Abstract
BACKGROUND: Ovarian cancer patients with chemotherapy-resistant residual microscopic disease in the peritoneal cavity have a considerable need for new treatment options. Alpha-emitting radionuclides injected intraperitoneally may be an attractive therapeutic option in this situation as they are highly cytotoxic, while their short range in tissues can spare surrounding radiosensitive organs in the abdomen. Herein we evaluate the therapeutic efficacy of a novel α-emitting compound specifically designed for intracavitary radiation therapy. METHODS: The α-emitter 224Ra was absorbed on calcium carbonate microparticles. Immunodeficient, athymic nude mice with human ovarian cancer cells growing intraperitoneally were treated with different activity levels of 224Ra-microparticles. Tumor growth, survival, and tolerance of the treatment were assessed. Two tumor models based on the cell lines, ES-2 and SKOV3-luc, with different growth patterns were studied. RESULTS: In both models, intraperitoneal treatment with 224Ra-microparticles gave significant antitumor effect with either considerably reduced tumor volume or a survival benefit. An advantageous discovery was that only a few kilobecquerels per mouse were needed to yield therapeutic effects. The treatment was well tolerated up to a dose of 1000 kBq/kg with no signs of acute or subacute toxicity observed. CONCLUSIONS: Intraperitoneal α-therapy with 224Ra-microparticles demonstrated a significant potential for treatment of peritoneal micrometastases in ovarian carcinoma.
Introduction
Among gynecological malignancies, ovarian cancer continues to be the most lethal type and is currently estimated to account for 5% of all cancer deaths in the female population [1]. Standard therapy with cytoreductive surgery in combination with platinum-based chemotherapy fails to prevent relapse within 3 years of the initial treatment in 75% of patients [2]. The prognosis is particularly dismal for women with platinum-resistant disease, where a minority of patients respond to subsequent therapies [3]. Considerable research effort is currently focused on alternative treatment strategies to eliminate residual disease in the peritoneal cavity due to the close correlation between the residual disease and poor prognosis for these patients [2] and because relapse mainly is confined to the abdominal cavity [3].
Therapeutic radionuclides include both α- and β-particle emitters. Alpha-particles have a short penetration depth of only a few cell diameters in tissue (50-100 μm), whereas β-particles can penetrate several millimeters. Alpha-emitting radionuclides are potent cytotoxic agents due to the large amount of energy they deposit over a short distance, resulting in high linear energy transfer. Just a few α-particle traversals of a cell nucleus are sufficient for cell kill because of their high capacity of inflicting nonrepairable DNA double-strand breaks. In contrast, low linear energy transfer β-radiation requires more than 1000 hits for lethal DNA damage. Due to their physical characteristics, α-emitting radionuclides are well suited for treatment of micrometastatic disease. Adjuvant intraperitoneal (IP) therapy with α-emitters may therefore be beneficial for ovarian cancer patients since hallmarks of the disease include dissemination within the abdominal cavity and residual micrometastases after cytoreductive surgery in a high number of patients.
In theory, α-emitting radionuclides are more suitable for elimination of micrometastatic disease in the peritoneal cavity than β-emitting radionuclides, although most clinical data exist for β-emitters. IP therapy with the β-emitting 32P-colloid was used in treatment of ovarian cancer during the 1980s. It was shown to be as effective as adjuvant cisplatin [4] but resulted in higher incidence of late bowel complications, most likely caused by a combination of heterogeneous distribution, relatively long half-life (14.3 days), and significant range of the 32P-radiation. More recently, several clinical trials of IP therapy with β-emitting radionuclides coupled to monoclonal antibodies have been conducted [5]. Unfortunately, the only phase III trial on IP radioimmunotherapy utilizing the β-emitting conjugate 90Y-HMG1 failed to demonstrate improved survival or time to relapse [6]. An insufficient absorbed dose in the micrometastases to efficiently eliminate tumor cells was suggested as a possible explanation of the unfavorable result [5], [6]. It is presumed that the use of α-emitters can overcome some of the hurdles with prior β-therapies, both due to (1) the considerably shorter range of α-particles that prevents irradiation of deeper regions of sensitive abdominal organs, such as the small intestine, and (2) the much higher relative biological effectiveness of the radiation. The two IP α-radioimmunotherapies that so far have been investigated clinically both showed minor treatment-related toxicity in phase I studies [7], [8]. However, no publication on antitumor activity exists to date, but the conclusion from the phase I study with the α-emitter 211At conjugated to antibody fragments was that the treatment could achieve therapeutically relevant absorbed doses in microscopic tumor nodules [7].
IP therapy with α-emitters has previously been examined in murine models with two carrier types: nano- to microsized particles [9], [10], [11], [12] and monoclonal antibodies [13], [14], [15], [16], [17], [18], [19], [20], [21]. With particles as carriers for radionuclides, it is possible to choose a size that facilitates a high retention of the particles in the peritoneal cavity [22], [23] and thus contribute to the therapeutic radiation being delivered in the target location. In contrast, antibodies can rapidly leak into the circulation, but they have the potential advantage of cell specific targeting.
A method for labeling of calcium carbonate (CaCO3) microparticles with the α-emitter 224Ra was recently reported [24]. Radium-224 has attractive properties for α-therapy as it produces four α-particles per decay and has a convenient half-life of 3.6 days. After IP administration of the 224Ra-labeled microparticles in athymic nude mice, the radioactivity mainly remained intra-abdominally with a promising distribution [24]. In the current study, the therapeutic potential of IP-administered 224Ra-labeled microparticles was explored in murine models of IP growing tumors, and a preliminary evaluation of the tolerability of the treatment was performed.
Materials and Methods
Preparation of 224Ra-labeled CaCO3 microparticles
The preparation of 224Ra-labeled CaCO3 microparticles has been described in detail elsewhere [24]. In brief, CaCO3 microparticles were prepared by a spontaneous precipitation method which yielded particles with individual diameters ranging from 3 to 15 μm. Radium-224 was extracted from a generator based on 228Th immobilized on an actinide resin, and a solution of 224Ra in 0.1 M HCl and 0.5 M NH4OAc was used for radiolabeling. The microparticles were mixed in a solution of 0.5% bovine serum albumin in Dulbecco’s PBS containing 0.3 wt% barium and sulfate and the desired amount of 224Ra solution. After radiolabeling, the microparticles were dispersed to a concentration of approximately 12.5 mg/ml in a sucrose solution (pH 7.5) containing 94 mg/ml sucrose (Sigma Ultra, St. Louis, MO) and 2.1 mg/ml Na2SO4 (Alfa Aesar, Karlsruhe, Germany).
