Abstract
Advanced hepatocellular carcinoma (HCC) has limited therapeutic options. Immunotherapy is a promising treatment, while sorafenib is a first-line drug-based treatment for advanced HCC. However, the efficacy of sorafenib and immunotherapy in combination, have not been clearly evaluated. Sorafenib treatment has been shown to promote immunosuppression by increasing hypoxia in orthotopic HCC models. Here, we found that sorafenib treatment in mice with orthotopic HCC increased the expression of inhibitor programmed death-ligand 1 (PD-L1) and T-regulatory cells in tumor tissues. We pulsed dendritic cells with exosomes derived from tumor cells (DC-TEX) and found that the number of T-regulatory cells decreased and the number of CD8+T cells increased. However, combining DC-TEX and sorafenib did not prolong survival in these mice. Moreover, we found that the number of PD-1+CD8+T cells significantly increased after DC-TEX treatment. Therefore, we next added PD-1 antibody (PD-1 Ab) to the treatment regimen to block the PD-1/PD-L1 pathway, and found that the exhausted CD8+T cells were restored, without affecting the number of T-regulatory cells. Thus, our data suggest that the combination of DC-TEX and PD-1 Ab enhanced the efficacy of sorafenib, but treatment with either DC-TEX or PD-1 Ab alone, did not.
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide with its annual incidence increasing globally [1]. For patients with early-stage HCC, surgical resection and liver transplantation are standard primary treatments [2]. Unfortunately, one-third of patients with early-stage HCC are asymptomatic; most patients are diagnosed with advanced-stage HCC [3]. For these patients, the efficacy of regular chemotherapy or radiotherapy is low. Recently, sorafenib, a promising drug was considered a milestone in targeted therapy for patients with advanced-stage HCC [4].
Sorafenib, a multitargeted tyrosine kinase inhibitor, exhibits a better antitumor efficacy and is a first-line treatment for advanced-stage HCC [5]. In a multicenter, double-blind trial, 602 patients with advanced-stage HCC were randomly assigned to receive either sorafenib or placebo. The median overall survival was 10.7 months and 7.9 months in the sorafenib group and placebo group, respectively (P < .001) [6]. Unfortunately, the median overall survival was modestly increased by just three months with patients developing resistance to sorafenib. The mechanisms of resistance to sorafenib are likely multifactorial [7], and one of mechanism was associated with the increase in tissue hypoxia [8], [9]. Hypoxia caused the resistance to sorafenib treatment by creating an immunosuppressive microenvironment [10], [11]. The immunosuppressive microenvironment caused by sorafenib treatment was shown that the number of CD4+CD25+ regulatory T cells (Tregs) was significantly increased [12]. Tregs are a sub-population of T cells that maintain immune tolerance, autoimmunity and inhibit immune responses [13]. Tregs decrease the antitumor immunity in patients with HCC. A significantly high number of Tregs is presented in HCC tumor tissues compared with normal tissues. High tumor-infiltrating Tregs are an independent factor of poor prognosis [14]. Duda et al found that the occurrence of increased intratumoral hypoxia after sorafenib treatment facilitated Tregs to accumulate. In addition, the tumor tissues in murine orthotopic HCC models expressed the Programmed Death ligand-1 (PD-L1) [15]. Therefore, an urgent need exists to discover effective therapeutic strategies that can improve the suppressive tumor environment created by sorafenib resistance.
Dendritic cells (DCs) are antigen-presenting cells that uptake tumor-associated antigens and subsequently induce tumor-specific T cell responses to remove tumor cells [16]. Palucka et al demonstrated that the antitumor effect of DCs loaded with tumor-associated antigens was in part, due to the decreased number of Tregs in tumor tissues and in circulation [17]. Tumor cell-derived exosomes induced a higher immune response than tumor cell-lysates in murine orthotopic HCC models and improved the tumor immune microenvironment by increasing the number of CD8+T cells and decreasing the number of Tregs in tumor tissues [18]. Exosomes are small vesicles about 30-100 nm in size and are secreted by different cell types including tumor cells. Tumor-derived exosomes contain tumor-associated antigens including TSG101, Alix, Hsp 60, Hsp70, Hsp90 and CD9, that can activate DCs to induce the specific antitumor response [19]. The antitumor effect of exosome-pulsed DCs to induce specific T cell responses has been demonstrated in both mice and humans [20]. However, tumor antigen-specific T cells become partially exhausted upon chronic exposure to tumor antigens, and express the Programmed Death 1 (PD-1) receptors [21]. PD-1 is an immunoinhibitory receptor that is mainly expressed on activated T cells. PD-1 together with PD-L1 impairs the effector functions of CD8+T cells, including proliferation, cytokine production and cytolysis and then induces an exhaustion-like state to escape immune surveillance [22]. Some studies have shown that blocking the PD-1 axis reversed the dysfunction and exhaustion of activated T cells and presented a significant benefit for the tumor microenvironment [23], [24].
Therefore, we hypothesized that exosome-pulsed DCs (DC-TEX) induce antitumor responses and change the tumor microenvironment by decreasing Treg accumulation in tumor tissue after sorafenib treatment. We speculate that blocking the PD-1/PD-L1 axis can restore the function of exhausted CD8+T cells. We addressed this hypothesis by combining DC-TEX and the PD-1 antibody (PD-1 Ab) with sorafenib and observing the effects on tumors in mice with orthotopic HCC.
