Abstract
In 2003, we proposed the hypersystemizing theory of autism. The theory proposes that the human mind possesses a systemizing mechanism (SM) that helps identify lawful regularities (often causal) that govern the input-operation-output workings of a system. The SM can be tuned to different levels, from low to high, with a normal distribution of individual differences in how strongly people search for such input-operation-out-put regularities in any data that is systemizable. Evidence suggests that people with autism are on average hypersystemizers, scoring higher than average on the systemizing quotient and on performance tests of systemizing. In this article, we consider the neural basis behind the SM, since there has been little consideration of the brain basis of systemizing. Finally, we discuss directions for future work in this field.
Keywords: autism, hypersystemizing, systemizing, systemizing mechanism
Abstract
En el año 2003 propusimos la teoría de la hipersistematización en el autismo. Esta teoría postula que la mente humana posee un mecanismo de sistematización (MS) que ayuda a identificar las reglas (a menudo causales) que gobiernan la entrada-operación-salida del funcionamiento de un sistema. El MS se puede ajustar a diferentes niveles, desde abajo hacia arriba, con una distribución normal de las diferencias individuales en la fuerza con la que las personas buscan tales reglas de entrada-operación-salida para cualquier dato que sea sistematizable. La evidencia sugiere que las personas con autismo son, en general, hipersistematizadoras, y que obtienen puntajes mayores que el promedio tanto para el cuociente de sistematización como para las pruebas de sistematización. En este artículo, se consideran las bases neurales más allá del MS, ya que ha existido poca atención para las bases cerebrales de la sistematización. Finalmente se discuten las directrices del trabajo a futuro en este campo.
Abstract
Nous avons proposé en 2003 la théorie de l'hypersystématisation dans l'autisme, qui propose que la pensée humaine possède un mécanisme systématisant (MS) permettant d'identifier les règles (souvent causales) qui sous-tendent le fonctionnement « donnée - opération - résultat » d'un système. Le MS peut être réglé à différents niveaux, de bas à élevé, avec une distribution normale des différences individuelles relatives à l'intensité de la recherche de telles règles concernant le fonctionnement donnée - opération - résultat pour toute donnée systématisable. Les données suggèrent que les autistes sont en général hypersystématiseurs, leurs scores au quotient de systématisation et aux tests de performance de systématisation étant plus élevés que la moyenne. Nous analysons dans cet article les fondements neuronaux du MS, les bases cérébrales de la systématisation ayant été peu prises en compte. Enfin nous évoquons les directions de travail à venir dans ce domaine.
Introduction
Autism spectrum conditions (henceforth autism) are a set of neurodevelopmental conditions involving difficulties in reciprocal social interaction and communication, alongside the presence of unusually narrow interests and repetitive behaviors.1 People with autism also have difficulties with adjusting to unexpected change and many also show sensory hypersensitivity. Underlying the behavioral phenotype of autism is a complex and heterogeneous array of developmental, genomic, neurobiological, and cognitive differences.2-5 Although by definition individuals with autism suffer from disabilities in social and communication domains, they also possess a different way of processing information and learning that may not lead to disability, but may be an example of “neurodiversity” and result in talent.6,7 A subset of individuals with autism show savantism—abilities restricted to specific domains that are both superior to the individual's other skills and superior to the majority of the population. In this article, we discuss the “hypersystemizing” theory of autism as a potential explanation between the link between autism and talent. First proposed in 2003 ,8 this theory posits that in autism, the systemizing mechanism (SM) is tuned to above-average levels. The SM processes information in a highly specific form: input-operation-output relations. Here, we extend this theory by examining the possible brain basis underlying component processes in the SM and discuss future directions and predictions made by the theory.
What is systemizing?