Animals and Tumor Models
Institutionally bred, 4- to 9-week-old, female athymic nude Foxnnu mice maintained under pathogen-free conditions with food and water supplied ad libitum weighing 17 to 24 g at the start of the experiment were used. The mice were ear-tagged for individual follow-up and randomly assigned to treatment groups. All procedures involving animals were approved by the National Animal Research Authority (permit ID 7274) and performed in compliance with regulations set by the same authority and the EU Directive 2010/63/EU on the protection of animals used for scientific purposes.
Xenografts were generated by a single IP injection of human ovarian epithelial adenocarcinoma cell lines SKOV-3luc (Bioware/Caliper Life Sciences, Hopkinton, MA) or ES-2 (ATCC, Wesel, Germany), cultured in McCoy’s 5A medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin at 37°C in a humid atmosphere with 5% CO2. The mice were monitored for changes in bodyweight, behavior, posture, and appearance a minimum twice per week and more frequently when they displayed signs indicating disease progression. Disease progression was observed as abdominal distensions due to buildup of ascites and/or palpable tumor nodules in the abdomen, often in combination with a decreased body condition score. Euthanasia by cervical dislocation was performed when mice reached endpoints defined as rapid weight loss (more than 10% loss within a week), abdominal distensions that impaired mobility or respiration, and/or a body condition score of two or less.
Therapy Studies
Two therapeutic studies with different setups and tumor models were performed. In the first study, mice inoculated with 5∙106 SKOV3-luc cells in 0.25 ml were treated 3 days later with IP injections of 224Ra-labeled CaCO3 microparticles in sucrose solution with activities of 65 kBq/kg (0.25-0.3 ml), 200 kBq/kg (0.35-0.4 ml), or three injections of 65 kBq/kg (0.25-0.4 ml). The latter group had a 48-hour interval between each injected fraction. Control animals received 0.9% NaCl (0.4 ml) or 200 mg/kg (0.35-0.4 ml) nonlabeled CaCO3 microparticles in sucrose solution. Each group consisted of eight mice. Tumor growth was monitored by bioluminescence imaging in an IVIS Spectrum in vivo imaging system (PerkinElmer, Waltham, MA). Each mouse was injected IP with 0.2 ml D-luciferin (Biosynth AG, Staad, Switzerland) dissolved in Dulbecco’s PBS (20 mg/ml) 10 minutes prior to imaging and anesthetized with sevoflurane during the imaging. At day 47 and 48 after cell inoculation, when mice in the control groups showed signs of disease progression, all animals were euthanized. During dissection, the presence of macroscopic tumors was assessed by careful inspection, and all visible tumors in the peritoneal cavity were removed and weighed. In addition, liver, spleen, stomach, intestines, and femur from three mice per group were excised and fixed in formalin-free fixative (Accustain, Sigma-Aldrich) for histopathological examination of tumor growth. At Capra Science (Ängelholm, Sweden), fixed tissues were embedded in paraffin wax, and 5-μm sections were stained with hematoxylin-eosin before microscopic examination by a pathologist.
In the second study, mice inoculated with 1×106 ES-2 cells in 0.35 ml were treated 1 day later with IP injections of 224Ra-labeled CaCO3 microparticles in sucrose solution. Groups of mice were given 150 kBq/kg (0.25-0.35 ml, 9 mice), 300 kBq/kg (0.3-0.35 ml, 9 mice), 1000 kBq/kg (0.4 ml, 3 mice), or two injections of 150 kBq/kg (0.3-0.4 ml, 9 mice) separated by 1 week. Control animals (10 mice) received 0.35 ml NaCl. The mice were sacrificed when they reached the predetermined endpoint and necropsied for gross pathological examination.
Toxicity Evaluation
In the SKOV3-luc study, hematology analyses were performed on blood samples drawn from the heart while the mice were under sevoflurane anesthesia prior to euthanasia. The samples were collected in EDTA-coated tubes, and platelets and white and red blood cells were counted in an ADVIA 120 Hematology System (Siemens AG, Munich, Germany) at Sentrallaboratoriet (Norwegian University of Life Sciences, Ås, Norway). In addition, the organs/tissues removed for histopathology were examined for abnormalities that may have been caused by the treatment.
Hematological analyses in the ES-2 study were performed on maximum 100 μl blood collected in EDTA-coated tubes from the vena saphena lateralis 13 days prior to and 13 and 26 days after treatment start. In each group, three to five mice were sampled at every time point. Platelets and white and red blood cells were counted in an automated veterinary hematology analyzer (scil Vet abc, ABX Diagnostics, Montpellier, France). In addition, the concentrations of clinical chemistry parameters urea, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase were measured in a Reflovet Plus clinical chemistry analyzer (Roche Diagnostics, Mannheim, Germany) on blood samples taken by heart puncture prior to euthanasia. The blood samples were collected in serum separation tubes containing a clot activator and left for minimum 30 minutes before centrifugation.
Statistical Analyses
All statistical analyses were performed in GraphPad Prism (version 7.03, GraphPad Software, La Jolla, CA). Blood parameters at sacrifice were analyzed by one-way ANOVA, and all experimental groups were compared to the control by Dunnett’s multiple-comparisons test. Analyses of hematological data over time were performed by two-way ANOVA and Tukey’s multiple-comparisons test. Survival curves were compared pairwise by log-rank tests. To adjust for multiple comparisons, the Bonferroni method was used with a family-wise significance level of .05. If the P value from each pairwise comparison was less than the Bonferroni-corrected threshold, the comparison was said to be statistically significant.