Materials and Methods
Cells and HCC Models
The hepa1-6 cell line was obtained from Boster Biological Technology Ltd. (Wuhan, China) and was cultured in DMEM medium (Invitrogen) with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin-streptomycin (Sigma, Munich, Germany). The murine DC2.4 cell line was provided by Professor Haifang Yin (Cell Biology and Research Centre of Basic Medical Science, Tianjin Medical University, Tianjin, China) and were cultured in DMEM medium with 10% FBS and 1‰ β-mercaptoethanol. The exosomes were derived from FBS by centrifugation. C57BL/6 mice (6 to 8 weeks old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. and were maintained in the Tianjin animal unit (SYXK 2011-0008) according to the ethics committee of the Chinese Academy of Medical Sciences and Peking Union Medical College Institute of Biomedical Engineering. We used tissue implantation to establish an orthotopic HCC model, as described [18].In briefly, we established subcutaneous HCC mouse models with hepa1-6 cell (5×106). When the longitudinal diameter of subcutaneous tumor reached 1cm, we cut the tumor tissue into 1mm3 and trasplanted it into left liver of mouse.
Treatment
For the established orthotopic HCC model, we confirmed tumor growth by MRI fourteen days after tissue implantation and then randomly divided the mice into different groups (Sorafenib, DC-TEX, PD-1 Ab, Sorafenib plus DC-TEX, Sorafenib plus PD-1 Ab, DC-TEX plus PD-1 Ab, Triple combination of Sorafenib, PD-1 Ab, and DC-TEX, Phosphate buffered saline, n = 6 mice/group). The tumor volume in each group was not significantly different. Sorafenib (Nexavar, Bayer Healthcare, Leverkusen, Germany) was dissolved in phosphate buffered saline (PBS) containing 1% Tween 80 and the final dose of 50 mg/kg was administered by gavage daily for three weeks. DCs were pulsed with exosomes (4×106, injected intravenously) three times, at 5-day intervals. PD-1 Ab (200 μg) was injected intraperitoneally three times at 5-day intervals (clone J43; BioXCell, West Lebanon, USA). After one week of treatment, the established orthotopic HCC model were to sacrifice and to analyze the variation. For survival analysis, the treatment was conducted as above described (n = 10 mice/group).
Flow Cytometry
We used flow cytometry (BD Biosciences, San Jose, CA, USA) count the number of Tregs cells and CD8+ T cells in the tumor tissues. The tumor tissues were digested using collagenase type IV (0.05 mg/mL; Gibco.) for 40 min at 37°C and then the tumor-infiltrating lymphocytes were harvested using 40% Percoll (Pharmacia, Sweden). The tumor-infiltrating lymphocytes were stained with FITC-conjugated anti-CD3e, FITC-conjugated anti-CD4, PE-conjugated anti-CD25, PE-conjugated anti-PD-1, APC-conjugated anti-CD8a and APC-conjugated anti-Foxp3. All the antibodies were purchased from eBioscience (San Diego, CA, USA).
Cytokines Analysis by Enzyme-Linked Immunosorbent Assay (ELISA)
We collected blood serum samples from orthotopic tumor-bearing mice to observe the levels of cytokines including IFN-γ, TNF-α, TGF-β, IL-2, IL-10 and AFP using the ELISA kits (eBioscience), according to the manufacturer's protocol.
Immunohistochemistry
Immunohistochemical staining was performed, as described previously [25]. Tumor tissues were embedded in paraffin and were cut into sections 4-μm in thickness. The sections were deparaffinized in xylene, rehydrated in gradient ethanol. Antigen retrieval was performed on these sections at 100°C for 30 min in citrate buffer. For blocking endogenous peroxidase activity, all slides were treated with 3% hydrogen peroxide for 10 min and were then blocked in 10% goat serum for 15 min. The sections were stained with mouse anti-PD-L1 monoclonal antibody (Abcam, 1:300) or anti-Foxp3 monoclonal antibody (Abcam, 1:200) overnight at 4°C. The following day, the sections were incubated with secondary antibody (biotinylated goat antirat IgG) for 30 min at room temperature and then stained with 3,30-diaminobenzidine tetrahydrochloride followed by counterstaining with hematoxylin.
Generation of Exosomes and Exosome-Loaded DCs
Exosomes were separated from the supernatants of hepa1-6 cells, as described previously [26]. To reduce the effects of exosomes from FBS, the FBS was centrifuged at 10,000×g for 60 min. After 48 h of culture, the supernatant was centrifuged at 2000×g for 20 min, 10,000×g for 30 min and 100,000×g for 70 min (Hitachi, Tokyo, Japan). The exosome pellet was washed twice with PBS and recovered by centrifugation at 100,000×g for 70 min. The concentration of exosomal proteins was determined by Bradford assay. DCs were co-cultured with exosomes for 48 h for in vivo DCs immunotherapy.
Statistical Analysis
Data are shown as the mean ± standard deviation for descriptive statistics. The differences between groups were determined using the Student's t-test or ANOVA. The survival analysis was calculated by the Kaplan–Meier method. All statistical analyses were performed using the SPSS 16.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered as a statistically significant difference based on a two-tailed test.
