Abstract
Widespread white matter abnormalities have been reported in schizophrenia, a disorder frequently characterised as a dysconnection syndrome. White matter connectivity in schizophrenia has been predominantly investigated using diffusion weighted imaging, with reductions in fractional anisotropy throughout the brain often interpreted as an indicator of abnormal myelination. However, diffusion weighted imaging lacks specificity and as such a number of microstructural factors besides myelin may be contributing to these results. We utilised multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) in medicated patients with chronic schizophrenia, stratified by treatment response status, and healthy controls, in order to assess myelin water fraction (MWF) in these groups. In addition, we assessed cognitive control using the Stroop task to investigate how response inhibition relates to myelination in patients and controls. Both treatment resistant (n = 22) and treatment responsive (n = 21) patients showed reduced MWF compared to healthy controls (n = 24) in bilateral fronto-occipital fasciculi, particularly evident in the vicinity of the striatum und extending to the cerebellum, with no difference between patient groups. Patients showed greater reaction time interference on the Stroop task compared to healthy controls, with no difference between patient groups. Stroop interference was significantly negatively correlated with MWF in the corpus callosum across groups, and MWF differences in this region mediated the behavioural group effects on the Stroop task. These findings support the suitability of mcDESPOT as a myelin-specific measure of abnormal connectivity in schizophrenia, and suggest that treatment resistant schizophrenia is not characterised by more severe abnormalities in myelination or cognitive control compared to treatment responsive schizophrenia.
Highlights
-
•
Treatment resistant and responsive schizophrenia patients show reduced myelin water fraction compared to healthy controls
-
•
Myelin water fraction in the corpus callosum is related to performance on a cognitive control task
-
•
Myelin water fraction in the corpus callosum mediates differences in a cognitive control task between patients and controls
1. Introduction
Schizophrenia, a debilitating psychotic disorder, has been widely described in terms of a dysconnection syndrome (Friston et al., 2016; Friston and Frith, 1995; Volkow et al., 1988). Symptoms of psychosis, including delusions and hallucinations, are in part suggested to emerge as a result of inadequate integration of neural processes in the brain. Both functional and structural connectivity dysfunctions have been observed in schizophrenia (Pettersson-Yeo et al., 2011) and it is by now widely established that white matter abnormalities are pervasive in the disorder (Klauser et al., 2017). These have been shown to be associated with abnormal functional activation (Marenco et al., 2012) and connectivity (Liu et al., 2011) as well as symptoms (Canu et al., 2015). The most consistent findings show white matter changes in frontal and temporal lobes, as well as in the corpus callosum and internal capsule (Kubicki et al., 2007; Samartzis et al., 2014; Wheeler and Voineskos, 2014).
Symptoms of psychosis are treated with antipsychotics which act via subcortical dopamine receptor blockade. However, symptomatic non-response to treatment remains an area of unmet clinical need, with around 30% of patients with a diagnosis of schizophrenia showing inadequate response despite optimal treatment (Lindenmayer, 2000; Mortimer et al., 2010). It is likely that this form of “treatment-resistant” schizophrenia (TRS) differs in terms of its underlying neurobiology from treatment responsive schizophrenia (Mouchlianitis et al., 2016). As a result, antipsychotic medication acting on the dopamine system may not be targeting the underlying cause of psychosis in TRS patients. It has been suggested that TRS is characterised by more severe or distinct patterns of structural dysconnectivity compared to treatment responsive schizophrenia (White et al., 2016), resulting in more severe cognitive deficits and psychotic symptoms. An association between good treatment response and improved white matter integrity has been previously shown (Garver et al., 2008), however it is unclear how myelination specifically relates to cognitive performance in schizophrenia as a function of treatment response.