Results
Antitumor Activity of 224Ra-labeled CaCO3 Microparticles in the SKOV3-luc Model of Intraperitoneal Micrometastases
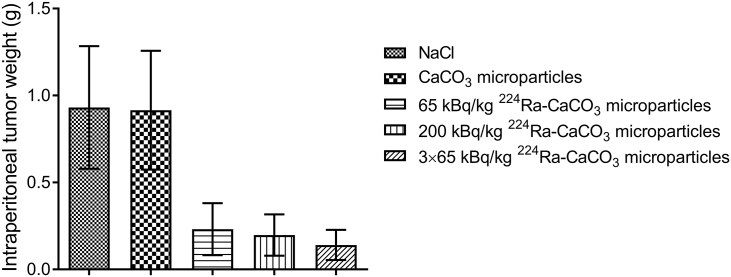
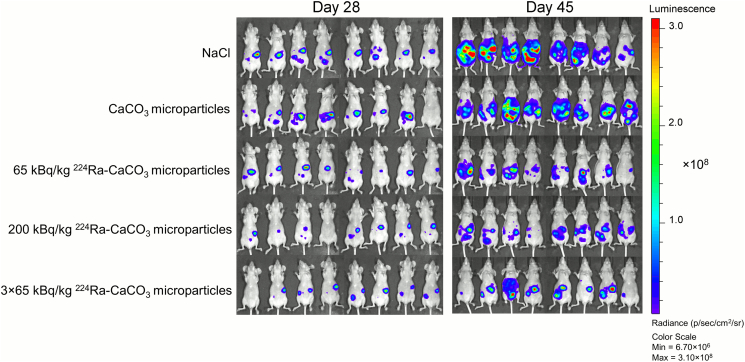
A substantial reduction of IP SKOV3-luc tumor growth was observed after treatment with the 224Ra-labeled CaCO3 microparticles. The average IP tumor weight was at least four times larger in the control groups than in all groups receiving 224Ra-labeled CaCO3 microparticles at days 47 and 48 after cell inoculation (Figure 1). Between the different radioactive treatment groups, there was a nonsignificant trend towards more pronounced tumor growth suppression at higher dose of 224Ra and by repeated treatment. The average IP tumor weights of the two control groups receiving either saline or nonlabeled CaCO3 microparticles were indistinguishable, indicating no antitumor effect of the microparticle carrier by itself. In some mice, tumor growth was observed as a subcutaneous xenograft at the injection site of the cell suspension. As these tumors were outside of the peritoneal cavity, their weights were not included in the total weights presented in Figure 1. The tumor growth was also monitored by in vivo bioluminescence imaging of all mice 28 and 45 days after inoculation of SKOV3-luc cells (Figure 2). At day 28, there was a tendency towards higher bioluminescent signal from the mice in the control groups compared to the 224Ra-treated groups. This trend was even more evident on day 45, and the bioluminescent signal clearly correlated with the IP tumor weight.
Figure 1.
Intraperitoneal tumor weight after mice bearing 3-day-old intraperitoneal SKOV3-luc xenografts received a single injection of saline, nonlabeled CaCO3 microparticles, or a low or high dose of 224Ra-labeled CaCO3 microparticles. One group received three injections of the low dose separated by 48-hour intervals. The intraperitoneal tumors were harvested and weighed 47 and 48 days after cell inoculation.
Figure 2.
Tumor growth monitored by bioluminescence imaging in the different therapy groups 28 and 45 days after SKOV3-luc inoculation. The mice are positioned in the same order at both time points.
The histopathological analysis showed that tumor cells and/or solid tumors were located at, or close to, the organ surfaces of all tissues investigated, except for femur/bone marrow where no tumors were observed. A lower prevalence of tumors in the 224Ra-treated groups versus the two control groups was confirmed. Solid tumors were more frequently observed in the control mice. Regarding the gastrointestinal tract, tumors were always associated with surrounding fat tissue, and the incidence was almost three times higher in the control compared to the active treatment groups. In addition, a histopathological analysis was carried out to identify possible treatment-related abnormalities. Overall, animals showed very similar tissue morphology, with minor deviations from normal. Distinct lesions were observed in only two animals: one mouse from the highest dose group and one from the group receiving repeated treatment (Supplementary Figure 1). The liver of the mouse from the highest dose group contained groups of “foamy cells” that may reflect hepatocyte degeneration (“feathery degeneration”) and/or macrophage-rich foci. In the mouse receiving repeated treatment, the liver showed inflammation of the portal tracts and piecemeal necrosis of adjoining hepatocytes, and the kidney contained tubules with cellular debris of the neutrophil granulocyte type, reflecting acute inflammation. It is not possible to conclude whether these lesions were caused by the radioactive treatment since they were not observed in animals of the two other groups.