Results
CD4+CD25+FoxP3+ Tregs in Tumor Tissues are Increased After Sorafenib Treatment
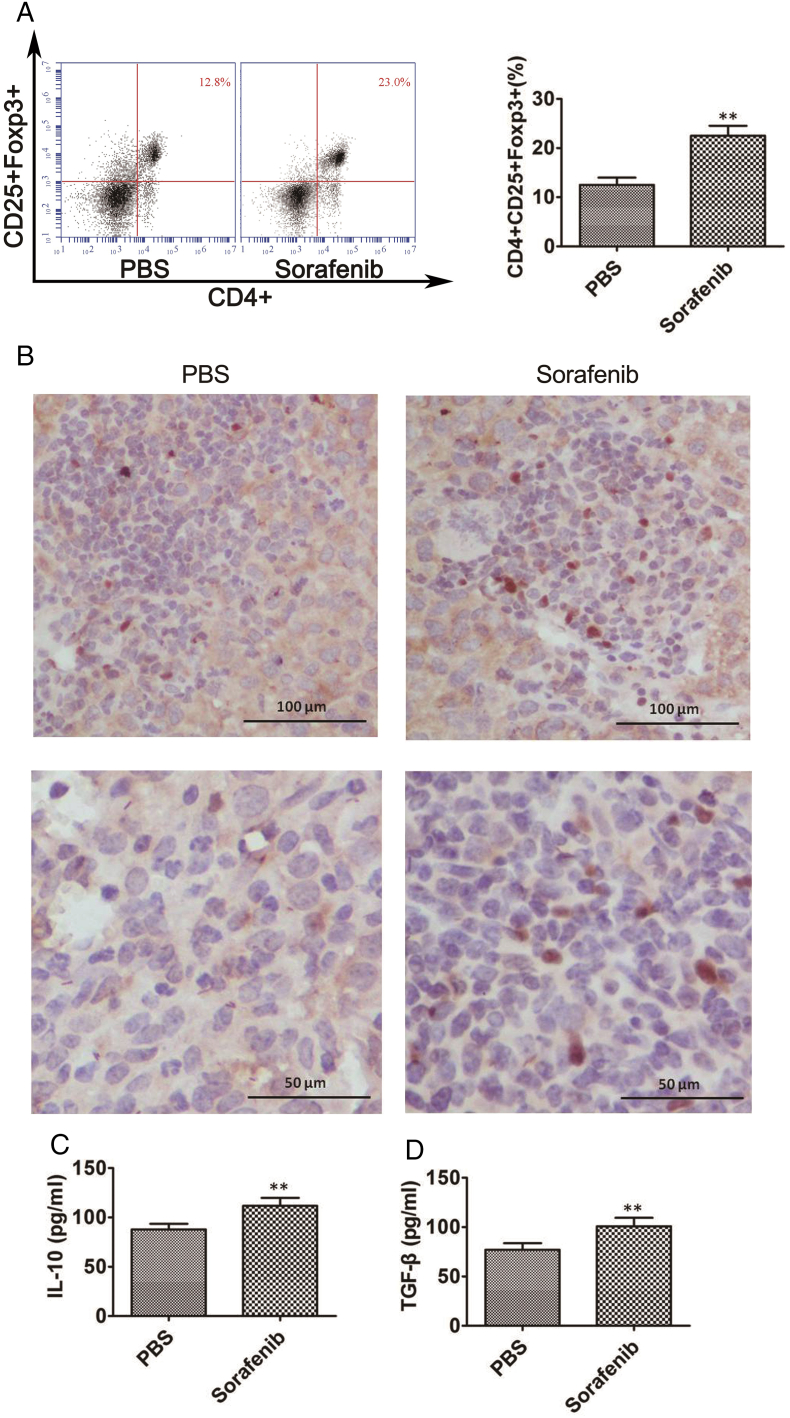
To observe any changes in the number of CD4+CD25+FoxP3+ Tregs in the orthotopic HCC tumor tissues after treatment with sorafenib, we treated the mice with sorafenib for 21 days and then evaluated the number of CD4+CD25+FoxP3+Tregs using flow cytometry and immunohistochemistry. We found that sorafenib treatment significantly increased the number of tumor-infiltrating CD4+CD25+FoxP3+ Tregs in the orthotopic tumor tissues (Figure 1A). Immunohistochemistry showed similar results (Figure 1B). In addition, we analyzed the levels of serum cytokines and found that the immunoinhibitory interleukin-10 (IL-10) (Figure 1C) and transforming growth factor-β (TGF-β) were significantly increased (Figure 1D). These results demonstrated that sorafenib treatment induced immunosuppression in mice with orthotopic HCC [15].
Figure 1.
Sorafenib treatment induced an immunosuppressive microenvironment in orthotopic HCC mice. We observed changes in the number of CD4+CD25+Foxp3+ Tregs in tumor tissues using flow cytometry and immunohistochemistry, and in the levels of serum IL-10 and TGF-β detected by ELISA. A. The number of CD4+CD25+Foxp3+ Tregs significantly increased after sorafenib treatment compared with the PBS group (n = 6 mice/group, **P < .01). B. Immunohistochemistry of FoxP3+Treg cells in tumor tissues. C-D. The levels of IL-10 and TGF-β significantly increased after treatment with sorafenib (n = 6 mice/group, **P < .01).
