apparent increase is that the cellular production of antioxidant molecules is increased as a compensatory mechanism against free radicals [9,25].
Many studies [[26], [27], [28]], have reported that the enhanced production of ROS was detected in AlP toxicity. ROS promote membrane lipid peroxidation in cardiac tissue due to presence of polyunsaturated fatty acids and oxygen [9].
In comparison to AlP intoxicated group, the antioxidant activity of Lam was observed. Lam increased CAT and GR activities, while it decreased the MDA level. Moreover, it decreased SOD activity, indicating that the AlP induced oxidative stress was too high [9].
Sheweita et al. [5] has reported that induction of SOD and CAT antioxidant activities is a cellular defence mechanism to withstand oxidative insult. In addition, GR increases the availability of reduced glutathione, which is a cellular antioxidants and NO, which is a vasodilator factor. Lam is capable to reduce lipid peroxidation of cell membrane and prevent free radicals induced damage through its antioxidant activity achieved by its active compounds [8,15,16,29].
Azad et al. [30] has reported that AlP cardio-toxicity can be caused by the oxidation of myocardial cell membranes and internal lipid structure. Moreover, antioxidant therapy has an important role in managing this oxidative insult.
Compared to control animals, AlP intoxicated group were characterized by marked histo-pathological abnormalities in cardiac tissues. These findings were in accordance with the findings of Shah et al. [31] who observed nearly the same histo-pathological changes. The observed AlP effect was evident in Akkaoui et al. [32] and Chugh et al. [33] studies. They reported that AlP intoxication was associated with left ventricular dysfunction, low ejection fraction, severe hypotension and electrocardiographic abnormalities [34,35].
AlP cardiac toxic effect was not found in Anand et al. [13] study. Even so, serum levels of cardiac enzymes were higher in AlP intoxicated rats. The absence of AlP toxic effect could be attributed to their usage of a single lethal dose (20 mg/kg body weight) that caused rapid animal death without eliciting inflammatory response.
While, Lam extract exerted protective effect against AlP–induced cardiac toxicity. It decreased muscle fiber disruption, necrosis, focal hemorrhagic areas between the muscle bundles, mononuclear cells infiltration and myocytes swelling. This is in agreement with Hashemzaei et al. [17] who stated that the polyphenol containing compounds e.g. Lam can stimulate mitochondrial metabolism, enhance the expression of respiratory chain components, and enhance oxygen tissue uptake. Moreover, it also can inhibit the main apoptotic pathway through modulating Akt protein expression.
5. Conclusion
In the present study, severe histological alterations were identified in AlP intoxicated group. Furthermore, our data highlighted the oxidative stress as a possible mechanism for AlP induced cardio-toxicity. Treatment with Lam could ameliorate the cardio-toxic effect of AlP due to its contents of antioxidant phytochemicals. Moreover, it has less adverse effects with lower economic burden than commercial drugs [36]. Hence, it can be used as adjuvant therapy in AlP induced cardio-toxicity. However, further echocardiographic studies evaluating cardio-toxic effects of AlP at different concentrations as well as the specific impact of Lam administration on cardiac functions are needed.