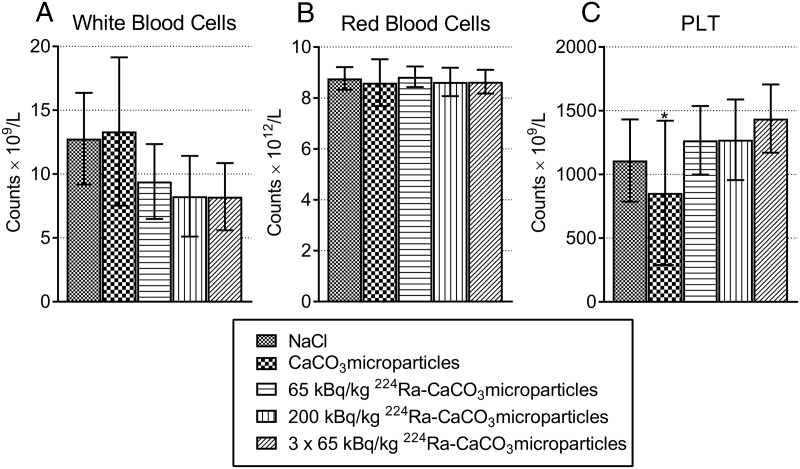
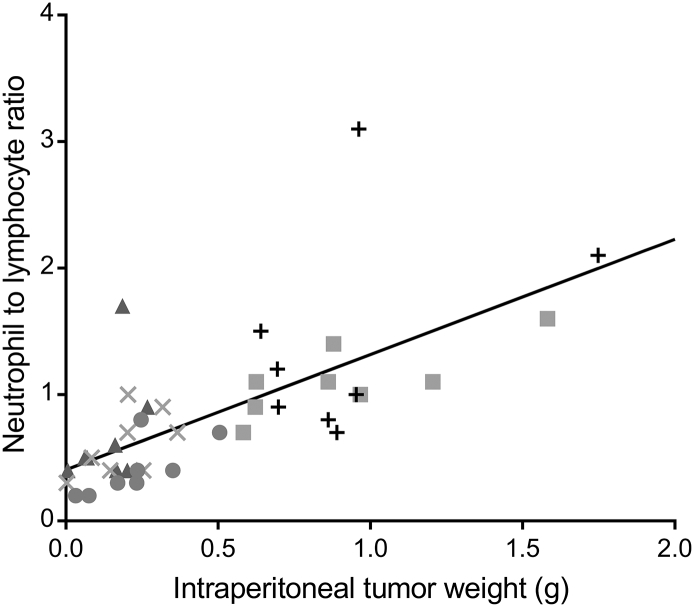
None of the mice had a weight loss larger than 3% after treatment start, and the majority experienced no weight reduction (Supplementary Figure 2). Blood samples taken for hematology evaluation at end point generally showed few differences between the groups (Figure 3), indicative of no or a moderate and reversible hematological toxicity of the radioactive treatment. Slightly higher white blood cell counts were observed in the controls compared to the 224Ra-treated groups. A clear correlation between IP tumor weight and the neutrophil to lymphocyte ratio was found (Figure 4). Elevated blood neutrophil to lymphocyte ratios were recently shown to be significantly associated with poorer overall and progression-free survival and correlated with advanced FIGO stage and more extensive ascites in ovarian cancer patients [25], and is known to have significance as a prognostic factor for various other cancers [26], [27]. It should, however, be emphasized that since these data were obtained in immunodeficient mice, they are not directly comparable to human studies.
Figure 3.
Hematological parameters white blood cell (A), red blood cell (B), and platelet (C) counts in the different therapy groups measured at end point 47 and 48 days after inoculation of SKOV3-luc cells. ⁎Large standard deviation probably due to some clotted samples.
Figure 4.
Relation of intraperitoneal tumor weight to neutrophil to lymphocyte ratio 47 and 48 days after SKOV3-luc inoculation. Each symbol gives the values for one mice receiving saline (+), unlabeled CaCO3 microparticles (■), 65 kBq/kg (●), 200 kBq/kg (×) or 3×65 kBq/kg 224Ra-labeled CaCO3 microparticles (▲) The solid line is the regression line showing the correlation between all intraperitoneal tumor weights and neutrophil to lymphocyte ratios.
Therapeutic and Hematological Effect of 224Ra-Labeled CaCO3 Microparticles in the ES-2 Ascites Model
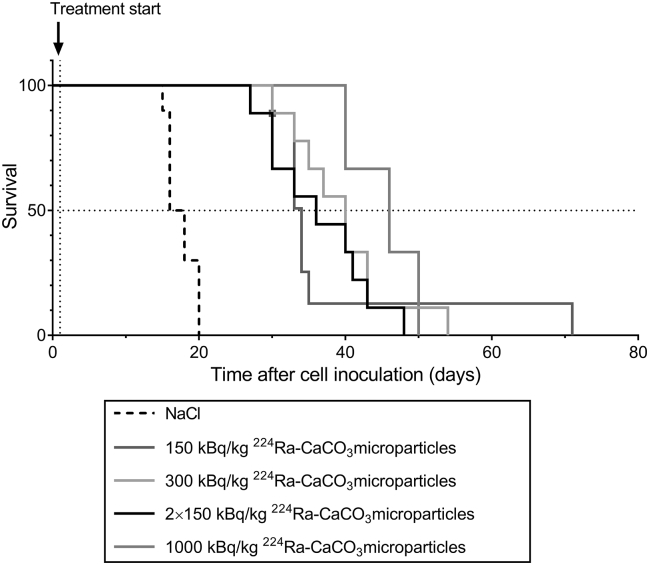
Mice inoculated with ES-2 cells were treated with 224Ra-labeled CaCO3 microparticles to investigate the therapeutic effect of the treatment in a cancer model that leads to aggressive tumor growth and ascites production. From the survival curves (Figure 5), it is seen that the median survival was prolonged from 17 days for the saline control group to 34, 40, 36, and 46 days in the groups treated with 150 kBq/kg, 300 kBq/kg, 2×150 kBq/kg, and 1000 kBq/kg 224Ra-labeled CaCO3 microparticles, respectively. A comparison of the curves revealed that all experimental groups differed significantly from the control group. The group given the highest dose consisted of only three mice and could not be compared statistically in a meaningful way with the other groups. None of the radioactive treatment groups were significantly different from each other, although there was a tendency for prolonged median survival with increased dosing.
Figure 5.
Survival of mice inoculated intraperitoneally with 1×106 ES-2 cells and treated 1 day later with intraperitoneal injections of saline or different doses of 224Ra-labeled CaCO3 microparticles. The fractionated treatment was given with a 1-week interval.