Exosomes Derived from Hepa1-6 Cells Act as Tumor Antigens to Activate T Cells
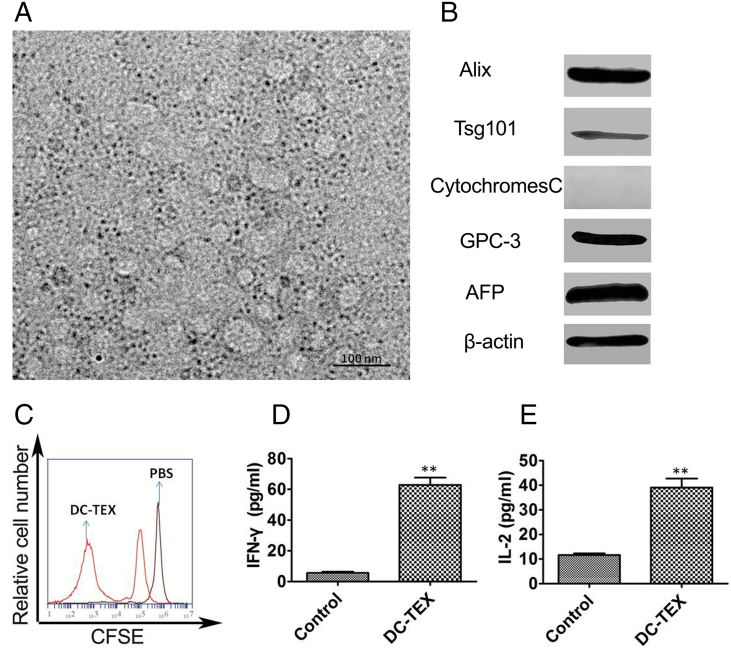
We next investigated the role of exosomes derived from hepa1-6 cells as tumor antigens. These are taken up by DCs which subsequently activate T cells. We measured the size of exosomes using transmission electron microscopy and found that they ranged between 30 and 100 nm (Figure 2A), consistent with other reports [27]. We confirmed the expression of exosome-specific surface markers including TSG101, Alix, GPC-3, and AFP using Western blots (Figure 2B). These tumor-associated antigens have the ability to activate T cells after being taken up by DCs. We have previously found that 40 μg/ml of exosomes co-cultured with DCs for 48 h exhibited a strong antitumor effect [18]. We selected this concentration of exosomes to activate T cells in vitro and observed a proliferation in T cells (Figure 2C). Moreover, we observed significantly increased levels of serum IL-2 and IFN-γ by ELISA (Figure 2D-E).
Figure 2.
Characteristics of exosomes and activation of T cells by DC-TEX. A. The size of exosomes was detected by transmission electron microscope. B The surface makers expressed on exosomes were confirmed by Western blot. C Exosomes were pulsed with DCs which then activated T cells. The naïve T lymphocytes were derived from inguinal lymph nodes of C57BL/6 mice and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and then co-cultured with DC-TEX for 72 h in a 1:10 ratio. The activated T lymphocytes were collected and analyzed using flow cytometry. D-E. Activated T lymphocytes secreted cytokines including IL-2 and IFN-γ (**P < .01).
DC-TEX Altered Immunosuppression But Did Not Increase the Efficacy of Sorafenib in Orthotopic HCC Mice
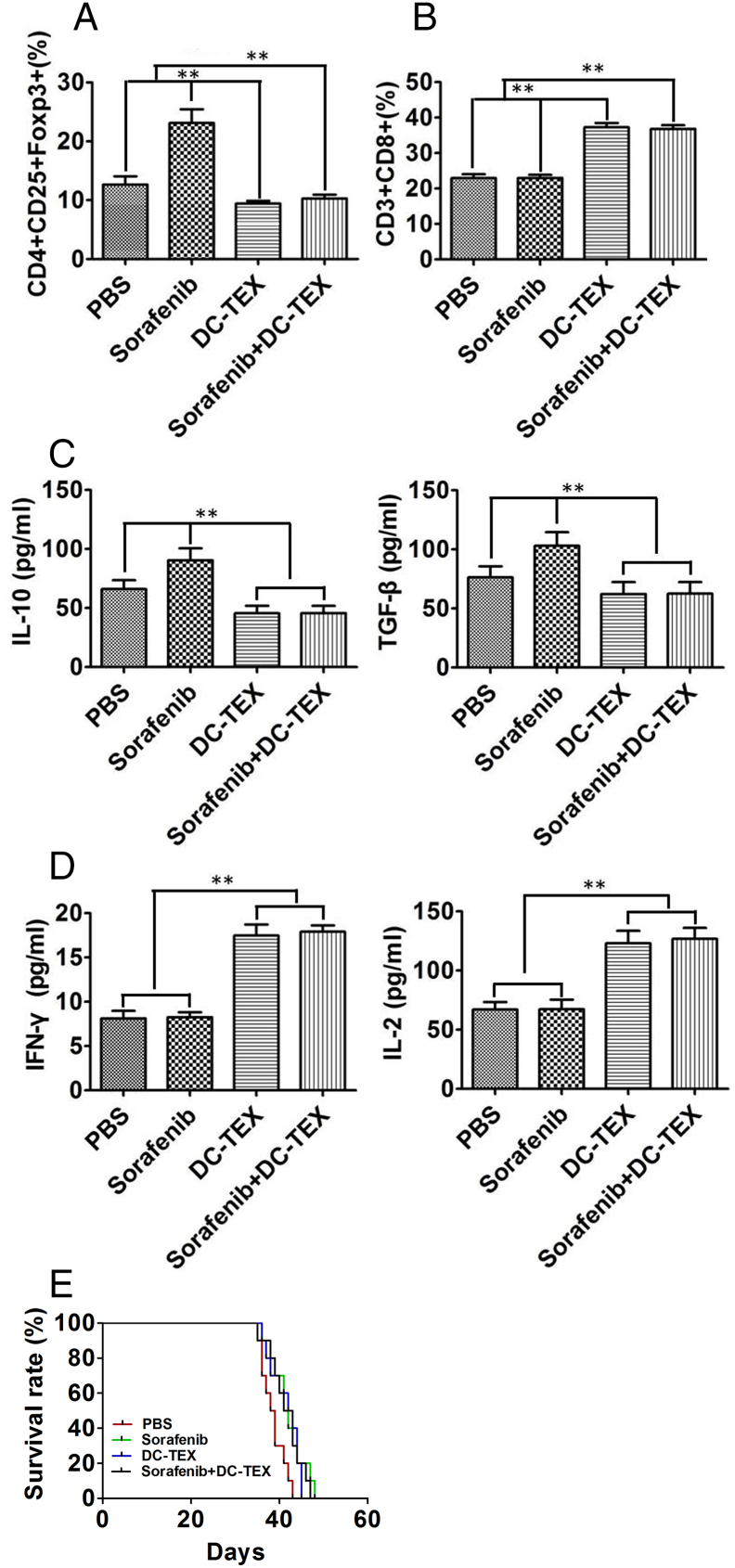
We treated the orthotopic HCC mice with DC-TEX three times at 5 day intervals and with sorafenib daily for 21 days. We then evaluated the immune microenvironment for any changes. Flow cytometry revealed that the number of CD4+CD25+FoxP3+ Tregs in tumor tissues significantly decreased after treatment with DC-TEX (Figure 3A). Furthermore, we observed a significant increase in CD8+T cells after treatment with DC-TEX (Figure 3B). We also observed a significant decrease in the levels of IL-10 and TGF-β (Figure 3C) after treatment with DC-TEX, while the levels of IFN-γ and IL-2 (Figure 3D) were significantly increased. These results