Overall there is a large body of evidence suggesting that patients with schizophrenia show widespread decreases in white matter integrity, usually on the basis of diffusion weighted imaging (Wheeler and Voineskos, 2014). The most commonly reported measure is fractional anisotropy (FA), which indexes the degree of directionality of diffusing water molecules. Changes in FA are attributed to changes in the microstructure of white matter tracts; however, these could in effect be indicators of numerous underlying features such as myelination, axon diameter, fibre density, axon number, or axonal membrane integrity. Multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) is a recently developed imaging technique which allows for the derivation of a whole-brain myelin water fraction (MWF) map with rapid acquisition times (Deoni et al., 2008). As a metric for the fraction of water trapped between the myelin sheaths around the neuronal axons, MWF has been shown to correlate strongly with histological measures of myelin content (Laule et al., 2006; Webb et al., 2003), and to provide higher specificity to myelin content as compared with diffusion measures (Mädler et al., 2008; Vavasour et al., 2011). Here we report the first study utilising mcDESPOT in a sample of patients with a diagnosis of schizophrenia, stratified by treatment response status, and a healthy control sample. In order to assess the relationship between myelination, treatment response, and cognitive function, we included a basic measure of executive control, the Stroop task. The task was chosen as it represents a widely used measure of executive control, which is known to be impaired in schizophrenia and has been shown to be related to functional outcome (Green et al., 2000). Impaired performance on the Stroop task in patients with schizophrenia was confirmed in a recent meta-analysis (Westerhausen et al., 2011).
The aims of this study were 1) to test the utility of mcDESPOT in detecting differences in myelination between patients with schizophrenia and healthy controls, 2) to investigate whether differences in myelination may account for differences in response to antipsychotic treatment, 3) to explore whether myelin content as measured by mcDESPOT modulates behavioural performance on a cognitive control task and 4) if this is differentially impacted across treatment refractory and treatment responsive patients with schizophrenia.
2. Material and methods
2.1. Participants
The study included 43 individuals with a diagnosis of schizophrenia (according to ICD-10 criteria) and 24 healthy controls matched for age, sex, and socioeconomic background. The patient sample included 22 patients who fulfilled criteria for treatment resistance (TRS), based on at least two prior drug trials of 4–6 weeks duration with no clinical improvement, persistence of illness for longer than five years with no period of good social or occupational functioning, and persistent psychotic symptoms as defined as a score of at least 4 (moderate) on at least two positive symptom items of the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). The remaining 21 patients fulfilled criteria for being in symptomatic remission (non-treatment resistant; NTR), as defined by a score of 3 or less on all items of the PANSS (Conley and Kelly, 2001), and these symptoms having been stable for at least 6 months (Andreasen et al., 2005). The two patient groups were matched for age, sex, duration of illness, medication type and dosage. Current clozapine use was an exclusion criterion for all patients; this was to maintain the homogeneity of the patient sample - fulfilling the standard criteria for treatment resistance - avoiding the introduction of sub-groups of patients refractory to clozapine (super-resistant patients). Chlorpromazine (CPZ) equivalent doses of medications were calculated using conversion tables. Exclusion criteria for all subjects were a history of neurological illness, current major physical illness, and drug dependency over the last six months. Exclusion criteria for HC were a history of psychiatric illness and a first-degree relative currently or previously suffering from a psychotic illness. All subjects had normal hearing and normal or corrected-to-normal vision. Demographic characteristics and clinical data are presented in Table 1. Ethical approval was provided by the London Camberwell St Giles Research and Ethics Committee. All participants provided informed written consent and were compensated for their time and travel.
Table 1.
Means and standard deviations of histogram data per group.
| HC |
NTR |
TRS |
||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | χ2(2) | P | |
| Mean | 24.19 | 0.98 | 23.33 | 1.30 | 23.31 | 1.24 | 7.61 | 0.022 |
| Variance | 24.02 | 4.57 | 28.93 | 4.71 | 27.90 | 5.00 | 13.49 | 0.001 |
| Mode (peak position) | 27.46 | 1.18 | 27.15 | 1.39 | 26.89 | 1.33 | 2.23 | > 0.05 |
| Mode frequency (peak height) | 0.17 | 0.04 | 0.14 | 0.03 | 0.14 | 0.03 | 6.98 | 0.031 |
HC: healthy controls. NTR: non-treatment resistant schizophrenia. TRS: treatment resistant schizophrenia. χ2: Kruskall-Wallis Chi squared test statistic.
2.2. MRI acquisition and processing
Scans were acquired on a 3T GE Excite II MR scanner (GE Healthcare, USA) with an 8-channel head coil. The mcDESPOT protocol consisted of a spoiled gradient recalled echo (SPGR) sequence across nine flip angles α (TR = 8.0 ms; TE = 3.6 ms; α = [2, 3, 4, 5, 6, 7, 9, 13, 18]°; matrix = 128 × 128), and a balanced steady-state free procession (bSSFP) sequence across eight flip angles, acquired at radiofrequency phase cycling patterns of 0° and 180° to correct for off-resonance effects (TR = 3.8 ms; TE = 1.9 ms; α = [12, 16, 21, 27, 33, 40, 51, 68]; matrix = 128 × 128). In addition an inversion recovery SPGR was acquired for estimating the flip angle error caused by transmitting B1 field inhomogeneity (TR = 8.0 ms; TE = 3.6 ms; α = 5°; matrix = 220 × 110). The field of view was 220 × 220 cm and the voxel size was 1.7 mm isotropic. The full mcDESPOT sequence lasted approximately 13 min.