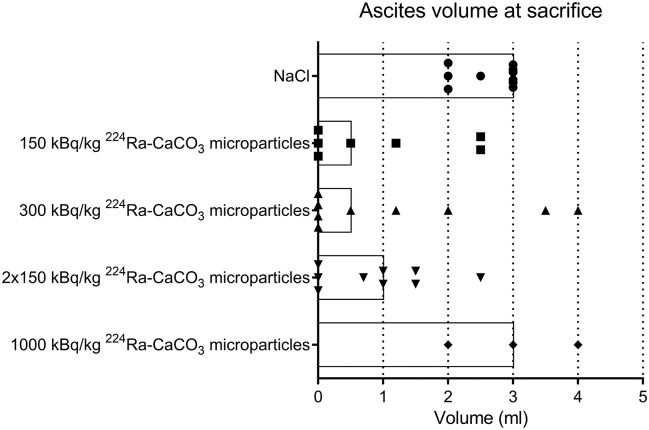
Measurements of ascites at time of sacrifice (Figure 6) showed a lower median volume in the 224Ra-treated groups compared to the saline control, except for the highest dose group which only consisted of three mice. In the control, all animals (10/10) had 2 ml or more ascites at sacrifice, whereas when all mice treated with 224Ra-labeled CaCO3 microparticles are pooled, only 32% (9/28) had 2 ml or more ascites. In addition, as many as 10 of the mice treated with 224Ra-labeled CaCO3 microparticles (36%) had no visible ascites at sacrifice. Clearly, there was larger intragroup variation in ascites volume after the radioactive treatments compared with the control. In contrast to the control group, there were incidences of organ abnormalities in many of the mice given 224Ra-labeled CaCO3 microparticles. At the necropsies, discolored and/or enlarged livers, kidneys, spleens, and/or ovaries were observed in these mice, suggestive of metastasis or tumor ingrowth in the respective organs at longer survival times.
Figure 6.
Variation in measured ascites volume at time of sacrifice for mice inoculated intraperitoneally with 1×106 ES-2 cells and treated 1 day later with intraperitoneal injections of saline or different doses of 224Ra-labeled CaCO3 microparticles. Each symbol represents the ascites volume for one mouse, and the length of the bar shows the median ascites volume in the different treatment groups.
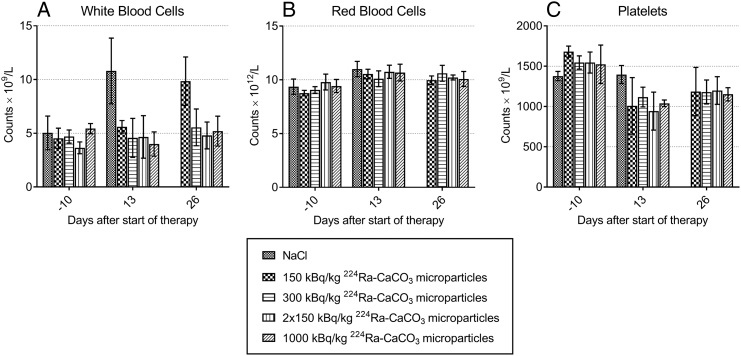
No mice experienced weight loss after the 224Ra treatment (Supplementary Figure 3). White and red blood cells and platelets were measured 10 days prior to and 13 and 26 days after treatment start (Figure 7). No reduction in white blood cell counts was observed compared to before treatment start. On the contrary, a significant increase in white blood cells was seen both for the saline group on day 13 and the lowest dose group on day 26. These time points coincided with when the sampled mice from these groups started to experience disease progression. For red blood cells, no notable differences from the saline control were seen in any of the treatment groups. The mean platelet counts were slightly reduced after therapy start in the radioactive treatment groups, although the decrease was not statistically significant. Platelet counts on day 13 in the group receiving fractionated treatment differed significantly from the saline control group on the same day but not from the platelet counts in the control measured prior to therapy start.
Figure 7.
Hematological parameters white blood cell (A), red blood cell (B), and platelet (C) counts determined at different times after intraperitoneal injections of saline or different doses of 224Ra-labeled CaCO3 microparticles in mice inoculated with ES-2 cells. Error bars represent standard deviations.
Clinical chemistry analyses were performed at sacrifice (Supplementary Figure 4). For most of the parameters, some degree of intragroup variation was observed, but no significant differences were detected between the saline and radiotherapy groups. Outliers showing high values appeared in all groups and were therefore probably not treatment related but rather due to the respective organs in these mice being influenced by tumor growth.
Discussion
To our knowledge, this is the first study of microparticles labeled with 224Ra used as a microdevice for delivering α-particle radiation to the surfaces of the peritoneal cavity. Herein, we have shown significant therapeutic efficacy of the 224Ra-labeled CaCO3 microparticles in two different murine models of IP growing ovarian cancer. The radioactive treatment was given shortly after inoculation of cells in an attempt to mimic the clinical situation of residual micrometastatic disease after surgery. All observations from the studies suggest that the 224Ra-labeled CaCO3 microparticles are well tolerated.
An advantageous property with the 224Ra-labeled CaCO3 microparticles was the low amount of radioactivity resulting in significant antitumor effect. A few kilobecquerels of 224Ra per mouse were sufficient, which are substantially lower than the several hundred kilobecquerels of 213Bi [13], [14], 211At [15], [16], and 212Pb [18], [19], [20] needed in IP α-radioimmunotherapy of mice with IP tumors previously reported. It is difficult to directly compare the therapeutic benefit of the different α-therapies due to variations in cancer models and treatment schedules, although for survival studies a therapeutic index defined as the median survival of the treatment group divided by the median survival of the untreated group can give an indication. An overview of a few published studies of IP α-therapy in mice inoculated with ovarian or colorectal cancer cell lines and the obtained therapeutic indices are given in Table 1. It is clear from the table that similar therapeutic efficacy was achieved for the 224Ra-labeled CaCO3 microparticles as with α-radioimmunotherapy based on short-lived nuclides like 213Bi, 211At, and 212Pb while requiring substantially lower amounts of radioactivity.
Table 1.