suggested that treatment with DC-TEX improved the immune microenvironment. We next evaluated the efficacy of the combination of DC-TEX and sorafenib on survival time. We found that the combination treatment did not prolong the survival of the orthotopic HCC mice compared with mice treated with either sorafenib or DC-TEX alone (Figure 3E). Although the DC-TEX altered immunosuppression induced by sorafenib by decreasing the number of CD4+CD25+FoxP3+ Tregs in tumor tissues, the survival time was not significantly different.
Figure 3.
DC-TEX treatment altered immunosuppression and affected efficacy. We established orthotopic HCC mice by tissue implantation and collected the tumor tissues and serum, 7 days after treatment. A. We analyzed the change in the number of CD4+CD25+FoxP3+ Tregs. We collected the tumor tissues and digested samples with collagenase type IV and then analyzed the cells by flow cytometry. The DC-TEX treatment significantly decreased the number of CD4+CD25+FoxP3+ Tregs (n = 6 mice/group, **P < .01). B. The number of CD8+T cells in tumor tissues were significantly increased (n = 6 mice/group, **P < .01). C. The levels of serum cytokines IL-10 and TGF-β were significantly decreased by ELISA (n = 6 mice/group, **P < .01). D. Measurement of serum IFN-γ and IL-2 in all groups were significantly different (n = 6 mice/group, **P < .01). E. No significant differences in survival times were observed between the sorafenib and DC-TEX group (n = 6 mice/group, P > 05).
The Expression of PD-L1 in Tumor Tissues Increased After Sorafenib Treatment
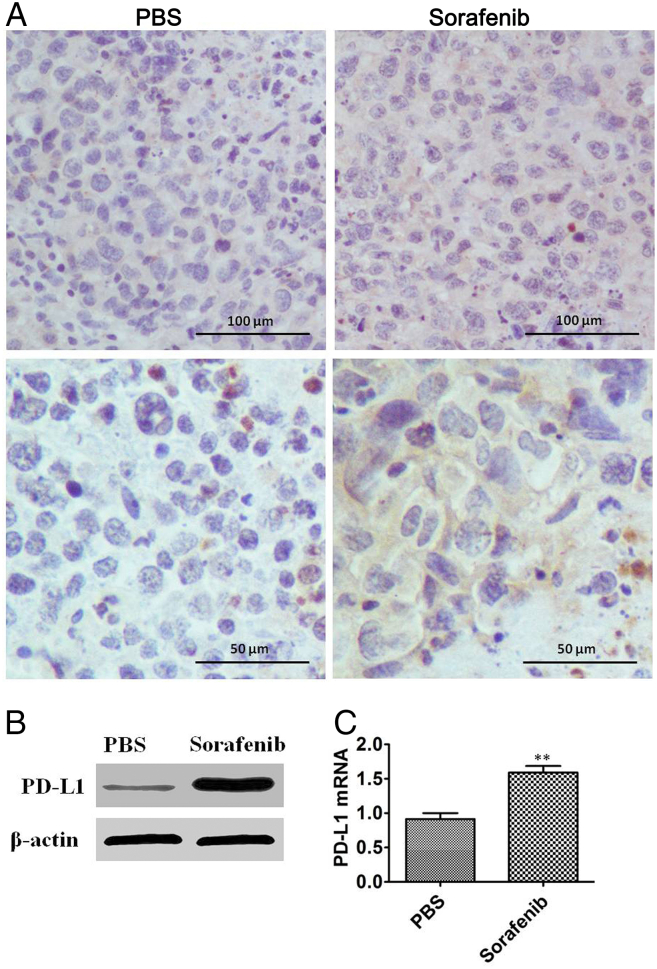
Sorafenib treatment has been shown to induce hypoxia and hypoxia is implicated in the increase in expression of PD-L1. We thus, evaluated if the hypoxia induced by sorafenib increased the expression of PD-L1 in orthotopic HCC tissues. We administered sorafenib for 21 days to orthotopic HCC mice. Subsequently, we collected the tumor tissues to analyze the PD-L1 expression using Western blots and immunohistochemistry. Our results revealed that sorafenib treatment increased the expression of PD-L1 in orthotopic HCC mice compared with mice in the control group (Figure 4A-B). In addition, we measured the mRNA levels of PD-L1 in tumor tissues by qPCR and found that the PD-L1 mRNA was up-regulated after sorafenib treatment (Figure 4C). These results demonstrate that the expression of PD-L1 in tumor tissue is affected by sorafenib.