All raw images were visually inspected for motion artefacts. Participants with missing scans or excessive motion were re-scanned either immediately or at their earliest convenience, resulting in a complete dataset. Each subject's scans were linearly coregistered, and non-brain parenchyma signal removed using BET (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl). mcDESPOT maps were processed using our previously described open-source software (Wood et al., 2016). Briefly, first T1 & B1 maps were calculated from the data (Deoni, 2007), then T2 & off-resonance maps (Deoni, 2009), and then finally Myelin Water Fraction maps using a three component model and Gaussian Region Contraction (Deoni et al., 2013). MWF maps were non-linearly registered to the MNI152 2 mm isotropic standard brain, and smoothed using a Gaussian kernel at 5 mm FWHM.
2.3. Statistical analysis
2.3.1. Whole-brain group comparisons
Whole-brain normalised MWF maps were subjected to non-parametric permutation tests using FSL's randomise (with 10,000 permutations), and significance values were corrected for multiple comparisons using threshold-free cluster enhancement (TFCE). Clusters were defined with an extent threshold of 50 voxels. We tested the effect of group (HC vs. NTR vs. TRS), adjusting for effects of age and sex.
2.3.2. White matter histogram analysis
In order to analyse the distribution of WMF values within the white matter specifically, we overlaid MWF maps with a white matter mask obtained from the Johns Hopkins University White Matter Atlas. Histogram analyses of white matter MWF were conducted by summing the number of voxels for 100 uniform bins between 0% and 30%. Each individual's histogram was normalised with respect to the area of the histogram. For each subject, the mean (first moment), variance (second moment), peak position (mode), and peak height (frequency of voxels in the modal bin) of the histogram were calculated and subsequently compared between groups using non-parametric Kruskal Wallis tests.
2.4. MWF and the Stroop effect
2.4.1. Stroop task
Subjects performed a standard verbal Stroop task inside the MRI scanner. The task consisted of naming the font colour of colour words that were either congruent (e.g. the word “yellow” printed in yellow) or incongruent (e.g. the word ‘yellow’ printed in blue). Thirty-three congruent, 33 incongruent, and 34 fixation trials were presented in randomised order, each with a duration of 700 ms and inter-stimulus-interval of 2300 ms. This preceded the mcDESPOT scanning protocol.
Each subject's Stroop effect was defined as the difference between their mean reaction time (RT) on incongruent trials and their mean RT on congruent trials. The Stroop effect was compared between groups using one-way ANOVA.
We tested for correlations between whole-brain MWF and the RT Stroop effect across all subjects using randomise. For the resulting significant cluster, we extracted each subject's mean MWF and compared these between groups. We then conducted a mediation analysis to test whether MWF in this region mediated group differences in the RT Stroop effect. We used a bootstrap approach proposed by Preacher and Hayes (2004) in order to test for significance of the indirect effect of group on RT Stroop effect.