Overview of Study Details for Some Published Reports of Intraperitoneal α-Therapy in Mice Inoculated Intraperitoneally with Ovarian or Colorectal Cancer Cell Lines
| Product | T½ | Dose | Cell Line | # Cells × 106 | Treatment Day After Cell Inoculation | Therapeutic Index | Ref. |
|---|---|---|---|---|---|---|---|
| 211At-trastuzumab | 7.2 h | 1.1 MBq | LS174T | 100 | 3 | 2.3±0.0* | [28] |
| 212Pb-panitumumab | 10.6 h | 740 kBq | LS174T | 100 | 3 | 2.6±1.7† | [29] |
| 212Pb-trastuzumab | 10.6 h | 370 kBq | LS174T | 100 | 3 | 2.7±0.7‡ | [28] |
| 212Pb-376.96 | 10.6 h | 510 kBq | ES-2 | 0.6 | 4 | 2.5 | [20] |
| 212Pb-376.96 | 10.6 h | 700 kBq | A2780cp20 | 2 | 10 | 1.9 | [20] |
| 213Bi-trastuzumab | 46 min | 18.5 MBq | LS174T | 100 | 3 | 2.8±1.0§ | [28] |
| 224Ra-CaCO3microparticles | 3.6 days | 8 kBq | ES-2 | 1 | 1 | 2.4 | |
| 225Ac-trastuzumab | 9.9 days | 3 × 4 kBq | SKOV3-NMP2 | 5 | 9 | 2.8 | [21] |
| 227Th-trastuzumab | 18.7 days | 18.5 kBq | LS174T | 100 | 3 | 2.1 | [28] |
| 227Th-trastuzumab | 18.7 days | 3 × 10 kBq | SKOV3-luc | 10 | 17 | 1.8 | [17] |
The therapeutic index is defined as the median survival of the treatment group divided by the median survival of the untreated group. It is given for the dose level reported to have the highest efficacy while at the same time not causing acute toxic events in the mice.
Number of studies (n) = 2.
n = 2.
n = 7.
n = 7.
This finding is assumed to be beneficial for translation into clinical applications as it can reduce radiation exposure to personnel during both preparation and administration of the product. Furthermore, 213Bi, 211At, and 212Pb are all radionuclides with short half-lives and therefore need to be prepared on site. Ra-224, on the other hand, with its 3.6-day half-life, makes centralized production before shipment to the hospital possible. In addition to logistical difficulties with short half-lives, 213Bi and 211At also both suffer from scalability issues. The high activities needed with 213Bi are demanding to handle for most institutions and make it expensive to use, while with 211At, availability is a major concern. A challenge with the 224Ra-series, which includes the 212Pb progeny, is the highly energetic 2.6 MeV γ-emission from one of the daughters which requires extensive shielding. Due to the at least 10-fold reduced amount of activity with 224Ra compared to 212Pb, the amount of γ-radiation is strongly reduced, offering improved ease of handling.
A minimum doubling in median survival was also reported with IP radioimmunotherapies with 225Ac and 227Th (Table 1), both long-lived nuclides with multiple α-emitting daughters. Similar activity levels of 225Ac and 227Th were needed to yield comparable therapeutic indices to the 224Ra-labeled CaCO3 microparticles in this study. Due to their significantly longer half-lives, on a radionuclide atom basis, the 224Ra therapy was more efficient.
There was a tendency towards better efficacy at higher doses of 224Ra-labeled CaCO3 microparticles, but no clear dose-response was observed. One explanation could be the aggressiveness of the ES-2 model. The 224Ra treatment could eliminate ascites in 36% of the mice (Figure 6) but was not able to provide curative effects due to tumor ingrowth in vital organs. As tumor cells spread via the circulation and/or grow into deeper layers of the organs, the treatment is not able to reach it as it is strictly local even if the radioactive dose is increased. In the study with the less aggressive SKOV3-luc model, a clear dose-response relationship was also absent and could therefore indicate that the microparticle distribution in the peritoneal cavity was suboptimal. In studies performed with 211At bound to polystyrene microparticles, higher cure rates in mice with IP tumors were obtained than after treatment with β-emitting 32P-colloid [12]. Interestingly, when the antitumor effect of the 211At-microparticles was compared with unspecific 211At-labeled antibody, similar survival but no clear dose-response relationship was observed [10]. This can indicate that the benefit of longer residence time in the peritoneal cavity with microparticles as carriers was not fully exploited with a short-lived nuclide like 211At or that the distribution of the microparticles in the peritoneal cavity was not optimal.
Heterogeneous particle distribution is a potential weakness with the type of IP therapy described in this study. Imaging of patients with gynecological tumors revealed that it was indeed the case with β-emitting 32P-colloid [30]. Persistent aggregates resulted in radiation “hot spots”, and this could be one cause of the frequent incidences of bowel obstructions in patients receiving this treatment. The risk of inducing such local toxicity due to microparticle aggregates is lower when utilizing α-emitters because of their limited range in tissue, but a suboptimal dispersion can reduce therapeutic efficacy as some areas of the peritoneal cavity may not be properly irradiated. The behavior of the noble gas radionuclide 220Rn produced from the decay of 224Ra can potentially counteract some of the disadvantages related to microparticle aggregation. When 224Ra decays, 220Rn may diffuse away from the microparticles. Its short half-life (56 seconds) will prevent any significant redistribution of 220Rn from the peritoneal cavity. The 220Rn emanation might therefore improve therapeutic efficacy by contributing to a more uniform dose deposition and at the same time reducing “point source toxicity” from microparticle aggregates.
Residue from the CaCO3 microparticles was observed at necropsy up to day 7 in the previously reported biodistribution studies [24] but was not seen in the therapy studies performed here when the mice were sacrificed between 26 and 70 days after administration. Even though particle residue was seen at the early time points, they did not cause adhesions, and the lack of a permanent implant or other signs of remaining residue after day 26 suggests that the CaCO3 microparticles are well tolerated and supports the general notion of CaCO3 being a biocompatible and biodegradable material. Biodegradable polymer microparticles, on the other hand, caused adhesions in mice 14 days after IP injection [31]. In 1 out of 12 patients, a laparoscopy 7 months after IP administration of similar polymer microparticles showed extensive adhesions, foreign body giant cell reaction, and residual polymer filaments [32].
The mice in this study did not experience weight loss after administration of the 224Ra-labeled CaCO3 microparticles, and no other biological or dose-limiting toxicities were observed. No indications of either transient or persistent influence on hematological parameters caused by the 224Ra treatment were detected. In previous biodistribution studies, it was found that some 224Ra was released from the microparticles and redistributed to bones [24], but the results from the hematology analyses performed here confirm that this level of release did not cause bone marrow toxicity. The only change in blood parameters detected was not treatment related but rather an increase in white blood cell counts that correlated with disease progression.