References
- 1.Behera C., Krishna K., Bhardwaj D.N., Rautji R., Kumar A. A case of accidental fatal aluminium phosphide poisoning involving humans and dogs. J. Forensic Sci. 2015;60(3):818–821. doi: 10.1111/1556-4029.12709. [DOI] [PubMed] [Google Scholar]
- 2.El Naggar A.R.M., El Mahdy N.M. Zinc phosphide toxicity with a trial of tranexamic acid in its management. J. Adv. Res. 2011;2(2):149–156. [Google Scholar]
- 3.Mehrpour O., Jafarzadeh M., Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh. Hig. Rada Toksikol. 2012;63(1):61–73. doi: 10.2478/10004-1254-63-2012-2182. [DOI] [PubMed] [Google Scholar]
- 4.Bumbrah G.S., Krishan K., Kanchan T., Sharma M., Sodhi G.S. Phosphide poisoning: a review of literature. Forensic Sci. Int. 2012;214(1–3):1–6. doi: 10.1016/j.forsciint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Sheweita S., Salama B., Hassan M. Erectile dysfunction drugs and oxidative stress in the liver of male rats. Toxicol. Rep. 2015;(June (2)):933–938. doi: 10.1016/j.toxrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folgi S., Nieri P., Breschi M. The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. FASEB J. 2004;18(April (6)):664–675. doi: 10.1096/fj.03-0724rev. [DOI] [PubMed] [Google Scholar]
- 7.Chugh S.N., Arora V., Sharma A., Chugh K. Free radical scavengers & lipid peroxidation in acute aluminium phosphide poisoning. Indian J. Med. Res. 1996;104(August):190–193. (PMID: 8840658) [PubMed] [Google Scholar]
- 8.Naser Ashraf Y. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats. Toxicol. Rep. 2014;(September):682–691. doi: 10.1016/j.toxrep.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zafiropoulos A., Tsarouhas K., Tsitsimpikou C., Fragkiadaki P., Germanakis I., Tsardi M. Cardiotoxicity in rabbits after a low-level exposure to diazinon, propoxur, and chlorpyrifos. Hum. Exp. Toxicol. 2014;33(December (12)):1241–1252. doi: 10.1177/0960327114532384. [DOI] [PubMed] [Google Scholar]
- 10.Ander B.P., Dupasquier C.M., Prociuk M.A., Pierce G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003;8(Winter (4)):164–172. (PMID: 19649216) [PMC free article] [PubMed] [Google Scholar]
- 11.Germanakis I., Tsarouhas K., Fragkiadaki P., Tsitsimpikou C., Goutzourelas N., Champsas M.C. Oxidative stress and myocardial dysfunction in young rabbits after short term anabolic steroids administration. Food Chem. Toxicol. 2013;61(November):101–105. doi: 10.1016/j.fct.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Bumbrah G.S., Krishan K., Kanchan T., Sharma M., Sodhi G.S. Phosphide poisoning: a review of literature. Forensic Sci. Int. 2012;214(1–3):1–6. doi: 10.1016/j.forsciint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Anand R., Binukumar B.K., Gill K.D. Aluminum phosphide poisoning: an unsolved riddle. J. Appl. Toxicol. 2011;31(6):499–505. doi: 10.1002/jat.1692. [DOI] [PubMed] [Google Scholar]
- 14.Razis Abdull, Faizal Ahmad, Ibrahim Din, Muhammad Kntayya. Saie health benefits of moringa oleifera. Asian Pac. J. Cancer Prev. 2014;15(20):8571–8576. doi: 10.7314/apjcp.2014.15.20.8571. [DOI] [PubMed] [Google Scholar]
- 15.Sharma N., Gupta P.C., Rao ChV. Nutrient content, mineral, content and antioxidant activity of Amaranthus viridis and Moringa oleifera leaves. Res. J. Med. Plant. 2012;6(3):253–259. [Google Scholar]
- 16.Suphachai Charoensin. Antioxidant and anticancer activities of Moringa oleifera leaves. J. Med. Plant Res. 2014;8(February (7)):318–325. [Google Scholar]
- 17.Hashemzaei M., Barani A., Iranshahi M., Rezaee R., Tsarouhas K., Tsatsakis A. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2016;46:110–115. doi: 10.1016/j.etap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Dua R., Gill K.D. Effect of aluminium phosphide exposure on kinetic properties of cytochrome oxidase and mitochondrial energy metabolism in rat brain. Biochim. Biophys. Acta. 2004;1674(1):4–11. doi: 10.1016/j.bbagen.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Bancroft J.D., Stevans A., Turner D.R. 4th edition. Churchill Livingstone Edinburgh; London, Melbourne, New York: 1996. Theory and Practice of Histological Techniques. ISBN-13: 978-0443047602. [Google Scholar]