3. Results
3.1. mcDESPOT group effects
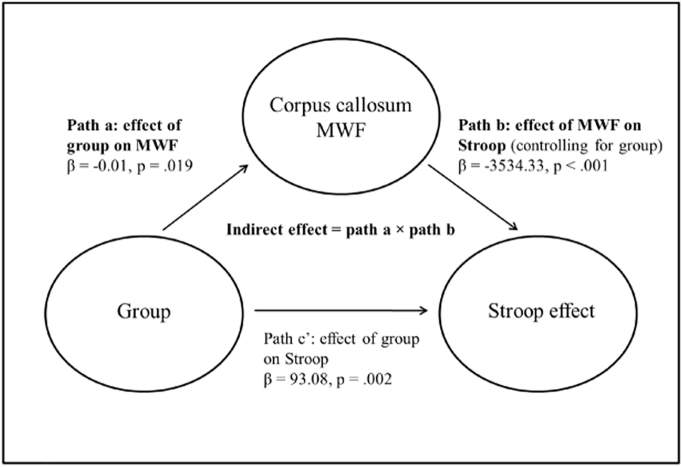
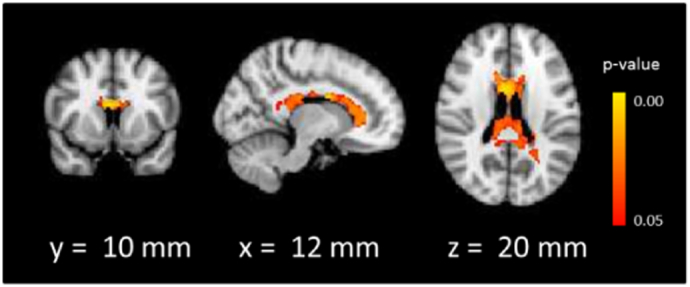
At whole-brain level, controlling for sex and age, there was a significant effect of group in four clusters (Fig. 1): two large clusters covering much of the right (MNI: x = 44, y = −68, z = −36, p = 0.016) and left (MNI: x = −32, y = −18, z = −2, p = 0.010) subcortical white matter including the inferior fronto-occipital fasciculi, particularly in the vicinity of the striatum and extending to the cerebellum, one cluster containing the left putamen (MNI: x = −26, y = 6, z = −6, p = 0.035), and a small cluster in the right subcallosal cortex (MNI: x = 8, y = 18, z = −24, p = 0.026). In each of these clusters, post-hoc t-tests revealed that both NTR and TRS patients showed reductions compared to HC, all ps < 0.05, with no difference detected between the two patient groups, all ps > 0.05. Mean MWF within these clusters ranged from 6% to 19% - lower than the expected range for white matter - indicating that group differences may extend to grey matter regions.
Fig. 1.
Main effect of group on myelin water fraction.
3.2. White matter histogram analysis
Group histograms (depicted in Fig. 2) show that voxel count peaks in the expected range for white matter (25–30%), consistent with previous mcDESPOT reports. Both patient groups show shifts slightly downwards and to the left, suggesting that less voxels within the white matter fall into the normal region of MWF values.
Fig. 2.
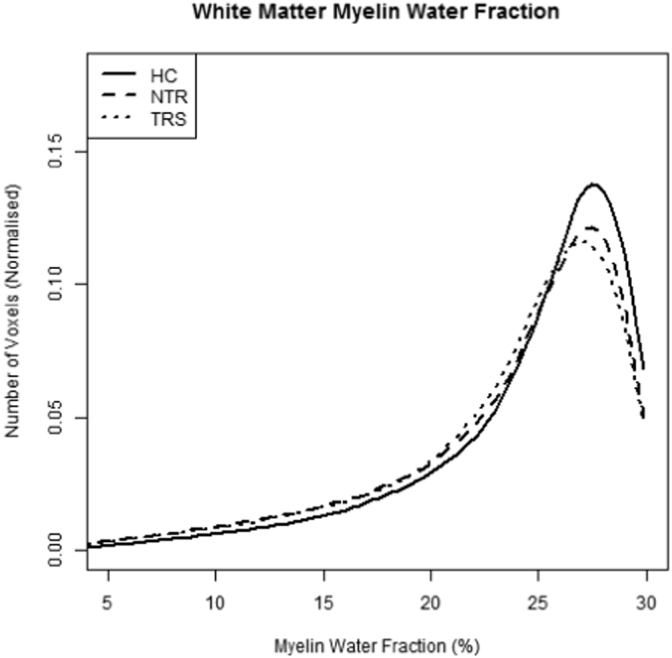
Normalised histograms of white matter myelin water fraction by group.
Post-hoc Mann-Whitney tests (Bonferroni corrected for multiple comparisons) showed that histogram means were significantly lower in TRS compared to HC (W = 374, p = 0.045), and marginally lower in NTR compared to HC (W = 353, p = 0.064). The variance was greater in both NTR and TRS compared to HC, all ps < 0.05. Peak height was reduced in both patient groups compared to HC, although these tests did not survive corrections for multiple comparisons. There were no differences between NTR and TRS groups on any of the histogram measures (Table 1).
3.3. MWF and the Stroop effect
Stroop data from one HC and one TRS subject were not available due to technical issues. There was a main effect of group on RT Stroop effect. Pairwise comparisons revealed that both TRS (M = 186.66 ms; SD = 128.26 ms) and NTR (M = 169.78 ms; SD = 129.95 ms) showed a greater Stroop effect than HC (M = 85.14 ms; SD = 71.87), all ps < 0.05 (Bonferroni corrected), with no difference between the two patient groups, p = 0.627.