The systemizing mechanism
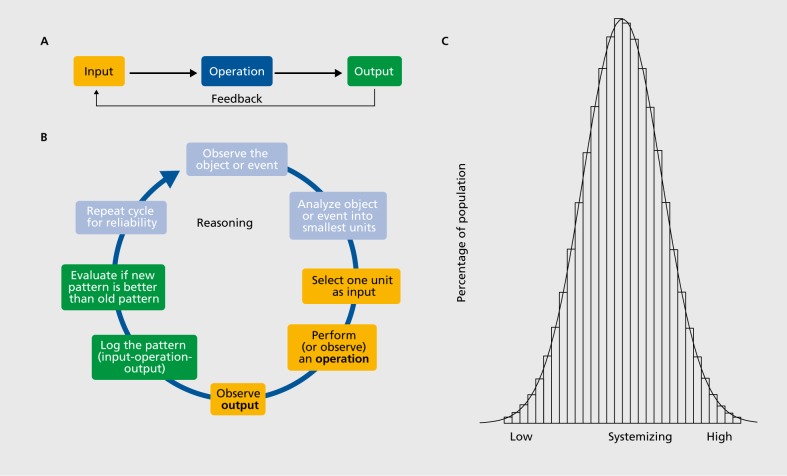
The hypersystemizing theory of autism postulated the existence of the systemizing mechanism (SM) in the human mind,8 representing relationships between three types of data: input-operation-output. This is very different to association learning, seen across multiple species, where the brain is not focused on tracking how an operation changes input-output relationships. The SM focuses on one detail (the input), then observes what happens to the input when it is manipulated by just one factor (the operation), while holding all other factors constant, and then logs the result of the transformation of the input by the operation (the output). When these three elements (input, operation, and output) line up, humans see a lawful, potentially causal pattern. If these same three steps are repeated over and over again and the same causal pattern is observed, the human mind infers a rule or a law that is true. Thus, the function of the SM is to identify laws, rules, and/or regularities that govern a system, so as to understand how that system works and predict what it will do. (Figure 1A) shows the basic cognitive architecture of the SM. Looking more closely at the cognitive processes within the SM, we propose that the three key steps of input-operation out put actually involve at least eight steps, with the critical three highlighted in yellow and the part labeled “feedback” highlighted in green in Figure 1B.
Figure 1. Panel A shows the three basic steps in the systemizing mechanism. Panel B shows the systemizing mechanism refined into eight component steps. Panel C shows a hypothetical example of how systemizing (as typically measured by the systemizing quotient [SQ]) varies within the population as a normal distribution.
Systemizing as a term refers to both the drive to identify such lawful patterns and the cognitive processes involved in identifying such phenomena. A drive is shorthand for a driver of behavior, something that pushes the organism to act in certain ways—otherwise known as motivation. More widely discussed drives in a range of species include hunger, thirst, and sex, pushing us to seek out food, drink, or a mate. In humans, when we observe change—for example, the leaves on a branch, or the waves in the sea moving when the wind blows, or the wheels of a bicycle rotating when the pedal is pushed—we have a drive to search for a law that can explain that change. It is a drive to explain or experiment or understand the mechanism behind changing events.
All humans are postulated to have a SM, so we can all be said to be “systemizers,” such that we all have this drive to identify lawful regularities, some of which may be causal. However, some of us have a stronger drive to systemize than others, and these individual differences fall on a bell curve in the population. This means most of us are systemizing at an average level, some people are below average at it: hardly doing it at all, and finding systemizing challenging. In contrast, some people are above average in this, finding systemizing intuitive and easy and are attracted to look for patterns wherever they exist, as clues to how things work. See (Figure 1C).
The philosopher George Boole, in his book in 1854, entitled An Investigation of the Laws of Thought9 can be said to have described the logic of the SM. He used different terminology for the three key components in systemizing: he called the input IF, the operation AND, and the output THEN. The essence of the SM is these three steps plus the feedback loop as a fourth step. The feedback loop is important because it allows for a comparison of the output (is the output better or worse?) and it allows for repetition (repeating the same three steps to ensure you get the same result each time). Boole was the first to formalize what we do when we think about the world systematically, through the use of “logic gates.” The following is an example of systemizing using IF, AND, THEN (Boolean) logic: “IF a mushroom has characteristic X (the gray one with the little black specks on) AND I eat it, THEN I will get sick.” Let's call this System A. In the three-step notation, we could compare three different systems, A, B, and C:
| System A | System B | System C |
| Input = Mushroom X | Input = Mushroom Y | Input = Mushroom X |
| Operation = Eat | Operation = Eat | Operation = Don't eat |
| Output = Get sick | Output = Stay healthy | Output = Stay healthy |
In System B, we tweaked the input from System A, and in System C, we tweaked the operation from System A. Both of these small variations led to a different outcome (output). When we systemize, we are looking for such invariant patterns in the data, and we do this because it gives us control over one domain (whether a natural or a “man-made” event or object) in the world. In addition, it allows us to predict and understand how that domain works.