Figure 4.
Expression of PD-L1 in tumor tissues of orthotopic HCC mice. Sorafenib was administered for 21 days in mice with orthotopic HCC and 7 days after treatment, the tumor issues were collected. A. Representative images of immunohistochemical staining of PD-L1 in the PBS and sorafenib treatment groups. B. Representative images of PD-L1 by Western blotting in the PBS and sorafenib treatment groups. C. qRT-PCR assay for PD-L1 mRNA expression in tumor tissue from the PBS and sorafenib treatment groups (n = 6 mice/group, **P < .01).
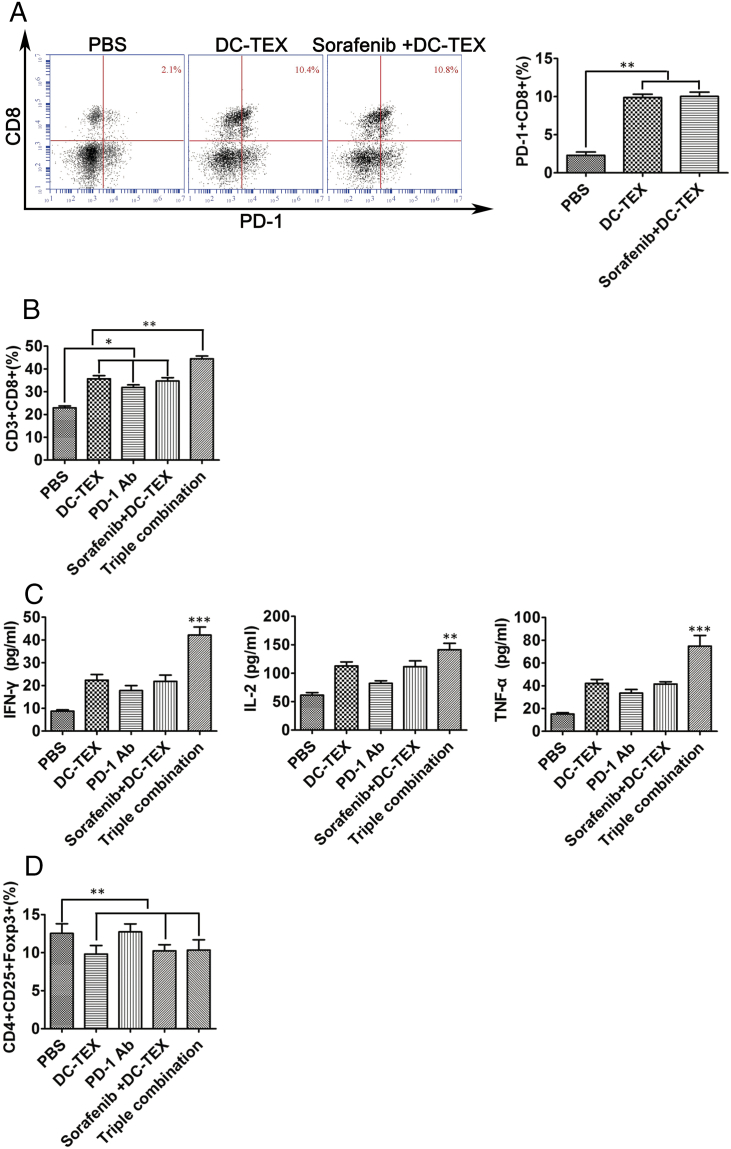
The Expression of PD-1+CD8+T Cells Increased After Treatment with DC-TEX in Orthotopic HCC Mice
DC-TEX treatment increased the number of CD8+T cells and suppressed tumor growth in orthotopic HCC mice [18]. PD-1 was mainly expressed on activated T lymphocytes, including the CD8+T cells. We analyzed the number of PD-1+CD8+T cells using flow cytometry in tumor tissues and found that the PD-1+CD8+T cells were significantly increased compared with the control group (Figure 5A). However, these cells exhibited aberrant proliferation by Flow cytometry and secretion of cytokines by ELISA. This is most likely because of the expression of PD-L1. PD-1 Ab blocked the axis of PD-1/PDL-1 and reversed the number of exhausted T cells. After using PD-1 Ab, the number of CD8+T cells significantly increased (Figure 5B) and the levels of IFN-γ, IL-2 and TNF-α increased (Figure 5C). The addition of PD-1 antibody did not change the number of CD4+CD25+FoxP3+ Tregs (Figure 5D).
Figure 5.
The number of PD-1+CD8+T cells vary before and after treatment with DC-TEX. The established orthotopic HCC mice were treated with DC-TEX and 7 days after treatment, the tumor tissues and serum were collected for analysis. A. The number of PD-1+CD8+T cells significantly increased in the DC-TEX treatment group (n = 6 mice/group, **P < .01). B. After treatment with PD-1 Ab to block PD-L1, the number of CD8+T cells significantly increased (n = 6 mice/group, **P < .01). C. Serum IFN-γ, IL-2 and TNF-α levels in the DC-TEX plus PD-1 Ab group significantly increased (n = 6 mice/group, **P < .01). D. Flow cytometry revealed that PD-1 Ab had no effect on CD4+CD25+Foxp3+ Tregs in tumor tissues (n = 6 mice/group, P > .05).