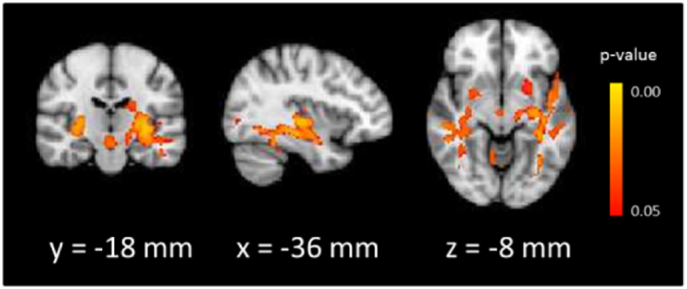
A large cluster consisting of the corpus callosum (genu, body, and splenium) was negatively related with RT Stroop effect across all subjects, such that higher MWF values were associated with a smaller Stroop effect (Fig. 3). We extracted mean MWF from within this cluster for each subject for further analyses. The negative correlation was evident within each group separately (HC: R = −0.35, p = 0.09; NTR: R = −0.50, p = 0.021; TRS: R = −0.59, p = 0.005). Pairwise comparisons of mean MWF in the corpus callosum between HC and the two patient subgroups did not survive Bonferroni corrections for multiple comparisons; hence NTR and TRS patients were pooled into a single schizophrenia (SZ) group for the mediation analysis. We tested whether corpus callosum MWF mediated group differences in the Stroop effect following the Baron and Kenny approach (Baron and Kenny, 1986; Fig. 4) (Baron and Kenny, 1986). Regressing the Stroop effect on group (HC vs. SZ) showed a significant effect of group (beta = 93.08, sd = 28.93, p = 0.002). Regressing corpus callosum MWF on group showed a significant effect of group, (beta = −0.01, sd = 0.01, p = 0.019), with higher MWF in HC (M = 0.20, SD = 0.1) compared to SZ (M = 0.19, SD = 0.17). Finally, regressing the Stroop effect on both group and corpus callosum MWF revealed a significant effect of MWF (beta = −3534.33, sd = 765.24, p < 0.001). The effect of group (beta = 60.42, sd = 26.13, p = 0.02), though significant, was reduced compared to the simple model regressing the Stroop effect on group alone. An analysis of the indirect effect (defined as the product between the effect of group on MWF and the effect of MWF on RT Stroop, controlling for group) was performed with non-parametric bootstrapping of the sampling distribution using 5000 bootstrap samples (Preacher and Hayes, 2004). This revealed a significant indirect effect (95% confidence interval [4.69, 67.50]); suggesting that there was a partial mediation of the effect of group on Stroop effect by corpus callosum MWF.
Fig. 3.
Significant correlation between Stroop effect and myelin water fraction.
Fig. 4.
Mediation diagram of the association between group, Stroop effect, and callosal myelin water fraction.
3.4. Relationship with clinical variables
For each of the four significant clusters from the whole-brain MWF group analysis as well as the corpus callosum cluster from the Stroop analysis, we performed an exploratory analysis of correlations of MWF with PANSS positive symptom score, PANSS negative symptom score, illness duration, and CPZ equivalent medication dosages, both across all patients as well as within the two patient groups separately. No correlation was significant, all ps > 0.05 (uncorrected for multiple comparisons).
4. Discussion
In this study we report the application of mcDESPOT (Deoni et al., 2008) to a sample of patients with a diagnosis of schizophrenia, stratified by treatment response, and healthy controls. We show that this method is sensitive to reductions in myelin water fraction (MWF) in schizophrenia compared to healthy controls, with the greatest effect evident in areas surrounding bilateral striatum, particularly the inferior fronto-occipital fasciculus, as well as the cerebellum. Reductions in the patient groups were bilateral but with larger clusters observed within the left cerebral hemisphere. Mean MWF in the significant areas were below the expected range for white matter, thus suggesting that the reductions included both white and grey matter voxels. Histograms of MWF restricted to the white matter tracts allowed us to more closely compare the distribution of values specifically in the white matter between the three groups, confirming that patients' histograms were shifted towards lower MWF values, with a greater variance compared to healthy controls. MWF did not, however, distinguish between the two patient subgroups, consisting of treatment resistant (TRS) and treatment responsive (NTR) schizophrenia patients. This suggests that underlying abnormal myelination is not a driving force behind antipsychotic treatment resistance at the chronic stage of the illness.