There are two ways to systemize anything. The first is when you simply look at something (eg, a wave in the ocean) and observe an operation happening—you literally see something changing or “operating” on the input (eg, the height of a wave increases) to deliver the output (eg, the wave travels further). The observational approach to systemizing is indispensable, especially if the system you're trying to understand is too big (like the changing size of waves in the ocean) or too distant (like the changing shape of the moon) for a person to manipulate. The observational method lies at the heart of classification, since when we classify both natural or “man-made” entities (apples, birds, cars, planes, etc.), we are assembling all the pieces in the system using rules in order to understand, for example, that “IF a bird has a black head AND a red belly, THEN it is a bullfinch,” or “IF it is a Black Renault Laguna AND has the registration plate number AUE7YJ, THEN it is Nicholas' car,” or “IF an apple has an all-green skin AND tastes tart (AND has a hard feel, AND a crisp bite), THEN it's a Granny Smith.” Each type of bird or apple has a set of characteristics that can be arranged logically to enable the systemizer to identify what's special about each apple and to understand how each apple differs from every other one. By systemizing the natural world, systemizers can derive knowledge about WHERE and WHEN to plant flowers in the garden. Here's a WHERE example in two contrasting systems:
| System A | System B |
| Input: Foxgloves | Input: Foxgloves |
| Operation: Plant in damp shady spot | Operation: Plant in dry sunny spot |
| Output: Tall flowers | Output: Short flowers |
To take another example, systemizing another aspect of the natural world—the sea: a strong systemizer might want to predict high and low tide according to the time of the year. The tide tables one can buy are usually in the form of a spreadsheet, and this is exactly how the information is represented in the mind of a strong systemizer, arranging the tidal information as a system (eg, IF on Saturday the tide height is low AND the day changes to Sunday, THEN the tide height will rise).
The second way to systemize is when you can perform an operation (ie, you can manipulate something to change the input, so you are controlling the “operating” on the input), to deliver an output. For example, noticing that “IF my iPhone is off AND I hold down the top button,” THEN “the phone will turn on.” This little sequence delivers a 100% reliable and consistent rule. In this case, the rule is a causal one (holding down the top button will cause the iPhone to switch on).
How does systemizing relate to the phenotype of autism?
In autism, we see two key features that are probably driven by hypersystemizing, where the SM is tuned to high levels. The first is extremely repetitive behavior, which in the early clinical literature was viewed as pathological. Under the hypersystemizing theory, this same behavior can be seen as the person with autism repeating their observations to confirm a rule. An example might be a child with autism who repetitively flicks light switches on and off in the house to confirm which lights go on and off, or who turns on the bathroom faucet and runs outside to observe the water flow coming out of the drain pipe, repetitively changing the position of the faucet and observing the results. A lower-level “stereotypy” might be a child flicking a piece of string repetitively using his fingers, close to his eyes, to observe the effects, or a child spinning the wheels of a car close to his peripheral vision, observing the patterns of the rotating object. Other children with autism become “obsessed” with watching washing machines rotating through their cycle or spinning their whole body very fast or watching electric fans rotating. These are all repeating, highly systemizable domains. Lining objects up in rigid patterns, such as lining up colored bricks, and becoming upset if anyone disrupts the pattern, may be another “symptom” of autistic behavior that is in fact a marker of the SM being tuned to very high levels.
A second characteristic of autism that can be viewed as the result of the SM being tuned to very high levels are “obsessional interests.” For example, many young children become fascinated with a class of objects, such as dinosaurs, needing to classify every dinosaur that ever lived according to its characteristics and to do this exhaustively. Once the category has been totally systemized (ie, there is no new information to be added), then the child might move on to a new “obsessional” interest. Or the child might obsessively collect pellet guns, taking them apart to see the length of the spring and reassembling them to fire pellets in order to see the lawful relationship between the size of the spring and the distance the pellet will travel when fired. Or the child might collect other kinds of facts, such as baseball statistics on teams in the league or players in the team, because these facts are lawful and unchanging and so constitute a system and allow predictions to be made. In autism, we see these strong interests followed to an extreme extent.