- 20.Okhawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Catalase Aebi H. In: 2nd ed. Bergmeyer H.U., editor. Vol. 2. Verlag Chemie; Weiheim: 1974. pp. 673–684. (Methods of Enzymatic Analysis). ISBN: 978-0-12-091302-2. [Google Scholar]
- 22.Nishikimi M., Roa N.A., Yogi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg D.M., Spooner R.J. In: 3rd edn. Bergmeyen H.V., editor. Vol. 3. Verlog Chemie; Deerfield beach, FI: 1983. pp. 258–265. (Methods of Enzymatic Analysis). [Google Scholar]
- 24.Karami-Mohajeri S., Jafari A., Abdollahi M. Comprehensive review of the mechanistic approach and related therapies to cardiovascular effects of aluminium phosphide. Int. J. Pharmacol. 2013;9(8):493–500. [Google Scholar]
- 25.Douarre C., Sourbier C., Dalla Rosa I., Brata Das B., Redon C.E., Zhang H. Mitochondrial topoisomerase Iis critical for mitochondrial integrity and cellular energy metabolism. PLoS One. 2012;7(7):e41094. doi: 10.1371/journal.pone.0041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noeman S.A., Hamooda H.E., Baalash A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011;3(August (1)):17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nehru B., Anand P. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J. Trace Elem. Med. Biol. 2005;19(2–3):203–208. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Baeeri M., Shariatpanahi M., Baghaei A., Ghasemi-Niri S.F., Mohammadi H., Mohammadirad A. On the benefit of magnetic magnesium nanocarrier in cardiovascular toxicity of aluminium phosphide. Toxicol. Ind. Health. 2013;29(March (2)):126–135. doi: 10.1177/0748233711425074. (0.1177/0748233711425074. Epub 2011 Nov 10) [DOI] [PubMed] [Google Scholar]
- 29.Singhai S.G.R. Antioxidant activity of moringa oleifera using successive solvent extraction technique. Int. J. Theor. Appl. Sci. 2016;8(1):23–27. Special Issue-NCRTASTSSN No. (Print): 0975-1718. ISSN No. (Online): 2249-3247. [Google Scholar]
- 30.Azad A., Lall S.B., Mittra S. Effect of N-acetylcysteine and L-NAME on aluminium phosphide induced cardiovascular toxicity in rats. Acta Pharmacol. Sin. 2001;22:298–304. [PubMed] [Google Scholar]
- 31.Shah V., Baxi S., Vyas T. Severe myocardial depression in a patient with aluminium phosphide poisoning: a clinical, electrocardiographical and histopathological correlation. Indian J. Crit. Care Med. 2009;13(January–March (1)):41–43. doi: 10.4103/0972-5229.53117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akkaoui M., Achour S., Abidi K., Himdi B., Madani A., Zeggwagh A.A. Reversible myocardial injury associated with aluminium phosphide poisoning. Clin. Toxicol. (Phila) 2007;45(September (6)):728–731. doi: 10.1080/15563650701517350. [DOI] [PubMed] [Google Scholar]
- 33.Chugh S.N., Chugh K., Ram S., Malhotra K.C. Electrocardiographic abnormalities in aluminium phosphide poisoning with special reference to its incidence, pathogenesis, mortality and histopathology. J. Indian Med. Assoc. 1991;89:32–35. (PMID: 2056173) [PubMed] [Google Scholar]
- 34.Bhasin P., Mittal H.S., Mitra A. An echocardiographic study in aluminium phosphide poisoning. J. Assoc. Phys. India. 1991;39:851. [Google Scholar]
- 35.Mehrpour O., Farzaneh E., Abdollahi M. Successful Treatment of aluminium phosphide poisoning with digoxin: a case report and review of literature. Int. J. Pharmacol. 2011;7(November):761–764. [Google Scholar]
- 36.Oikeh E.I., Omoregie E.S., Oviasogie F.E., Oriakhi K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci. Nutr. 2015;4(July (1)):103–109. doi: 10.1002/fsn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]