We used a standard Stroop task as a measure of executive control and found a correlation with corpus callosum MWF, whereby greater MWF values were associated with a smaller reaction time interference effect. This association was observable both across all subjects and within all groups separately. A mediation analysis showed that MWF in the corpus callosum partially mediated the group difference on the Stroop task, such that lower MWF in patients accounted in part for the greater interference effects seen in this sample compared to controls.
The findings of reduced MWF in patients with schizophrenia compared to healthy controls are in line with a large body of evidence suggesting impaired white matter integrity in the illness (Fitzsimmons et al., 2013; Kubicki et al., 2005; Pettersson-Yeo et al., 2011). The proliferation of diffusion weighted imaging reports in recent years has shed enormous light on disturbances of white matter in psychosis and schizophrenia on a whole-brain basis, yet a remaining disadvantage of the technique is the large number of microstructural factors which could contribute to the signal. Multicomponent relaxation imaging allows for a more myelin-specific quantification of the dysfunction in vivo. The mcDESPOT protocol is such a technique which has recently been validated pre-clinically (Wood et al., 2016), and to our knowledge this is the first report to use this technique in chronic patients with a diagnosis of schizophrenia, with an additional stratification by treatment response status. The mechanisms underlying resistance to antipsychotic medication are as yet not well understood (Mouchlianitis et al., 2016), but the lack of response despite adequate dopamine D2 receptor occupancy in TRS (Coppens et al., 1991; Wolkin et al., 1989) suggests that striatal hyperdopaminergia may not be the principal aetiology of psychotic symptoms in this patient subgroup. One study investigating white matter microstructure found reduced fractional anisotropy and increased radial diffusivity in the corpus callosum in chronic treatment resistant patients as compared to healthy controls (Holleran et al., 2014); however this study did not include a remitted patient group and as such the specificity of the finding to TRS is unclear. On a functional connectivity level, a recent report by White et al. (2016) suggested that divergent pathophysiologies of striatal resting-state connectivity are present in treatment resistant and treatment responsive schizophrenia. In addition, Sarpal and colleagues demonstrated that functional striatal resting-state connectivity may be predictive of treatment response in first-episode psychosis patients (Sarpal et al., 2015). Our data suggest that these findings of functional connectivity differences are not mirrored by myelin water fraction differences. In this chronic patient sample, the effects of illness chronicity and exposure to medication can by definition not be entirely disentangled from the effects of interest and therefore remain as potential confounds. However, the absence of group difference on these variables as well as the lack of association with myelin water fraction suggest that it is unlikely that these effects are masking true differences in myelination between treatment resistant and responsive patients. Nevertheless, future research could usefully examine these issues by applying the technique to patients at an earlier stage of the illness.
Cognitive deficits are considered a core feature of TRS (Buckley and Shendarkar, 2005), and as such we aimed to investigate whether a lack of cognitive control specifically was associated with treatment resistance. The presence of cognitive control deficits, accompanied by abnormal activation of networks underlying cognitive control, has been widely established in schizophrenia (Edwards et al., 2010; Fornito et al., 2011) and the extent of the dysfunction has been linked to symptom severity (Woodward et al., 2003). An exacerbated inability to exert cognitive control may thus result in the persistence of symptoms even if a subcortical dopamine dysfunction is normalised. Cognitive control further relies on intact connectivity of the underlying neural network (Cole et al., 2012; Hwang et al., 2010); hence we aimed to assess how both cognitive and structural mechanisms relate to treatment response. We did not observe differences in reaction time interference on the Stroop task between the two patient groups. Thus on a behavioural level, treatment resistance does not seem to be associated with exacerbated cognitive control deficits at the chronic stage of the illness. However, callosal myelin reductions in schizophrenia patients partially mediated performance differences compared to healthy individuals. Impaired white matter integrity in the corpus callosum reductions have been widely observed in the illness, but this is the first report of a direct link between a measure of callosal myelination and cognitive control function in schizophrenia.
This study was performed in a relatively small sample. Due to the risk of effect size overestimation associated with small sample sizes, the results warrant replication in future multi-centre studies with larger samples. However, recruitment and neuroimaging assessment in clinical populations can be particularly challenging and typically require a large amount of resources, and as such preliminary assessments in smaller samples can be useful. Suckling et al. (2014) recently demonstrated that effect sizes achieved with the DESPOT sequence in a calibration study on six participants was highly predictive of effect sizes observed in a large-scale multi-centre imaging study. Thus, while acknowledging the need to further validate mcDESPOT imaging as a sensitive method for studying schizophrenia, our results may be useful for supporting the design and execution of future studies.