A final example of hypersystemizing in autism is savant skills. Some children with autism are calendrical calculators, but effectively they have latched onto a single system (the calendar), analyzed it (consciously or otherwise) to identify the lawful regularities that govern calendars, such that they can then make predictions (which day of the week a date in the past or the future will fall) with 100% accuracy. Or the child may become gifted in mathematics or music, because they have obsessively systemized that domain, even if their other areas of skill or knowledge are way behind this particular one.
What is the neural basis of systemizing?
The literature on sensory hypersensitivity in individuals with autism may be relevant to understanding the neural basis of the SM, as it may be that part of what makes individuals with autism on average stronger systemizers could be heightened initial low-level processing of sensory/perceptual input.10 Heightened sensory processing may mean more input/data or more detail or superior attention to detail, these differences require further research in order to be understood. At a slightly later point in perceptual processing, Mottron's concept of “veridical mapping”11,12 may be relevant as a mechanism by which sensory input is compared in order to identify isomorphic relationships. His related notion of enhanced perceptual functioning in autism13 may be a useful starting point regarding how low-level sensory and perceptual systems in the brain are important for the SM. Mottron suggests that cortical areas subserving sensory and perceptual functions are enhanced in autism and give rise to superior perceptual abilities. Several pieces of evidence suggest that individuals with autism have enhanced sensory and perceptual responses in low-level sensory cortices across a range of tasks and modalities.10,11 This may result in an amplification of the sensory and perceptual input to enable better systemizing, although this alone is not sufficient for systemizing.
A second useful view is in terms of attention and how this can modify and enhance function of primary sensory cortices.14,15 Attention to detail and a cognitive style associated with a local processing bias is enhanced in autism.16-19 Therefore, in addition to enhanced responsivity in the neural circuitry handling basic sensory and perceptual processing, it is likely that heavily biased attentional processes that promote a local detail-oriented focus could further amplify processing in such sensory and perceptual circuitry. In autism, attention and orienting to the nonsocial world is biased at very earlyages,20 and this could have marked effects on how neural circuits are sculpted. It will be important to examine how neural circuits involved in attention and low-level sensory and perceptual processes interact over the life span. In particular, we need to investigate how early biases in the way someone samples information from the environment may shape how such circuits are organized and lead to enhanced abilities relevant to systemizing.
A third aspect of the neural basis behind systemizing involves higher-level reasoning and rule learning. Many of the eight steps outlined in Figure 1B are part of reasoning (eg, identifying causal relations, applying IF-AND-THEN logic, identifying rules). No discrete neural “module” for reasoning or rule learning has yet been identified. Rather, such complex cognitive processes are thought to be implemented via large-scale neural circuits in lateral frontal and parietal areas.21-23 Such circuits are manifest in intrinsic patterns of functional organization of the brain.24 Crucially, the function of such lateral frontoparietal circuits cannot be ascribed to such specific functions but rather function within the general domain of cognitive control. However, these circuits have been studied through use of paradigms relevant to other types of reasoning (such as deductive, inductive, and analogical reasoning), and it remains to be seen if the very specific type of Boolean reasoning involved in systemizing (IF, AND, THEN) utilizes similar neural circuitry.
Fourth, because systemizing involves a drive to understand systems, necessarily there will be a motivational component, involving the brain's reward system. This largely constitutes striatal regions (nucleus accumbens, caudate, and putamen) and its connections with a variety of areas in cortex,25 via dopaminergic neurons. It will be important for future research to understand the neural circuitry behind reward and enhanced drive to systemize. We would predict this circuitry would involve striatal reward systems and their connections to cortical areas to be involved in the SM.