Radium-224 progeny can also cause toxicities if they are released from the CaCO3 microparticles in vivo. This can potentially be the case for 212Pb and 212Bi, as these daughters have long enough half-lives to be released into the systemic circulation before decay. After intravenous injection of 224Ra in adult beagles, an excess of 212Pb was found in red blood cells and liver, whereas 212Bi was found to accumulate in the kidneys [33], suggesting that these organs/tissues can be identified as at risk for experiencing toxicities due to relocalization of progeny from the peritoneal cavity. This is also in accordance with studies of IP or intravenous injections of 212Pb in immunocompetent mice, where treatment-related effects were found in the hematopoietic system, kidneys, and liver [34]. The findings were however not interpreted to be severe enough to impact organ function. Significantly higher doses of 212Pb were administered in these studies: 185 to 740 kBq compared to the maximum of approximately 25 kBq of 224Ra given here. Consequently, even in the worst case scenario of all 212Pb being released from the microparticles, it is perceived as unlikely that higher toxicities should be observed than what was reported as acceptable for the high doses of 212Pb. Nevertheless, these organs/tissues should be monitored carefully in future studies.
Exploring treatment options for ovarian cancer patients with α-emitters as the payload is of great interest since recurrent disease is regarded as chemoresistant. On the other hand, as α-particles adsorbed onto microparticles are not assumed effective on larger tumors, an optimal debulking surgery is a prerequisite for its success. Future work is required to determine if the combination of 224Ra-labeled CaCO3 microparticles with chemotherapy can add significantly to the therapeutic benefit, as has been shown previously for 212Pb-trastuzumab and platinum-based chemotherapy [35].
Conclusions
The combination of 224Ra and CaCO3 microparticles yields a construct which has demonstrated high therapeutic activity together with a favorable safety profile at effective doses, altogether making this novel α-particle radiation microdevice promising as a new modality for treating minimal residual cancers in body cavities.
Acknowledgements
The authors thank Stein Waagene (Department of Tumor Biology, The Norwegian Radium Hospital) and Marion Malenge (Oncoinvent AS) for assistance with the in vivo studies.
Footnotes
Funding: This study was supported by the Norwegian Research Council (grant numbers 237661 and 235531) and the private Norwegian company Oncoinvent AS.
Conflict of Interest: Authors S. W., T. B. B., and Ø. S. B. are employed and own stocks in Oncoinvent AS. R. H. L. is chairman of the board of Oncoinvent AS and a shareholder.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2017.12.011.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ushijima K. Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010;2010:497429. doi: 10.1155/2010/497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergote IB, Vergote-De Vos LN, Abeler VM, Aas M, Lindegaard MW, Kjørstad KE, Tropé CG. Randomized Trial Comparing Cisplatin with Radioactive Phosphorus or Whole-Abdomen Irradiation as Adjuvant Treatment of Ovarian Cancer. Cancer. 1992;69:741–749. doi: 10.1002/1097-0142(19920201)69:3<741::aid-cncr2820690322>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Meredith RF, Buchsbaum DJ, Alvarez RD, LoBuglio AF. Brief overview of preclinical and clinical studies in the development of intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2007;13:5643s–5645s. doi: 10.1158/1078-0432.CCR-07-0985. [DOI] [PubMed] [Google Scholar]
- 6.Verheijen RH, Massuger LF, Benigno BB, Epenetos AA, Lopes A, Soper JT, Markowska J, Vyzula R, Jobling T, Stamp G. Phase III Trial of Intraperitoneal Therapy with Yttrium-90-labeled HMFG1 Murine Monoclonal Antibody in Patients with Epithelial Ovarian Cancer After a Surgically Defined Complete Remission. J Clin Oncol. 2006;24:571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 7.Andersson H, Cederkrantz E, Bäck T, Divgi C, Elgqvist J, Himmelman J, Horvath G, Jacobsson L, Jensen H, Lindegren S. Intraperitoneal α-Particle Radioimmunotherapy of Ovarian Cancer Patients: Pharmacokinetics and Dosimetry of 211At-MX35 F(ab’)2–a Phase I Study. J Nucl Med. 2009;50:1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 8.Meredith R, Torgue J, Shen S, Fisher DR, Banaga E, Bunch P. Dose Escalation and Dosimetry of First-in-human α Radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med. 2014;55:1636–1642. doi: 10.2967/jnumed.114.143842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloomer WD, McLaughlin WH, Neirinckx RD, Adelstein SJ, Gordon PR, Ruth TJ, Wolf AP. Astatine-211–tellurium radiocolloid cures experimental malignant ascites. Science. 1981;212:340–341. doi: 10.1126/science.7209534. [DOI] [PubMed] [Google Scholar]
- 10.Larsen RH, Hoff P, Vergote IB, Bruland ØS, Aas M, De Vos L, Nustad K. α-Particle Radiotherapy with 211At-Labeled Monodisperse Polymer Particles, 211At-Labeled IgG Proteins, and Free 211At in a Murine Intraperitoneal Tumor Model. Gynecol Oncol. 1995;57:9–15. doi: 10.1006/gyno.1995.1093. [DOI] [PubMed] [Google Scholar]
- 11.Rotmensch J, Atcher RW, Schlenker R, Hines J, Grdina D, Block BS, Press MF, Herbst AL, Weichselbaum RR. The Effect of the α-Emitting Radionuclide Lead-212 on Human Ovarian Carcinoma: A Potential New Form of Therapy. Gynecol Oncol. 1989;32:236–239. doi: 10.1016/s0090-8258(89)80040-x. [DOI] [PubMed] [Google Scholar]
- 12.Vergote I, Larsen RH, de Vos L, Nesland JM, Bruland Ø, Bjørgum J, Alstad J, Tropé C, Nustad K. Therapeutic Efficacy of the α-Emitter 211At Bound on Microspheres Compared with 90Y and 32P Colloids in a Murine Intraperitoneal Tumor Model. Gynecol Oncol. 1992;47:366–372. doi: 10.1016/0090-8258(92)90141-5. [DOI] [PubMed] [Google Scholar]