In summary, the current study demonstrates that mcDESPOT is a suitable method to detect myelin alterations in schizophrenia, clarifying the nature of the changes reported in earlier DTI studies, and this may be a relevant marker in terms of cognitive control performance in patients. However, we did not find more severe myelin abnormalities in treatment resistant patients compared to treatment responsive patients, suggesting that primary differences in treatment response and functional dysconnectivity are not driven by alterations in myelination. Further research could usefully address potential confounds of medication exposure and illness duration by studying these measures in a longitudinal setup in first-episode psychosis.
Acknowledgements
This research was funded by a European Research Council Grant to SSS (grant number 311686), and developed by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London and a joint infrastructure grant from Guy's and St Thomas' Charity and the Maudsley Charity. LDV is supported by a Medical Research Council studentship. All authors report that they have no biomedical financial interests or potential conflicts of interest with respect to the content of this manuscript.
References
- Andreasen N.C., Carpenter W.T., Jr., Kane J.M., Lasser R.A., Marder S.R., Weinberger D.R. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiatr. 2005;162(3):441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Buckley P.F., Shendarkar N. Treatment-refractory schizophrenia. Curr. Opin. Psychiatry. 2005;18(2):165–173. doi: 10.1097/00001504-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Canu E., Agosta F., Filippi M. A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr. Res. 2015;161(1):19–28. doi: 10.1016/j.schres.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Yarkoni T., Repovš G., Anticevic A., Braver T.S. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J. Neurosci. 2012;32(26):8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley R.R., Kelly D.L. Management of treatment resistance in schizophrenia. Biol. Psychiatry. 2001;50(11):898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- Coppens H.J., Slooff C.J., Paans A.M., Wiegman T., Vaalburg W., Korf J. High central D 2-dopamine receptor occupancy as assessed with positron emission tomography in medicated but therapy-resistant schizophrenic patients. Biol. Psychiatry. 1991;29(7):629–634. doi: 10.1016/0006-3223(91)90132-6. [DOI] [PubMed] [Google Scholar]
- Deoni S.C. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI) J. Magn. Reson. Imaging. 2007;26(4):1106–1111. doi: 10.1002/jmri.21130. [DOI] [PubMed] [Google Scholar]
- Deoni S.C. Transverse relaxation time (T2) mapping in the brain with off-resonance correction using phase-cycled steady-state free precession imaging. J. Magn. Reson. Imaging. 2009;30(2):411–417. doi: 10.1002/jmri.21849. [DOI] [PubMed] [Google Scholar]
- Deoni S.C., Matthews L., Kolind S.H. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn. Reson. Med. 2013;70(1):147–154. doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C., Rutt B.K., Arun T., Pierpaoli C., Jones D.K. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn. Reson. Med. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- Edwards B.G., Barch D.M., Braver T.S. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front. Hum. Neurosci. 2010;4:32. doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons J., Kubicki M., Shenton M.E. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr. Opin. Psychiatry. 2013;26(2):172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yoon J., Zalesky A., Bullmore E.T., Carter C.S. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 2011;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K., Brown H.R., Siemerkus J., Stephan K.E. The dysconnection hypothesis (2016) Schizophr. Res. 2016;176(2):83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome. Clin. Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Garver D.L., Holcomb J.A., Christensen J.D. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int. J. Neuropsychopharmacol. 2008;11(1):49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. National Institute of Mental Health; 2000. Neurocognitive Deficits and Functional Outcome in Schizophrenia: Are we Measuring the “Right Stuff”? [DOI] [PubMed] [Google Scholar]