Finally, mathematical reasoning and numerical representations have also been studied in cognitive neuroscience and are relevant here since people with autism are disproportionately represented among talented mathematicians.26 The intraparietal sulcus (IPS) in posterior parietal cortex is a critical node in numerical representations and processing.27 Several studies have also implicated the region beneath the IPS in temporoparietal cortex (ie, angular gyrus, supramarginal gyrus) in numerical calculations (mental arithmetic).27,28 Overdevelopment of this region may also reflect a frontoparietal shift in developmental specialization underlying arithmetic skills.28,29
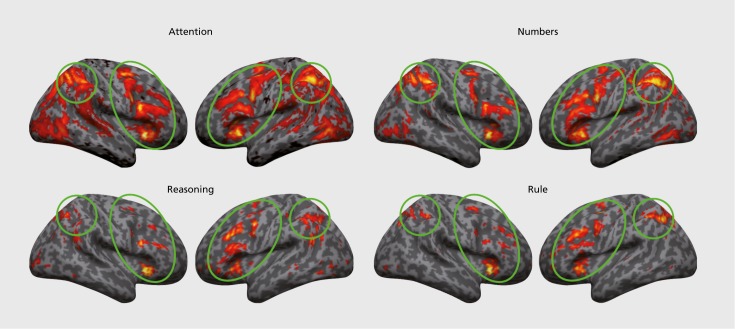
To provide a more informed layout of the potential neural circuits involved in cognitive processes related to systemizing, such as reasoning, rule learning, attention, and numerical representations, we used Neurosynth30 to conduct large-scale meta-analyses of studies, using these key words. In Figure 2, we show forward inference maps—ie, probability of activation given the term; P(ActivationlTerm)—from Neurosynth that may help guide future work on the topic. As expected, although we can link many cognitive terms such as “reasoning,” “rule,” “numbers,” and “attention” to the SM, the probability of recruiting common, large-scale, lateral frontoparietal circuitry given each of these terms is high. As a start for hypothesizing cortical circuitry relevant to the SM, these data indicate that lateral frontoparietal circuits are highly important. We should be mindful that by searching with such broad cognitive terms (reasoning, attention, etc.) we have probably cast too broad a net, and the neural circuitry of the SM is probably far more specific and possibly far more localized.
Having laid out some initial ideas regarding the neural circuitry potentially underlying systemizing, it may be helpful to discuss existing work in autism that may be relevant. Here, we discuss studies of the Embedded Figures Task (EFT) in autism, since this task essentially involves taking a system apart to identify the component parts of the system. The figure is said to be “embedded,” and the analytic process to solve the task could be said to be “disembedding.” Early studies demonstrated that individuals with autism display strengths in performance on this task.18,31 This prompted imaging investigations in order to uncover the potential neural bases for such enhanced performance on this task. In an early study, we discovered that when individuals with autism perform the EFT, many areas within the lateral frontoparietal circuit shown in (Figure 2) were activated similarly in autism and controls. However, despite the presence of intact activation throughout much of this lateral frontoparietal circuit, there were small specific areas within this circuit that showed a decrease in activation in autism. Furthermore, other posterior areas overlapping with low-level visual perceptual areas were enhanced in activity in autism,32 which is congruent with Mottron's proposal of enhanced functioning of low-level circuitry in autism.11 Although this study did not look at connectivity between such low-level systems and higher-level lateral frontoparietal circuits, the interactions between these circuits could be important for understanding some of the strengths individuals with autism can exhibit in nonsocial domains of functioning.
Figure 2. Lateral frontoparietal control circuits involved in common processes related to systemizing. This fi gure shows forward inference (P(Activation|Term)) meta-analytic maps from Neurosynth (http://neurosynth.org) for terms “attention,” “reasoning,” “number,” and “rule.” Areas circled in green form lateral frontoparietal control circuits that are common across all terms.
Later studies on the EFT in autism largely corroborated and extended this work. For example, Manjaly and colleagues also identified enhanced occipital cortex activity in autism, alongside reduced activity in lateral frontoparietal areas.33 Damarla and colleagues also similarly showed that areas of parietal and occipital cortex were increased in activity in autism relative to controls, whereas other frontal and parietal areas were decreased in activity in autism.34 These studies are therefore finding a replicable pattern in autism, relative to controls, during performance of the EFT. Each of these studies was carried out in older adults and adolescents with autism. In contrast, a study by Lee and colleagues used a much younger sample population, individuals aged 7 to 12 years. Whereas they also uncovered areas of decreased activity across the lateral frontoparietal circuit, they did not highlight any evidence for enhanced activity in occipital areas linked with low-level visual perception. The authors noted that children with autism performed the task equivalently to matched controls but exhibited a generally reduced recruitment of cortical areas, which again may be congruent with the idea that at the neural level, the handling of such a task was conducted in a more efficient fashion.35
Finally, in a study by Spencer and colleagues, we were able to identify only one area in temporal cortex that differentiated autism from controls, with the autism group showing enhanced activation.36 This study did not find evidence of any difference in lateral frontoparietal circuitry reported in other work, nor did it identify any enhancement of occipital cortex activation in autism. The findings of this study are incompatible with most of the other work on the topic36 and may reflect the heterogeneity within the autism population, particularly when generalizing across relatively small-scale studies. Overall, the example of EFT studies in the literature points toward the idea that both low-level sensory/perceptual systems and higher-level lateral frontoparietal circuits are involved in systemizing in autism. More work is needed to expand on this literature and identify what may be distinct neural correlates behind enhanced systemizing in autism.