PD-1 Ab plus DC-TEX Elevated the Efficacy of Sorafenib in HCC Mice
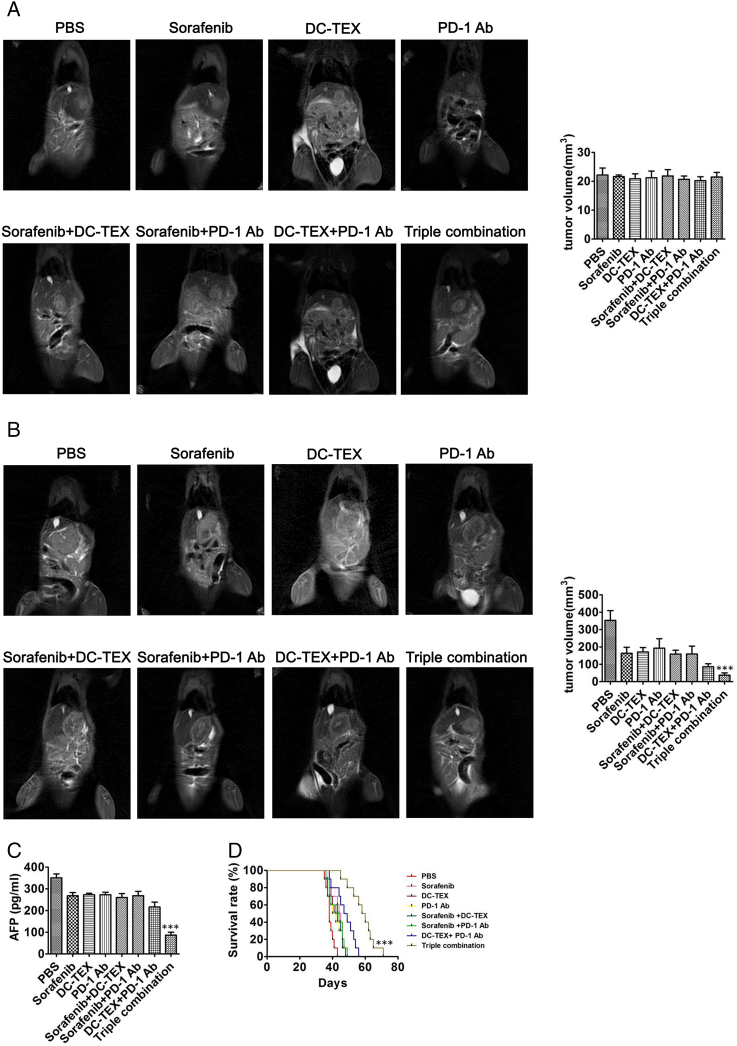
Our study confirmed that DC-TEX did not increase the efficacy of sorafenib in HCC orthotopic mice. We next assessed the efficacy of the triple combination of PD-1 Ab, DC-TEX and sorafenib on mice with orthotopic HCC. After fourteen days, we confirmed tumor growth by MRI. Before treatment, MRI showed no significant differences in tumor sizes in all groups (Figure 6A). After one week of treatment, we found that the triple combination treatment of PD-1 antibody, DC-TEX and sorafenib significantly suppressed tumor growth (Figure 6B). In addition, mice administered the triple combination treatment exhibited significantly decreased serum AFP levels, as measured by ELISA. In contrast, the combination treatment of DC-TEX and sorafenib did not significantly decrease AFP levels (Figure 6C). Furthermore, we found that the triple combination of PD-1 Ab, DC-TEX and sorafenib treatment significantly prolonged the survival time of mice with orthotopic HCC compared with DC-TEX alone, sorafenib alone or DC-TEX and sorafenib (Figure 6D).
Figure 6.
The antitumor effect of PD-1 Ab, sorafenib and DC-TEX in HCC mice. The established HCC orthotopic mice were administered PD-1 Ab, sorafenib and DC-TEX alone or in combination. A. We observed no significant differences in tumor sizes by MRI in the different groups before treatment (n = 6 mice/group, P > .05). B Measurement of tumor volume after treatment for 7 days. The triple combination treatment exhibited a significantly superior effect (n = 6 mice/group, **P < .01). C. Serum AFP levels using ELISA significantly decreased in the triple combination group (n = 6 mice/group, **P < .01). D. The triple combination treatment group exhibited a significantly prolonged survival time (n = 10 mice/group, **P < .01).
Discussion
Sorafenib inhibits tumor cell proliferation and tumor angiogenesis by blocking the Raf/MEK/ERK signaling pathway and targeting the tyrosine kinase receptor vascular endothelial growth factor receptor-2 and platelet-derived growth factor receptor β. [28], [29].Unfortunately, HCC patients treated with sorafenib often develop resistance.
Previous studies showed that sorafenib decreased the tumor vascular density, and increased hypoxia in tumor tissues in HCC. Hypoxia is a hallmark of tumor tissue and [30], [31], [32], [33] and promotes immunosuppression. Hypoxia induced by sorafenib also promotes immunosuppression in HCC via the SDF1α/CXCR4 axis [34]. HCC is highly immunogenic and recruits CD4+CD25+Tregs and inhibitory receptors to the tumor environment [35]. The number of CD4+CD25+Tregs correlates with survival; a low number of Tregs is an indicator of a better prognosis in clinics [36], [37]. In our study, mice with orthotopic HCC treated with sorafenib exhibited a significantly increased number of CD4+CD25+Tregs compared with those in the control group. This result was further compounded by increased intratumoral hypoxia in mice with orthotopic HCC. DC-TEX treatment altered the immunosuppressive environment of the tumors by increasing the number of CD8+T cells and decreasing the number of CD4+CD25+Tregs in HCC mice [18], [38]. We observed a dramatic reduction in the number CD4+CD25+Tregs in tumor tissues and the serum levels of IL-10 and TGF-β after DC-TEX treatment, but the combined treatment of sorafenib and DC-TEX did not prolong the survival time compared with treatment with sorafenib or DC-TEX alone.