- Holleran L., Ahmed M., Anderson-Schmidt H., McFarland J., Emsell L., Leemans A.…Barker G.J. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology. 2014;39(4):944–954. doi: 10.1038/npp.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K., Velanova K., Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J. Neurosci. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Flszbein A., Opfer L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Klauser P., Baker S.T., Cropley V.L., Bousman C., Fornito A., Cocchi L.…Henskens F. White matter disruptions in schizophrenia are spatially widespread and topologically converge on brain network hubs. Schizophr. Bull. 2017;43(2):425–435. doi: 10.1093/schbul/sbw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., McCarley R.W., Shenton M.E. Evidence for white matter abnormalities in schizophrenia. Curr. Opin. Psychiatry. 2005;18(2):121. doi: 10.1097/00001504-200503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., McCarley R., Westin C.-F., Park H.-J., Maier S., Kikinis R.…Shenton M.E. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007;41(1):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C., Leung E., Li D.K., Traboulsee A., Paty D., MacKay A., Moore G.R. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult. Scler. J. 2006;12(6):747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J.-P. Treatment refractory schizophrenia. Psychiatry Q. 2000;71(4):373–384. doi: 10.1023/a:1004640408501. [DOI] [PubMed] [Google Scholar]
- Liu H., Fan G., Xu K., Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J. Magn. Reson. Imaging. 2011;34(6):1430–1438. doi: 10.1002/jmri.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mädler B., Drabycz S.A., Kolind S.H., Whittall K.P., MacKay A.L. Is diffusion anisotropy an accurate monitor of myelination?: correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn. Reson. Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Marenco S., Stein J.L., Savostyanova A.A., Sambataro F., Tan H.-Y., Goldman A.L.…Apud J.A. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37(2):499–507. doi: 10.1038/npp.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer A., Singh P., Shepherd C., Puthiryackal J. Clozapine for treatment-resistant schizophrenia: national institute of clinical excellence (NICE) guidance in the real world. Clin. Schizophr. Relat. Psychoses. 2010;4(1):49–55. [PubMed] [Google Scholar]
- Mouchlianitis E., McCutcheon R., Howes O.D. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016;3(5):451–463. doi: 10.1016/S2215-0366(15)00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev. 2011;35(5):1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Samartzis L., Dima D., Fusar-Poli P., Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J. Neuroimaging. 2014;24(2):101–110. doi: 10.1111/j.1552-6569.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- Sarpal D.K., Argyelan M., Robinson D.G., Szeszko P.R., Karlsgodt K.H., John M.…Lencz T. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am. J. Psychiatr. 2015;173(1):69–77. doi: 10.1176/appi.ajp.2015.14121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J., Henty J., Ecker C., Deoni S.C., Lombardo M.V., Baron-Cohen S.…Ooi C. Are power calculations useful? A multicentre neuroimaging study. Hum. Brain Mapp. 2014;35(8):3569–3577. doi: 10.1002/hbm.22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasour I.M., Laule C., Li D.K., Traboulsee A.L., MacKay A.L. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J. Magn. Reson. Imaging. 2011;33(3):710–718. doi: 10.1002/jmri.22441. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wolf A.P., Brodie J.D., Cancro R., Overall J.E., Rhoades H., Van Gelder P. Brain interactions in chronic schizophrenics under resting and activation conditions. Schizophr. Res. 1988;1(1):47–53. doi: 10.1016/0920-9964(88)90039-4. [DOI] [PubMed] [Google Scholar]
- Webb S., Munro C.A., Midha R., Stanisz G.J. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magn. Reson. Med. 2003;49(4):638–645. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- Westerhausen R., Kompus K., Hugdahl K. Impaired cognitive inhibition in schizophrenia: a meta-analysis of the Stroop interference effect. Schizophr. Res. 2011;133(1):172–181. doi: 10.1016/j.schres.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Wheeler A.L., Voineskos A.N. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.P., Wigton R., Joyce D.W., Collier T., Fornito A., Shergill S.S. Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology. 2016;41(5):1274–1285. doi: 10.1038/npp.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin A., Barouche F., Wolf A.P., Rotrosen J., Fowler J.S., Shiue C.-Y.…Brodie J.D. Dopamine blockade and clinical response: evidence for two biological subgroups of schizophrenia. Am. J. Psychiatr. 1989;146(7):905–908. doi: 10.1176/ajp.146.7.905. [DOI] [PubMed] [Google Scholar]
- Wood T.C., Simmons C., Hurley S.A., Vernon A.C., Torres J., Dell'Acqua F.…Cash D. PeerJ Preprints. vol. 4. 2016. Whole-brain ex-vivo quantitative MRI of the cuprizone mouse. (e2323v2321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward T.S., Ruff C.C., Thornton A.E., Moritz S., Liddle P.F. Methodological considerations regarding the association of Stroop and verbal fluency performance with the symptoms of schizophrenia. Schizophr. Res. 2003;61(2):207–214. doi: 10.1016/s0920-9964(02)00211-6. [DOI] [PubMed] [Google Scholar]