Future work, predictions, and conclusions
Clearly, more research is needed in many areas. First, it will be important to enhance our understanding of how systemizing at a cognitive level may be implemented in the brain. Although we have laid out some ideas, these ideas should be taken as an initial roadmap, which must be further tested to better understand how individuals with autism and enhanced systemizing abilities develop such high levels of skill in specific topics. For instance, how do lateral frontoparietal, reward, and low-level sensory and perceptual systems interact during systemizing? Can the components of the SM be broken down further into discrete elements with specific neural bases? How does systemizing and its underlying neural basis develop in autism?
Second, we need studies examining potential dissociations between the motivational components of systemizing (ie, the drive to systemize) versus ability levels, which may better indicate skill at implementing the cognitive components involved in systemizing. Although we highlight these as two components of systemizing, they are probably tightly connected. That is, individuals with enhanced drive to systemize will probably develop enhanced skill due to practice and increased levels of experience. However, it remains to be seen if there are situations where these two components do not align. It is possible that some individuals with autism have an intact or even enhanced drive to systemize, yet may not show enhanced performance in systemizing. Conversely, there may be other individuals with very little drive to systemize, yet when given the chance to implement such processes, can do so at high levels.
Third, we need to understand the brain basis of “autistic obsessions.” When a person with autism “latches” on to a particular system (eg, types of cars, mathematical rules, names of dinosaurs, putting electrical light switches into fixed positions, watching the wheel of a toy car going round and round), and/or becomes “obsessed” with the systematic, repeatable information, what is happening in the brain? As that person is mapping the rules or laws in the system, do neural circuits become highly sculpted for this specific domain of obsession/expertise? More relevant to the SM, is it the same process involved each time a person with autism becomes “obsessed” with a new topic?
Fourth, autism is a very heterogeneous set of conditions and it may be that no overarching explanation can fully account for all individuals on the spectrum.3 Keeping in mind that heterogeneity is the rule rather than the exception, future work will be needed to understand how systemizing is differentially present in different types of individuals. Furthermore, it could also be that there are different neural bases in different types of autistic individuals that are considered high systemizers. Ultimately, what is needed is a more nuanced understanding of how to apply these theoretical ideas at the level of the individual, rather than making blanket statements about all individuals with an autism diagnosis.
Finally, it will be important to design functional magnetic resonance imaging studies contrasting typically developing individuals and autistic individuals perfomiing multiple types of tasks relevant to systemizing. We would predict that the same neural circuitry is activated during performance of even very different types of tasks, revealing circuitry for the SM, and that this circuitry may differ in individuals with autism. In particular, it will be important to conduct careful imaging studies of individuals with islets of ability or savantism. It may be that studying extreme cases, such as those with remarkable precocity in maths, music or art, will shed light on the neural basis of the SM, even in autistic individuals who are not savants.
Acknowledgments
SBC was supported by the Autism Research Trust and the Medical Research Council during the period of this work. The research was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East of England at Cambridgeshire and Peterborough National Health Service NHS) Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. None of the authors has any financial or other conflicts of interest to declare.
Contributor Information
Simon Baron-Cohen, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom.
Michael V. Lombardo, Department of Psychology, Center for Applied Neuroscience, University of Cyprus, Nicosia, Cyprus.
REFERENCES
- 1.Lai MC., Lombardo MV., Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 2.Geschwind DH., Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Happe F., Ronald A., Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 4.Lai MC., Lombardo MV., Chakrabarti B., Baron-Cohen S. Subgroupingthe autism “spectrum”: reflections on DSM-5. PLoS Biol. 2013;11(4):e1001544. doi: 10.1371/journal.pbio.1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombardo MV., Pierce K., Eyler LT., et al. Different functional neural substrates for good and poor language outcome in autism. Neuron. 2015;86(2):567–577. doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron-Cohen S., Ashwin E., Ashwin C., Tavassoli T., Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron-Cohen S. Editorial perspective: Neurodiversity - a revolutionary concept for autism and psychiatry. J Child Psychol Psychiatry. 2017;58(6):744–747. doi: 10.1111/jcpp.12703. [DOI] [PubMed] [Google Scholar]
- 8.Baron-Cohen S., Richler J., Bisarya D., Gurunathan N., Wheelwright S. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):361–374. doi: 10.1098/rstb.2002.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boole G. An investigation of the Laws of Thought. London and Cambridge, UK: Walton & Maberly; 1854 [Google Scholar]
- 10.Robertson CE., Baron-Cohen S. Sensory perception in autism. Nat Rev Neurosci. 2017;18(11):671–684. doi: 10.1038/nrn.2017.112. [DOI] [PubMed] [Google Scholar]
- 11.Mottron L., Bouvet L., Bonnel A., et al. Veridical mapping in the development of exceptional autistic abilities. Neurosci Biobehav Rev. 2013;37(2):209–228. doi: 10.1016/j.neubiorev.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Mottron L., Dawson M., Soulieres I. Enhanced perception in savant syndrome: patterns, structure and creativity. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1385–1391. doi: 10.1098/rstb.2008.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mottron L., Dawson M., Soulieres I., Hubert B., Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 14.Somers DC., Dale AM., Seiffert AE., Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA. 1999;96(4):1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopfinger JB., Buonocore MH., Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 16.Plaisted K., O'Riordan M., Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. J Child Psychol Psychiatry. 1998;39(5):765–775. [PubMed] [Google Scholar]
- 17.O'Riordan MA., Plaisted KC., Driver J., Baron-Cohen S. Superior visual search in autism. J. Exp Psychol Hum Percept Perform. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- 18.Jolliffe T., Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J Child Psychol Psychiatry. 1997;38(5):527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 19.Robertson CE., Kravitz DJ., Freyberg J., Baron-Cohen S., Baker CI. Tunnel vision: sharper gradient of spatial attention in autism. J Neurosci. 2013;33(16):6776–6781. doi: 10.1523/JNEUROSCI.5120-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones W., Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel V. Anatomy of deductive reasoning. Trends Cogn Sci. 2007;11(10):435–441. doi: 10.1016/j.tics.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Prado J., Chadha A., Booth JR. The brain network for deductive reasoning: a quantitative meta-analysis of 28 neuroimaging studies. J Cogn Neurosci. 2011;23(11):3483–3497. doi: 10.1162/jocn_a_00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badre D., Kayser AS., D'Esposito M. Frontal cortex and the discovery of abstract action rules. Neuron. 2010;66(2):315–326. doi: 10.1016/j.neuron.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent JL., Kahn I., Snyder AZ., Raichle ME., Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haber SN., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron-Cohen S., Wheelwright S., Burtenshaw A., Hobson E. Mathematical talent is linked to autism. Hum Nat. 2007;18(2):125–131. doi: 10.1007/s12110-007-9014-0. [DOI] [PubMed] [Google Scholar]
- 27.Ansari D. Effects of development and enculturation on number representation in the brain. Nat Rev Neurosci. 2008;9(4):278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- 28.Rivera SM., Reiss AL., Eckert MA., Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex. 2005;15(11):1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- 29.Houde O., Rossi S., Lubin A., Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci. 2010;13(6):876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 30.Yarkoni T., Poldrack RA., Nichols TE., Van Essen DC., Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah A., Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 32.Ring HA., Baron-Cohen S., Wheelwright S., et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122(pt 7):1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- 33.Manjaly ZM., Bruning N., Neufang S., et al. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35(1):283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damarla SR., Keller TA., Kana RK., et al. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3(5):273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee PS., Foss-Feig J., Henderson JG., et al. Atypical neural substrates of Embedded Figures Task performance in children with autism spectrum disorder. Neuroimage. 2007;38(1):184–193. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer MD., Holt RJ., Chura LR., et al. Atypical activation during the Embedded Figures Task as a functional magnetic resonance imaging endophenotype of autism. Brain. 2012;135(pt 11):3469–3480. doi: 10.1093/brain/aws229. [DOI] [PMC free article] [PubMed] [Google Scholar]