The hypoxia induced by sorafenib treatment in HCC mice also increased the expression of the PD-L1. PD-L1 expressed on HCC cells induced T-cell apoptosis, which was markedly reduced after addition of an antibody that blocked PD-L1 [39]. Indeed, a recent study used PD-L1 deficient mice to demonstrate that PD-L1 plays a key role in regulating the accumulation and deletion of CD8+ T cells in the liver [40]. PD-L1 acts with the inhibitory receptor PD-1 and decreases the antitumor immunity of T cells [41]. Several studies showed that the expression of PD-1 was up-regulated in tumor antigen-specific CD8+ T cells after treatment with tumor vaccine [42], [43]. Exosomes derived from hepa1-6, as a source of antigens enhanced the DC-based antitumor immunity in orthotopic HCC mice by changing the microenvironment of the tumor [18]. In our study, the inhibitory receptor PD-1 was also up-regulated in CD8+ T cells after treatment with exosomes, consistent with what was previously observed. PD-L1 induced CD8+T cell exhaustion, where the T cells express PD-1 via the PDL1/PD-1 axis in HCC [44]. This explains in part, why the combination of sorafenib and DC-TEX did not prolong survival in orthotopic HCC mice, even though treating the mice with DC-TEX changed the immunosuppressive microenvironment of the tumors by decreasing the number of CD4+CD25+Tregs. Blocking PD-1 improved the antitumor effects of CD8+T cells by inhibiting the PD-1/PD-L1 pathway and thus restoring exhausted CD8+T cells in HCC mice [45]. Although blocking the PD-1 and PD-L1 axis played an important role in controlling tumor growth, the combination of sorafenib and PD-1 antibody did not show superior antitumor effects compared with sorafenib or PD-1 antibody alone. Previous work demonstrated that the hypoxia induced by sorafenib treatment increased the expression of PD-L1 and the chemokine ligand 12 (SDF-1a). The SDF-1a/CXCR4 axis can be activated by hypoxia and thus induce tumor immunosuppression through regulatory T cells [34].
Based on the above study, the antitumor effect of sorafenib and PD-1 antibody was possibly counteracted by the increased number of Tregs. Therefore, we administered the triple treatment of sorafenib, DC-TEX and PD-1 antibody in orthotopic HCC mice and found that the triple combination exhibited superior antitumor effects.
Conflicts of Interest Statement
No author has any conflict of interest to declare. All authors have read the journal's policy on conflicts of interest and the journal's authorship agreement.
Footnotes
Financial Support: This work was supported by the Peking Union Medical College Innovation Research Team Fund, the Union Young Fund of Peking Union Medical College (3332016105) and the sci-tech development project of Shandong Medicine and Health (2016WS0552).
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 2.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The L, European Organisation For R, Treatment Of C EASL-EORTC clinical practice guidelines: management of Hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;1:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Gadaleta‐Caldarola G, Divella R, Mazzocca A, Infusino S, Ferraro E, Filippelli G, Daniele A, Sabbà C, Abbate I, Brandi M. Sorafenib: the gold standard therapy in advanced Hepatocellular carcinoma and beyond. Future Oncol. 2015;11(16):2263–2266. doi: 10.2217/fon.15.161. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Chang LJ, Liu C, Nelson DR. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2013;62:737–746. doi: 10.1007/s00262-012-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP. CXCR4 inhibition in tumor microenvironment facilitates anti-PD-1 immunotherapy in sorafenib-treated HCC in mice. Hepatology. 2015;61(5):1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merad M, Salmon H. A dendritic-cell brake on antitumour immunity. Nature. 2015;523(7560):294–295. doi: 10.1038/523294a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64(2):456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 19.Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, Xiang J, Wu Z, Jiang G, Cao L. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6(30):29877–29888. doi: 10.18632/oncotarget.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. https://doi.org/10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser AD, Schuster K, Gadiot J, Borkner L, Daebritz H, Schmitt C, Andreesen R, Blank C. Reduced tumor-antigen density leads to PD-1/PD-L1- mediated impairment of partially exhausted CD8+T cells. Eur J Immunol. 2012;42(3):662–671. doi: 10.1002/eji.201141931. [DOI] [PubMed] [Google Scholar]
- 22.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cang Y, Zhang J, Nicholas SA, Bastien J, Li B, Zhou P, Goff SP. Deletion of DDB1 in mouse brain and lens leads to p53-dependent elimination of proliferating cells. Cell. 2006;127:929–940. doi: 10.1016/j.cell.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 27.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, Samuel R, Hiddingh L, Roberge S, Koppel C, Lauwers GY. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromalderived factor 1 a/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59:1435–1447. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in Hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera R, Ararat M, Eksioglu EA, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol. 2010;72:293–301. doi: 10.1111/j.1365-3083.2010.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley LM, Dalton DK, Croft M. A direct role for IFNgamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 39.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1is induced in hepatocytes by viral infection and by interferon-α and -γand mediatesTcell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-1determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 41.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-1is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8+ T cells induced by melanoma vaccines. Cancer Res. 2014;74:1045–1055. doi: 10.1158/0008-5472.CAN-13-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta‐Estremera SM, Greeley NR, Nitti G, Peng W. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, Romero P. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces antitumor immunity. Eur J Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]