- 13.Derrien A, Gouard S, Maurel C, Gaugler MH, Bruchertseifer F, Morgenstern A, Faivre-Chauvet A, Classe JM, Chérel M. Therapeutic Efficacy of Alpha-RIT Using a 213Bi-Anti-hCD138 Antibody in a Mouse Model of Ovarian Peritoneal Carcinomatosis. Front Med (Lausanne) 2015;2 doi: 10.3389/fmed.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milenic D, Garmestani K, Dadachova E, Chappell L, Albert P, Hill D, Schlom J, Brechbiel MW. Radioimmunotherapy of Human Colon Carcinoma Xenografts Using a 213Bi-labeled Domain-Deleted Humanized Monoclonal Antibody. Cancer Biother Radiopharm. 2004;19:135–147. doi: 10.1089/108497804323071904. [DOI] [PubMed] [Google Scholar]
- 15.Elgqvist J, Andersson H, Bäck T, Hultborn R, Jensen H, Karlsson B, Lindegren S, Palm S, Warnhammar E, Jacobsson L. Therapeutic Efficacy and Tumor Dose Estimations in Radioimmunotherapy of Intraperitoneally Growing OVCAR-3 Cells in Nude Mice with 211At-labeled Monoclonal Antibody MX35. J Nucl Med. 2005;46:1907–1915. [PubMed] [Google Scholar]
- 16.Palm S, Bäck T, Claesson I, Danielsson A, Elgqvist J, Frost S, Hultborn R, Jensen H, Lindegren S, Jacobsson L. Therapeutic efficacy of astatine-211-labeled trastuzumab on radioresistant SKOV-3 tumors in nude mice. Int J Radiat Oncol Biol Phys. 2007;69:572–579. doi: 10.1016/j.ijrobp.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Heyerdahl H, Abbas N, Sponheim K, Mollatt C, Bruland Ø, Dahle J. Targeted Alpha Therapy with 227Th-trastuzumab of Intraperitoneal Ovarian Cancer in Nude Mice. Curr Radiopharm. 2013;6:106–116. doi: 10.2174/18744710113069990018. [DOI] [PubMed] [Google Scholar]
- 18.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. α-Particle Radioimmunotherapy of Disseminated Peritoneal Disease Using a 212Pb-labeled Radioimmunoconjugate Targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 19.Boudousq V, Bobyk L, Busson M, Garambois V, Jarlier M, Charalambatou P, Pélegrin A, Paillas S, Chouin N, Quenet F. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using 212Pb. PLoS One. 2013;8:e69613. doi: 10.1371/journal.pone.0069613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasten BB, Arend RC, Katre AA, Kim H, Fan J, Ferrone S, Zinn KR, Buchsbaum DJ. B7-H3-targeted 212Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl Med Biol. 2017;47:23–30. doi: 10.1016/j.nucmedbio.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted Actinium-225 in Vivo Generators for Therapy of Ovarian Cancer. Cancer Res. 2003;63:5084–5090. [PubMed] [Google Scholar]
- 22.Au JLS, Lu Z, Wientjes MG. Versatility of Particulate Carriers: Development of Pharmacodynamically Optimized Drug-Loaded Microparticles for Treatment of Peritoneal Cancer. AAPS J. 2015;17:1065–1079. doi: 10.1208/s12248-015-9785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai M, Lu Z, Wang J, Yeh TK, Wientjes MG, Au JLS. Effects of carrier on disposition and antitumor activity of intraperitoneal Paclitaxel. Pharm Res. 2007;24:1691–1701. doi: 10.1007/s11095-007-9298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westrøm S, Malenge M, Jorstad IS, Napoli E, Bruland ØS, Bønsdorff TB, Larsen RH. Ra-224 Labeling of Calcium Carbonate Microparticles for Internal α-Therapy: Preparation, Stability and Biodistribution in Mice. J Label Compd Radiopharm. 2018 doi: 10.1002/jlcr.3610. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang QT, Zhou L, Zeng WJ, Ma QQ, Wang W, Zhong M, Yu YH. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Ovarian Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cell Physiol Biochem. 2017;41:2411–2418. doi: 10.1159/000475911. [DOI] [PubMed] [Google Scholar]
- 26.Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B. Prognostic Role of Neutrophil-to-lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 28.Milenic DE, Baidoo KE, Kim YS, Barkley R, Brechbiel MW. Comparative studies on the therapeutic benefit of targeted α-particle radiation therapy for the treatment of disseminated intraperitoneal disease. Dalton Trans. 2017;46:14591–14601. doi: 10.1039/c7dt01819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milenic DE, Baidoo KE, Kim YS, Barkley R, Brechbiel MW. Targeted α-Particle Radiation Therapy of HER1-Positive Disseminated Intraperitoneal Disease: An Investigation of the Human Anti-EGFR Monoclonal Antibody, Panitumumab. Trans Oncol. 2017;10:535–545. doi: 10.1016/j.tranon.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan WD, Zimmerman RE, Bloomer WD, Knapp RC, Adelstein SJ. Therapeutic Intraperitoneal 32P: A Clinical Assessment of the Dynamics of Distribution. Radiology. 1981;138:683–688. doi: 10.1148/radiology.138.3.7465847. [DOI] [PubMed] [Google Scholar]
- 31.Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J Biomed Mater Res A. 2006;77:351–361. doi: 10.1002/jbm.a.30654. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong DK, Fleming GF, Markman M, Bailey HH. A phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer) in recurrent ovarian cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;103:391–396. doi: 10.1016/j.ygyno.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd RD, Mays CW, Taylor GN, Atherton DR, Bruenger FW, Jones CW. Radium-224 retention, distribution, and dosimetry in beagles. Radiat Res. 1982;92:280–295. [PubMed] [Google Scholar]
- 34.Milenic DE, Molinolo AA, Solivella MS, Banaga E, Torgue J, Besnainou S, Brechbiel MW, Baidoo KE. Toxicological Studies of 212Pb Intravenously or Intraperitoneally Injected into Mice for a Phase 1 Trial. Pharmaceuticals (Basel) 2015;8:416–434. doi: 10.3390/ph8030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milenic DE, Baidoo KE, Shih JH, Wong KJ, Brechbiel MW. Evaluation of Platinum Chemotherapy in Combination with HER2-targeted α-particle Radiation. Cancer Biother Radiopharm. 2013;28:441–449. doi: 10.1089/cbr.2012.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures