Abstract
Pancreatic cancer is typically characterized by its aggressive tumor growth and dismal prognosis. Approximately 30% of patients with pancreatic cancer present with locally advanced disease, broadly defined as having a tumor-to-artery interface >180°, having an unreconstructable portal vein or superior mesenteric vein and no signs of metastatic disease. These patients are currently designated to palliative systemic chemotherapy, though median overall survival remains poor (approximately 11 months). Therefore, several innovative local therapies have been investigated as new treatment options for locally advanced pancreatic cancer (LAPC). This article provides an overview of available data with regard to morbidity and oncological outcome of novel local therapies for LAPC.
Keywords: locally advanced pancreatic cancer (LAPC), pancreatic cancer, local ablative therapies
1. Introduction
Pancreatic adenocarcinoma is one of the most aggressive forms of cancer and is projected to arise as the second leading cause of cancer-related deaths in Europe and the United States by 2030 [1]. The prognosis has hardly improved over the past two decades and remains dismal, with an overall 5-year survival rate of approximately 8% [2,3]. Surgical resection is the only treatment option with the potential for long-term survival and cure. Even after potential curative resection, most patients will eventually have recurrent disease, resulting in a 5-year survival of only 20% [4]. Because early symptoms are often vague and mild, roughly 30% of patients with pancreatic cancer present with locally advanced pancreatic carcinoma (LAPC) and approximately 50% with metastatic disease (mPC) [5].
Systemic chemotherapy is considered the standard of care for patients with LAPC (AJCC stage III) and mPC (AJCC stage IV) [6]. The FOLFIRINOX regimen (combination chemotherapy using fluorouracil, leucovorin (folinic acid), irinotecan, and oxaliplatin) has emerged over the last years as a therapy that improves survival (median overall survival (OS) 11.1 months for FOLFIRINOX vs. 6.8 months for gemcitabine for mPC), at the cost of a greater concomitant toxicity [7]. International medical oncology guidelines extrapolate these results and hence recommend the use of FOLFIRINOX as the standard of care for mPC and for LAPC patients with a good performance status and no major comorbidities [4,8,9].
Considering the poor survival of LAPC patients, a lot of research from the last decade focused on combining systemic chemotherapy with local ablative therapies, such as radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, irreversible electroporation (IRE), stereotactic body radiation therapy (SBRT), iodine-125 seed implantation, high-intensity focused ultrasound (HIFU), and photodynamic therapy (PDT). The ablative techniques all share the mutual goal to achieve local tumor control, as this likely impacts quality-of-life and survival.
Thermal ablation techniques, such as RFA, MWA and cryoablation, attempt to destroy tumor tissue by increasing or decreasing temperatures sufficiently to induce cellular injury [10]. Complete and adequate destruction requires that the entire tumor plus the ablative margin to be subjected to cytotoxic temperatures. In RFA, a high-frequency alternating current runs through one or more electrodes, leading to tumor destruction by coagulation and protein denaturation [10]. In MWA, the oscillation of polar molecules produces frictional heating, ultimately generating tissue necrosis [11]. Cryoablation utilizes argon or helium gases to induce the rapid freezing and thawing of target tissue [12]. Cellular destruction by cryoablation is caused by direct physical damage during freezing, and by vascular-mediated cytotoxicity occurring as a result of progressive vasoconstriction, occlusion and endothelial damage, resulting in tissue ischemia [12]. Another thermal, but non-needle guided, ablative technique is HIFU. HIFU destroys tumor cells by raising local tissue temperatures as high as 65˚C using focused ultrasound energy from an extracorporeal source [13].
In contrast to conventional radiotherapy, which has shown conflicting results for LAPC [14,15,16], SBRT permits the precise application of high-dose radiation to a limited target volume, reducing the radiation dose adjacent to healthy tissue and subsequently minimizing toxicity. Another form of irradiation studied for pancreatic cancer is iodine-125 seed implantation (brachytherapy). Iodine-125 seed is an isotope which provides gamma radiation for a short distance, resulting in the death of targeted cells [17]. It has been suggested as a distinct treatment option or for use in combination with other ablative therapies.
In PDT, localized tissue necrosis is caused by the activation of a photosensitizer using light of a specific wavelength [18]. The photosensitizer causes a cytotoxic effect by the generation of reactive oxygen species (ROS), such as singlet oxygen and free radicals, that mediate cellular toxicity [18].
In the field of LAPC, IRE is the ‘new kid on the block’. In IRE, multiple needle electrodes are placed in and around the tumor, either percutaneously or during laparotomy. High-voltage electrical pulses are delivered between each needle electrode pair, creating nanopores that irreversibly damage the cell membrane, leading to apoptosis. Since the working mechanism is primarily non-thermal, there is little concurrent damage to connective tissues such as collagen and elastin, so the mechanical integrity of tissue or important structures, like blood vessels and bile ducts, is preserved [19].
In reversible electroporation, in contrast to IRE, a transient state of cellular permeabilization is created using electric pulses. This allows for extracellular agents to access the cells. Reversible electroporation combined with chemotherapeutic agents (ECT) may improve their uptake, in particular for drugs that are poorly, or not, permeant [20].
This article aims to give an extensive overview of the available literature regarding the safety and oncological outcome of these local innovative ablative therapies in the treatment of patients with LAPC.
2. Search
A search was performed in October 2017 in PubMed for studies published in the English language. Studies were eligible if they reported safety and/or oncological outcomes of at least ten patients treated for LAPC. Case reports, (systematic) reviews, animal or experimental/preclinical studies, and studies reporting multiple tumor histologies or stages were excluded. Abstracts were merely included when their methodology and all relevant data could be adequately extracted.
3. Radiofrequency Ablation (RFA)
Six studies included at least ten patients; four prospective [21,22,23,24] and two retrospective [25,26]. The RFA procedures were performed during open laparotomy in five studies and using a percutaneous approach in one. All six studies were performed at the University of Verona Hospital Trust. Given the intersecting date ranges, the results likely stem from a (partially) overlapping patient cohort [21,22,23,25,26]. Patients in the study from D’Onofrio et al. were treated with percutaneous RFA [24]. Four studies reported on OS, ranging between 19.0 and 25.6 months [21,22,25,26]. The study from Cantore et al. compared upfront RFA plus palliative bypass surgery to RFA as a second-line treatment option after chemo(radio)therapy. The median OS from date of diagnosis was significantly higher for patients receiving RFA as a second-line treatment compared to upfront RFA (25.6 months vs. 14.7 months; p = 0.004). However, as the number of patients that did not qualify for RFA after chemo(radio)therapy is unclear, an important selection bias remains. Morbidity varied between 0 and 28% and 30-day mortality between 0 and 3%, which was caused by hepatic failure [21,22,23], sepsis following a duodenal perforation [21], severe acute pancreatitis [22], and duodenal hemorrhage [22]. The most common (serious) adverse events included pancreatic fistula [21,22,23,25], acute pancreatitis [21,22,23], portal vein thrombosis [21,22,23], duodenal injury [21,22,23,25], biliary injury [21,22,23], gastric ulcer or fistula [22,23,25], haemoperitoneum [21,22,23], and liver failure [21,22,23]. An overview of the literature on RFA is given in Table 1.
Table 1.
Safety and efficacy of radiofrequency ablation (RFA) for locally advanced pancreatic cancer (LAPC).
| Reference | Design | # pts | Age, yrs | Size, mm | Morbidity | 30-day Mortality | Median FU | Median OS |
|---|---|---|---|---|---|---|---|---|
| Cantore [21] ** | Prospective | 107 | N.S. | N.S. | 28.0% (n = 30) |
1.9% (n = 2) | N.R. | 25.6 months |
| D’Onofrio [24] ** | Prospective | 18 | mean 62.4 | mean 48.1 (25–86) | 0% | 0% | N.R. | N.R. |
| Frigerio [25] ** | Retrospective | 57 | med 63 | N.R. | 14% (n = 8) |
0% | N.R. | 19 months |
| Girelli [23] ** | Prospective | 50 | med 64.5 | med 40 (IQR 30–50) |
24% (n = 12) |
2% (n = 1) | 8 months | N.R. |
| Girelli [22] ** | Prospective | 100 | mean 64 | med 36 (IQR 30–45) |
26% (n = 26) |
3% (n = 3) | 12 months | 20 months |
| Paiella [26] ** | Retrospective | 30 | N.R. | N.R. | N.R. | 0% | 15 months | 19 months * |
pts = patients; FU = follow-up; OS = overall survival; med = median; IQR = interquartile range; N.S. = not specified (per group); N.R. = not reported; * median disease specific survival. ** All procedures in all studies were performed at one single center: the University of Verona Hospital Trust; given the overlapping date ranges patient data are likely (partially) re-reported.
4. Microwave Ablation (MWA)
Currently available data on microwave ablation for LAPC is limited. Lygidakis et al. studied the feasibility, safety, and efficacy of MWA in 15 patients with histologically proven LAPC [27]. In all patients, partial necrosis was achieved whilst no major procedure-related morbidity or mortality occurred. Carrafiello and colleagues retrospectively reviewed ten patients treated with percutaneous (n = 5) or laparotomic (n = 5) MWA [11]. The 9-month and 1-year local tumor progression rates per new response evaluation criteria in solid tumours (RECIST 1.1) were 37.5% (3/8) and 62.5% (5/8), respectively [28]. In 20% of the patients, minor complications were registered. Two grade 3 or more complications were registered: pancreatitis (grade 3; n = 1) and pseudoaneurysm of the gastroduodenal artery (grade 4; n = 1).
5. Cryoablation
Two studies compared cryoablation plus palliative bypass surgery (PBC group) to bypass surgery alone (PB group) [29,30]. Although both studies found tumor mass shrinkage in the PBC group, OS was not significantly different from the patients in the PB group (350 days versus 257 days, p = 0.124; 5 months versus 4 months, p > 0.05). The postoperative complication rate in the study from Li et al. was not significantly different between the two groups, except for delayed gastric emptying, which was higher in the PBC group (35.7% in PBC group vs. 5.3% in PB group) [29]. Main postoperative complications included pancreatic or biliary leakage, GI bleeding or obstruction, delayed gastric emptying, infection or intra-abdominal bleeding [29,30].
6. High-Intensity Focused Ultrasound (HIFU)
Ten studies were identified that reported on pain relief, morbidity or oncologic outcome after HIFU. Six studies were prospective [31,32,33,34,35,36] and four were retrospective [37,38,39,40]. Six studies reported on OS, which ranged between 6.0 and 14.0 months [31,32,35,37,39,40]. In the retrospective analysis from Ning et al., no significant survival benefit was found for patients treated with HIFU (median OS 8.3 months) compared to patients who did not receive HIFU treatment (median OS 7.3 months; p = 0.783) [37]. Gao et al. compared patients treated with HIFU alone to patients treated with HIFU and concurrent chemotherapy (gemcitabine 1000 mg/m2 over 30 min, weekly for 3 weeks every 28 days) [31]. The median OS was significantly longer for patients treated with both HIFU and chemotherapy (median OS 12.0 months versus 8.0 months; p < 0.05) [31]. Morbidity was relatively low, varying between 0–23.2% [31,32,33,35,37,39,41]. Increase of serum amylase was commonly seen after HIFU [31,34,36,37,40,41]. Other regularly encountered complications included skin burns or subcutaneous fat sclerosis [34,39,40,42], GI dysfunction (e.g., nausea, vomiting, loss of appetite) [31,34,36,37,41], GI ulcer or bleeding [32,34,37,41,42], abdominal pain [37,38,40,41], (mild) fever [34,36,37,40], pancreatic fistula or pseudocyst [33,34,39,40] and pancreatitis [33,34,36,42]. Eight studies reported on pain relief after HIFU, which is a major focus of HIFU treatment [31,32,33,34,35,36,38,39]. Pain relief was achieved in 78.6% to 87.5% of patients, either complete or partial (i.e., numerical (pain) rating scale (NRS) decrease by 2 or more) [31,35,36,39]. A summary of literature on HIFU is given in Table 2.
Table 2.
Safety and efficacy of high-intensity focused ultrasound (HIFU) for locally advanced pancreatic cancer (LAPC).
| Reference | # pts | Age, yrs | RECIST | Median OS | Morbidity | Pain Relief |
|---|---|---|---|---|---|---|
| Gao [31] | 39 | med 58 (42–79) | CR 0 PR 5 (12.8%) SD 25 (64.1%) PD 9 (23.1%) |
11.0 months | 12.8% | Total 31 (79.5%) Complete 9 (23.1%) Partial 22 (56.4%) † |
| Li [32] | 16 | mean 62 (49–72) | CR 0% * PR 43.7% SD 25% PD 31.3% |
14.0 months (from treatment) | 12.5% | Mean pre-VAS 5.1 Mean post-VAS 3.3 Median PRT 5.6 months |
| Ning [37] | 100 | N.S. | N.R. | 8.3 months | 23.2% | N.R. |
| Shi [38] | 71 | N.R. | N.R. | N.R. | N.R. | Pre-HIFU 70.42% painless Post-HIFU 92.96% painless |
| Sofuni [33] | 30 16 III |
N.S. | N.S. | N.R. | 10% | 66.7% (N.S.) ‡ |
| Sung [34] | 46 18 III |
N.S. | N.R. | N.S. | N.R. | Pre-VAS 4.9 Post-VAS 2.1 p < 0.001 (N.S.) |
| Wang [35] | 40 13 III |
N.S. | N.S. | 10 months | 0% | Total 35 (87.5%) (N.S.) Complete 9 (22.5%) Partial 26 (65%) Median PRT 10 weeks |
| Xiong [39] | 89 39 III |
N.S. | N.S. | 11.2 months | 11.2% | Total 54 (80.6%) (N.S.) Complete 21 (31.3%) Partial 33 (49.3%) † |
| Zhao H. [36] | 39 31 III |
N.S. | N.S. | N.S. | N.R. | Total 22 (78.6%) (N.S.) Complete 9 (32.1%) Partial 13 (46.4%) † |
| Zhao J. [40] | 38 | med 75 (62–80) | N.R. | 6.0 months vs. 10.3 months ~ | N.R. | N.R. |
pts = patients; N.S. = not specified (per stage); N.R. = not reported; III = stage 3 pancreatic cancer; VAS = visual analog scale; median PRT = median duration of pain relief time; RECIST 1.1 = new response evaluation criteria in solid tumours [28] * at 6 months after treatment; † NRS decrease by 2 or more; ‡ more than 30% improvement; ~ traditional HIFU 6 months vs. low power HIFU 10.3 months; p = 0.018.
7. Stereotactic Body Radiotherapy (SBRT)
Nineteen single arm studies have been published regarding SBRT for LAPC; 9 prospective [43,44,45,46,47,48,49,50,51] and 10 retrospective [52,53,54,55,56,57,58,59,60,61]. Some studies have overlapping study populations, as the retrospective analysis of Alagappan et al. [52] included 72 patients that were already included in other reports [46,47,49,50]. Furthermore, the study from Mellon et al. [57] updated the outcomes and toxicity using induction chemotherapy and SBRT from an earlier published report by Chuong et al. [54]. The treatment modalities included SBRT with a linear accelerator [43,46,50,51,52,54,57,59] or CyberKnife [45,47,48,49,52,53,55,56,59,60,61]. In two studies, the treatment modality was not specified [44,58]. Median radiation doses varied between 20 Gy, delivered in one fraction, and 45 Gy, in six fractions.
All studies provided data on OS (range: 10–20 months from diagnosis [43,44,46,47,48,49,50,52,53,54,55,56,57,58,61] and 6.2–12.5 months from SBRT [51,53,59,60]) after a median follow-up varying between 5 and 24 months. Local control varied between 40 and 89% [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,59]. Ten studies reported the number of patients who were eligible for resection after SBRT, which ranged from 0 to 20.3% [43,45,46,48,49,50,53,54,57,58]. An overview of the efficacy of SBRT for LAPC is given in Table 3. Complications from SBRT were divided into acute (within 3 months after SBRT) and late complications. Acute grade 3 or higher toxicity ranged between 0 and 28.4%, whilst late grade 3 or higher toxicity varied between 0 and 13% [43,45,46,47,48,49,50,51,53,54,55,56,57,58,59,60,61]. Frequently encountered mild to moderate complications include fatigue, abdominal pain and nausea. Other common complications were mostly GI-related, such as ulceration [46,49,50,53,57,58], gastritis or enteritis [43,46,49,58], duodenal stricture [49,53,60,61], bleeding from the gastro-intestinal tract [46,54,55,56,57,58], or anorexia [46,54,57,61].
Table 3.
Efficacy of stereotactic body radiotherapy (SBRT) for locally advanced pancreatic cancer (LAPC).
| Reference | Design | # pts | Age, yrs | Median Dose | Fractions | Median FU | Local Control | Median OS | Downstage |
|---|---|---|---|---|---|---|---|---|---|
| Alagappan [52] | Retrospective | 208 * | med 75.2 (IQR 65.9–86.1) |
25 Gy (103 pts) 33 Gy (105 pts) |
1 5 |
7.5 months | 87% | 14.0 months (OSd) | N.R. |
| Chang [53] | Retrospective | 77 56 III |
N.S. | 25 Gy (61 pts) 25 Gy + EBRT |
1 1 + 25 |
6 months | 87% (N.S.) | 6.7 months (OSt) ‡ 11.5 months (OSd) ‡ |
1 pt (N.S.) |
| Chuong [54] | Retrospective | 73 16 III |
N.S. | 30 Gy | 5 | 7.8 months | 1-year LC = 81% (N.S.) | 15 months (OSd) ‡ | 0 |
| Comito [43] | Prospective | 45 | mean 68 (40–87) | 45 Gy | 6 | 13.5 months | 89% | 15 months (OSd) | 3 pts |
| Dholakia [44] | Prospective | 32 | N.R. | 33 Gy | 5 | 13.4 months | 72% | 18.8 months (OSd) | N.R. |
| Gurka [45] | Prospective | 10 | mean 62.5 (50–79) | 25 Gy | 5 | N.R. | 40% | 12.2 months (N.S.) | 0 |
| Herman [46] | Prospective | 49 | med 67 (35–87) | 33 Gy | 5 | 13.9 months | max. 78% | 13.9 months (OSd) | 5 pts |
| Koong [47] | Prospective | 15 | med 62 (43–82) | 20 Gy | 1 | 5 months | max. 80% | 11 months (OSd) | N.R. |
| Mahadevan 2011 [56] | Retrospective | 39 | med 67 (44–88) | 24.92 Gy | 3 | 21 months | 85% | 20 months (OSd) | N.R. |
| Mahadevan 2010 [55] | Retrospective | 36 | med 65 (43–88) | 29.33 Gy | 3 | 24 months | 78% | 14.3 months (OSd) | N.R. |
| Mellon [57] | Retrospective | 159 49 III |
med 67.2 (47–85) | 40 Gy | 5 | 14.0 months | 1-year LC = 78% (N.S.) | 15.0 months (OSd) ‡ | 5 pts |
| Moningi [58] | Retrospective | 88 74 III |
N.S. | 33 Gy | 5 | 14.6 months | N.R. | 18.4 months (OSd) ‡ | 15 pts |
| Polistina [48] | Prospective | 23 | med 68 (44–75) | 30 Gy | 3 | 9 months | 82% | 10.6 months (OSd) | 2 pts |
| Rwigema [59] | Retrospective | 71 40 III |
N.S. | 24 Gy | 1–3 | 6.0 months | 53% | 6.2 months (OSt) ‡ | N.R. |
| Schellenberg 2008 [49] | Prospective | 16 | med 69 (39–87) | 25 Gy | 1 | 9.1 months | 81% | 11.4 months (OSd) | 0 |
| Schellenberg 2011 [50] | Prospective | 20 | med 63 (45–85) | 25 Gy | 1 | N.R. | 75% | 11.8 months (OSd) | 0 |
| Song [60] | Retrospective | 59 | med 62 (28–86) | 45 Gy | 5 (3–8) | 10.9 months | N.R. | 12.5 months (OSt) | N.R. |
| Tozzi [51] | Prospective | 30 | mean 67 (43–87) | 45 Gy | 6 | 11 months | 86% | 11 months (OSt) | N.R. |
| Zhu [61] | Retrospective | 417 218 III |
N.S. | 30–46.8 Gy | 5–8 | 11 months | N.R. | 10.0 months (OSd) ‡ | N.R. |
pts = patients; OSd = median overall survival from diagnosis; OSt = median overall survival from treatment (SBRT); III = number of patients with stage 3 pancreatic cancer (LAPC); N.R. = not reported; IQR = interquartile range; N.S. = not specified per stage; LC = local control; EBRT = external-beam radiotherapy; * 12 patients had metastatic disease; ‡ overall survival of patients with LAPC only.
8. Iodine-125 Seed Implantation
Two studies from Wang et al. treated a total of 28 patients (who were considered unresectable) during laparotomy with 125I seed implantation [62,63]. Median irradiation dose was 120 Gy (range 60–163 Gy). Seven patients received an additional 35–50 Gy external beam radiotherapy (EBRT) and ten patients received 2–10 cycles of adjuvant chemotherapy. The overall local control rate was 87.5% (n = 24), median OS 10.1 months. In the majority of patient pain relief was classified as good or medium (94.1%). Adverse events included a chylous fistula (n = 1), gastric ulcer (n = 1), radiation enteritis (n = 2), and transient fever (n = 10). In two patients, seeds migrated to the liver, however without any side effects.
Xu et al. investigated the combination of 125I seed implantation with cryosurgery in 49 patients with LAPC, of which 12 patients had hepatic metastases [64,65]. Seeds were implanted during cryosurgery in 35 patients, or 3–9 days after cryosurgery in 14 patients. Twenty patients received additional (1–4 cycles) chemotherapy. After a median follow-up (FU) of 18 months, the median survival was 16.2 months. The majority of patients experienced abdominal pain (n = 34) and/or fever (n = 26). Other adverse events included acute pancreatitis (n = 6), increased amylase levels (n = 25), abdominal bleeding (n = 3), pulmonary infection (n = 3), myocardial infarction (n = 1) and cerebral infarction (n = 1). Iodine-125 seed implantation has also been investigated in combination with RFA by Zou et al. [66]. Twenty-four patients with stage III pancreatic cancer, identified during laparotomy, were treated with intraoperative RFA plus seed implantation. Pain scores decreased significantly after the operation (p < 0.05). The median OS was 19 months. One patient experienced acute pancreatitis, which was related to the RFA procedure.
9. Irreversible Electroporation (IRE)
Fourteen single arm studies were published regarding IRE for LAPC, either as margin accentuation [67,68,69,70] or as primary tumor treatment [71,72,73,74,75,76,77,78,79,80]. Patients were treated percutaneously [69,71,72,73,74,75,77,80], laparoscopically [70], or during laparotomy [68,69,70,72,73,76,78,79]. After a median follow-up of 1–29 months, the median OS from diagnosis ranged between 15.3 and 27.0 months [68,70,74,75,76,77,78], whilst the median OS from IRE varied between 7.0 months and 14.2 months [67,73,74,75,77,81]. In the study from Martin et al., the difference in OS between patients treated with IRE for primary tumor control (median OS: 23.2 months) was compared to IRE as margin accentuation after resection (median OS: 28.3 months). However, this was not found to be statistically significant (p > 0.05) [68]. Three studies reported on the ability of resection after IRE, ranging between 6.0 and 10.3% [74,75,81]. Local progression was specified in 9 studies, varying between 0 and 27.8% [67,68,69,70,72,75,80,81]. Mansson et al. found a local failure rate of 58.3%, however, this number may have been overestimated since the authors rated every ablation zone growth as local progression [74]. Table 4 gives an overview of the efficacy of IRE for LAPC.
Table 4.
Efficacy of irreversible electroporation (IRE) for locally advanced pancreatic cancer (LAPC).
| Reference | # pts | Age, yrs | Size, mm | Approach | Treatment | Median FU | Median OS | Local Failure | Down-Stage | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Belfiore [71] | 29 | med 68.5 (55–81) |
N.R. | Perc | Local | 29 months | 14 months (OSt) | 3% | N = 3 | N.R. |
| Dunki–Jacobs [72] | 65 | N.R. | med 35 | Perc 12 Open 53 |
Local | 23 months | N.R. | 26% | N.R. | N.R. |
| Kluger [67] | 50 | med 66.5 (IQR 60.2–72.0) |
med 30 (IQR 17–50) |
N.R. | Margin 24 Local 29 |
8.69 months | 12.03 months (OSt) | 11% | N.R. | 6% (n = 3) * |
| Lambert [73] | 21 | 68.2 | 39 (21–65) | Perc 2 Open 19 |
Local | N.R. | 10.2 months (OSt) | N.R. | N.R. | 0 |
| Mansson [74] | 24 | med 65 (42–77) |
med 35 (15–45) |
Perc | Local | N.R. | 17.9 months (OSd) 7.0 months (OSt) |
58.3% | N = 2 | 4% (n = 1) |
| Martin 2012 [69] | 27 | med 61 (45–82) |
med 30 | Perc 1 Open 26 |
Margin 8 Local 19 |
90 days | N.R. | 0% | N.R. | 4% (n = 1) |
| Martin 2013 [70] | 54 | med 61 (45–80) |
N.R. | Open 52 Lap 2 |
Margin 19 Local 35 |
15 months | 20.2 months (OSd) | 27.8% | N.R. | 2% (n = 1) |
| Martin 2015 [68] | 200 | med 62 (27–88) |
med 28 | Open | Margin 50 Local 150 |
29 months | 24.9 months (OSd) | 6% | N.R. | 2% (n = 3) |
| Narayanan [75] | 50 | med 62.5 (46–91) |
mean 32 (15–80) |
Perc | Local | N.R. | 27.0 months (OSd) 14.2 months (OSt) |
18% | N = 3 | 6% (n = 3) |
| Paiella [76] | 10 | med 66 | med 30 (25–39) |
Open | Local | 7.6 months | 15.3 months (OSd) | N.R. | N.R. | 0 |
| Scheffer [77] | 25 | med 61 (41–78) |
med 40 (33–50) |
Perc | Local | 12 months | 17 months (OSd) 11 months (OSt) |
N.R. | N.R. | 0 |
| Vogel [78] | 15 | N.R. | N.R. | Open | Local | 24 months | 16 months (OSd) | N.R. | N.R. | 13% (n = 2) |
| Yan [79] | 25 | med 58 (49–80) |
med 42 (28–49) |
Open | Local | N.R. | N.R. | N.R. | N.R. | 4% (n = 1) |
| Zhang [80] | 21 | N.R. | med 35 (20–67) |
Perc | Local | 1 month | N.R. | 0 | N.R. | 0 |
pts = patients; perc = percutaneous; lap = laparoscopic; OSd = overall survival from diagnosis; OSt = overall survival from treatment; N.R. = not reported; med = median; IQR = interquartile range; *3 deaths were deemed IRE related.
Major complications (grade 3 or higher) varied between none to 30% [67,71,74,75,80], whilst overall morbidity ranged between 10 and 57% [68,69,72,73,74,76,77,78,79]. Most frequently encountered (major) complications were abdominal pain [75,77]; GI-related adverse events such as nausea and anorexia [67,68,70,72,77], delayed gastric emptying or ileus [67,69,70,72,74,77,78,79], and (perforated) ulcers with or without GI bleeding [67,74,77,78,79]; portal vein thrombosis [67,68,69,70,72,74,75,78,79]; biliary stricture; leakage or cholangitis [67,68,69,70,72,73,77,78]; pancreatic leakage; fistula or pancreatitis [70,73,74,75,76,77,78,79]; abscesses [67,73,76,77]; and ascites [67,68,69,70,72].
Two studies did not report mortality rates [71,72], whilst four studies did not have any 90-day mortality [73,76,77,80]. In the other studies, the mortality varied between 1 to 3 patients [67,68,69,70,73,74,75,78,79]. Mortality was caused by tumor progression [75,79], liver failure [68,78], hemorrhage from a GI ulcer [68,78], portal vein thrombosis [67,69,70], duodenal and bile duct necrosis [67], multisystem organ failure [67], cardiopulmonary arrest [67], pneumonia [74], and pulmonary embolism [68].
10. Photodynamic Therapy (PDT)
One study, performed by Huggett et al., was published investigating PDT for patients with LAPC [82]. An earlier study from the same group studied the photosensitizer meso-tetrahydroxyphenyl chlorin (mTHPC), however, this study also included patients with stage 1 and 2 pancreatic cancer, whilst data for stage III pancreatic cancer could not be extracted separately [83]. The study from Huggett et al. used the photosensitizer verteporfin in 15 patients treated for LAPC, with a median tumor size of 4.0 cm [82]. One and three months after IRE, 11 and 6, respectively, out of 13 patients had stable disease. The median OS after PDT was 8.8 months and from diagnosis 15.5 months. Adverse events included mild to moderate pain (n = 3), transient increase in amylase levels (n = 1), mild diarrhea (n = 1), persistent steatorrhea (n = 1), and subclinical inflammatory changes on computed tomography (CT) (n = 2).
11. Electrochemotherapy (ECT)
Experience with ECT for LAPC is limited. Granata et al. investigated the safety and feasibility of ECT in 13 patients with LAPC [84]. No electrochemotherapy-related serious adverse events occurred. A transient, self-limiting supraventricular arrhythmia was detected in one patient. In four patients, delayed gastric emptying occurred, however without clinical significant symptoms. Other complications included: pleural effusion, ascites, and splenic infarction without thrombosis of the splenic vessels. The same research group also published an article regarding early radiological assessment of LAPC treated with ECT, elaborating the cohort to 19 patients [84,85]. One patient died within 48 h after treatment with ECT because of a complication, which has not been discussed in detail. No significant reduction of largest diameter by CT scan and magnetic resonance imaging (MRI) was observed. According to RECIST criteria, all patients showed stable disease using MRI, while on CT imaging one patient showed progressive disease. According to Choi criteria, all patients were considered in partial response. Using functional MR derived parameters, a significant reduction of viable tumor tissue was observed.
12. Discussion
This study presents an overview of the available literature on local ablative therapies for LAPC. The most data was available for RFA, SBRT, IRE and HIFU. The main shortcoming affecting all techniques is the lack of randomized controlled trials determining the (additional) value of local ablative technique above systemic palliative chemotherapy alone. The comparative analysis of treatment options is also hampered by the fact that until recently there was no globally accepted standard of care for LAPC [86] Potential selection biases, such as the lack of consensus regarding resectability criteria and the heterogeneous use of (neo-)adjuvant chemo(radio)therapy plus consequential timing of the ablative procedure, further impede a reliable assessment [21,86,87,88]. Since protracted courses of neo-adjuvant therapy will exclude patients with early disease progression from receiving ablative therapy, the OS for the ablated group will likely increase given the biologically favorable nature of the tumor. Nonetheless the reported OS, especially after SBRT and IRE, remains promising. Besides the goal to achieve local tumor control, downstaging to resectable disease has also been reported after local ablative therapies as SBRT and IRE (see Table 3 and Table 4). Although the number of patients that were able to undergo resection is low, local ablative therapies can create curative potential for patients who were actually designated to palliative therapy only.
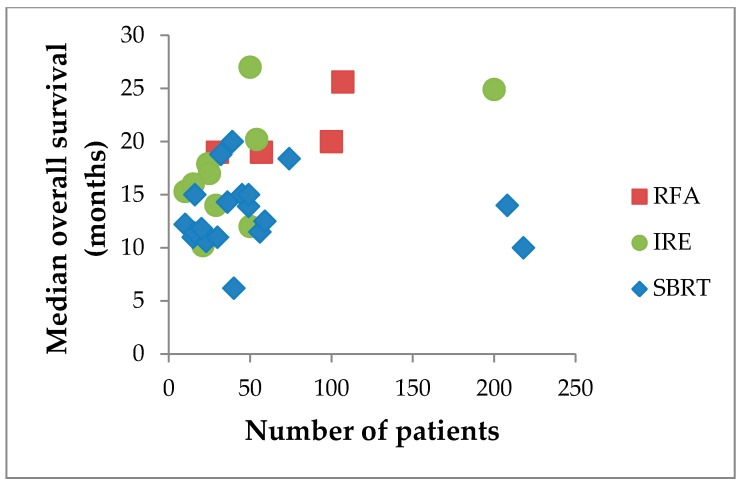
All local therapies described in this article have their own distinct advantages and disadvantages (Table 5). Although the survival benefit of adding traditional fractionated external-beam radiotherapy to chemotherapy regimens remains controversial [14,15,16], SBRT allows for limited fractions, decreased toxicity, and more promising survival outcomes (median OS reaching 20 months; Figure 1) [56,89,90]. Furthermore, patients can be treated in the outpatient setting because the technique is minimally invasive, with the exception of the need to implant fiducials [89]. The latter may be overcome by MRI-guided radiation therapy [91]. This technique continuously images soft-tissue during radiation treatment, allowing for accurate alignment of the tumor to the treatment beams, and does not require fiducials [91]. Physicians should be aware of the risk of late complications (i.e., >3 months after SBRT) and the inability of retreatment in the case of local failure. The literature on SBRT is heterogeneous with regards to delivered radiation doses and fractions, which confounds the comparison of SBRT to other local ablative therapies [90].
Table 5.
Overview of specific advantages and disadvantages for various techniques.
| Technique | Advantage | Disadvantage |
|---|---|---|
| RFA | Easily applicable; superior availability; low costs. Open approach allows for exploration of peritoneal cavity; percutaneous approach seems less invasive, however limited data (one study) for LAPC. Indication of RFA-based immunomodulation: general activation of adaptive immune response along with a decrease of immunosuppression [96]. |
Tumor debulking, since a safety margin is required to prevent thermal damage to critical structures such as large blood vessels and bile ducts. All available literature from one single center. Heat-sink effect, decreasing treatment efficacy of tumors surrounding large vessels. 30-day mortality (0–3%); relatively high complication rate: 0–28%. |
| MWA | Limited data for pancreatic cancer | |
| Cryoablation | Presumed abscopal effect, especially when combined with immunotherapy [97]. | Cryoshock syndrome. Hemorrhage induced by ice-ball cracking. Probe-size demands open approach. No survival benefit for cryoablation with palliative bypass surgery versus bypass surgery alone. |
| HIFU | No needles required. Effective technique for pain relief. |
Limited survival data. Complication rate: 0–23.2%. Risk of second and third degree skin burns and subcutaneous fat sclerosis. |
| SBRT | Noninvasive, except for the implantation of the fiducials (though, very low complication rate). Treatment in the outpatient setting. |
No uniform data with regard to radiation doses used, making comparisons difficult. Retreatment often impossible. Lower dose at the border of the tumor due to organs at risk (OARs). Risk of late complications (i.e., >3 months after SBRT): 0–13% (≥grade 3); acute complication rate: 0–28.4% (≥grade 3). |
| Iodine-125 seeds | Limited data for pancreatic cancer. Implantation demands open approach. |
|
| IRE | Deployable as primary tumor control or margin accentuation after resection. Treatment is repeatable. Preservation of critical structures, such as biliary ducts and large blood vessels. Not susceptible to heat-sink effect. Open approach allows for exploration of peritoneal cavity; percutaneous approach is less invasive. |
No uniform protocol. High learning curve. 90-day mortality (0–13%); relatively high complication rate: 0–30% (≥grade 3). |
| PDT | Preservation of connective tissues, maintaining the mechanical integrity of critical structures, such as intestines and blood vessels. | Limited data for pancreatic cancer. |
Figure 1.
Median overall survival in months after RFA*, SBRT and IRE per number of patients per study. * All procedures in all studies performed at one single center: the University of Verona Hospital Trust; given the overlapping date ranges patient data are likely (partially) re-reported.
Similar to SBRT, HIFU does not require needle placement. Although the number of articles on HIFU for LAPC is relatively high, the number of patients per series was limited and the majority focused on pain relief without reporting oncological outcomes [92]. Pain can effectively be relieved by HIFU in 78.6–87.5% of patients, albeit at the cost of major skin burns (grade 2 or 3) and/or subcutaneous fat sclerosis, [31,35,36,39].
Due to the anatomical location of pancreatic cancer, RFA in the pancreas is associated with a high morbidity and mortality, since heating can seriously damage critical blood vessels, bile ducts and gastro-intestinal structures [93]. For this reason RFA has been largely abandoned, with the exception of one group who advocates the use of a large safety margin to these critical structures. Hence the primary aim of the technique is cytoreduction [22,87]. The continuous cooling caused by nearby blood vessels may further negatively impact the effect of RFA (heat-sink effect) [93]. On the other hand, RFA is relatively easily applicable, has a superior availability, and low costs. Pancreatic RFA is mostly performed during open laparotomy, which has the advantage of the exploration of the peritoneal cavity to identify unsuspected disease and therefore withhold patients from unnecessary treatment with RFA. Although the percutaneous approach may be more suitable to palliate patients, only one study investigated this approach [24].
Similar to thermal ablation, IRE can also be performed during open laparotomy or percutaneously. IRE has the theoretical advantage that the goal is to radically ablate the entire macroscopically visible tumor and hence achieve local tumor control. Since the working mechanism of IRE is based on the destruction of cellular membranes, whilst preserving the extracellular matrix, blood vessels and bile ducts remain intact [93]. Moreover, since the working mechanism is not based on thermal energy, IRE is not affected by the heat-sink effect [93]. These two characteristics make the use of pulsed electrical fields a promising technique for LAPC. These statements seem to be supported by the OS found in literature (range 15.3–27.0 months; Figure 1) [94]. However, IRE is known to have a high learning curve and literature is heterogeneous with regards to the electrical settings used for the procedure [95]. This latter problem is currently being addressed by an international Delphi consensus study, aiming for a uniform applied protocol. The morbidity and mortality rates reported in the more recent prospective series seem to be higher than those from earlier retrospective reports, especially for the open approach [77].
The other techniques discussed in this review, i.e., PDT, MWA, cryoablation, ECT, and iodine-125 seed implantation, all proved feasible in the treatment of LAPC patients. However, the data is too limited to draw any hard conclusions with regards to safety and efficacy.
13. Future Perspectives
As stated before, the main shortcoming of all local therapies is the lack of randomized controlled trials, comparing the additional value of local therapy over systemic chemotherapy. The currently ongoing CROSSFIRE-trial (ClinicalTrials.gov number NCT02791503), is a randomized controlled phase III trial comparing the outcome of FOLFIRINOX plus IRE with FOLFIRINOX plus MR-guided SBRT on OS for patients with LAPC. The PELICAN-trial (Dutch Trial Register number NTR5517), is another ongoing randomized controlled trial that compares the outcome on OS for patients with LAPC treated with FOLFIRINOX or gemcitabine plus RFA to treatment with chemotherapy only. The randomized phase III trial, organized by the Stanford University, aims to determine the additional value of SBRT over FOLFIRINOX alone (ClinicalTrials.gov number NCT01926197). These trials will hopefully further define the exact role of local therapies for patients with LAPC.
The current focus of several studies is the immunogenic potential of local ablative therapies. After the destruction of the tumor with a local ablative technique, antigen presenting cells (APCs) infiltrate the ablation zone [98]. These APCs then activate the immune system, specifically helper and cytotoxic T cells [98]. This may induce a so-called ‘abscopal effect’, referring to the phenomenon where localized treatment of the primary tumor induces a forceful immune response, targeting occult distant micrometastases, potentially prolonging tumor control and survival [99]. Besides the activation of the immune response, small studies also have shown a decrease of immunosuppressive cells after treatment with local ablative techniques [96]. Combining local ablative techniques with immunotherapy may potentially boost the immune system to suppress pancreatic cancer [97]. Another direction for future studies on LAPC is the correlation of treatment effectiveness or survival with genetic alterations. There are four commonly known mutated genes in pancreatic cancer: KRAS, CDKN2A, TP53, and SMAD4 [3]. Studies of precursor lesions found KRAS mutations to be one of the earliest alterations in pancreatic tumorigenesis, along with CDKN2A [3,100,101,102]. In contrast, the inactivation of SMAD4 and TP53 were found in advanced pancreatic intraepithelial neoplasias grade 3 and invasive carcinomas [102]. A recent study from Paiella et al. showed patients with pancreatic cancer with a SMAD4 loss had a poorer prognosis after RFA [26]. In the future, it may be possible to identify subgroups of patients who will and will not benefit from local therapies.
Also currently investigated, though not addressed in this paper, are oncolytic viruses [103,104]. Viruses are designed with biological specificity to infect cancerous cells preferentially [104]. The direct working mechanism is overwhelming viral infection and lysis, which releases additional viral particles to infect neighboring cells and distant metastases [104]. Viral infections can also activate the immune system and aid the immune system to recognize and attack malignancies [104]. For instance, in the randomized phase II trial performed by Noonan et al., pelareorep (reolysin) was added to treatment with chemotherapy (carboplatin and paclitaxel) [103]. Pelareorep has cytotoxic effects on malignant cells with an activated RAS signaling pathway, due to mutations in the RAS proto-oncogene [103]. No survival benefit was found for patients treated with pelareorep, which could be due to a neutralized effect in the context of other (unknown) mutations. Although patients receiving pelareorep did not experience a survival benefit, a number of immune biomarkers were found that were associated with an improved disease control rate or progression free survival [103].
14. Conclusions
In conclusion, this review gives an overview of the currently available ablative techniques for the treatment of patients with LAPC, with their distinct advantages and disadvantages. Although the safety profile is generally defined as good, major adverse events can occur and even mortality has been reported for all techniques. In the absence of randomized controlled trials, high-quality evidence for any survival benefit over chemotherapy alone is lacking. Only one series, comparing IRE plus chemotherapy to chemotherapy alone, has used case matching and multivariate analysis to compare two historical cohorts [70]. Although OS was superior in the IRE group, several confounders remain. Nonetheless, the promising survival, especially for studies employing SBRT and IRE, warrant the setup of randomized controlled phase II and III trials to compare the available local ablative therapies and to assess the additive value of local therapy over chemotherapy alone.
Acknowledgments
No funding to disclose.
Author Contributions
A.R. performed the literature search, analyzed the data, and wrote the paper. L.V., R.P., H.S., and M.M. contributed to manuscript editing and final approval.
Conflicts of Interest
M.M. is a paid consultant for AngioDynamics. The other authors declare no conflict of interest.
References
- 1.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T., Wood L.D., Itoi T., Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 4.Ducreux M., Cuhna A.S., Caramella C., Hollebecque A., Burtin P., Goere D., Seufferlein T., Haustermans K., Van Laethem J.L., Conroy T., et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26(Suppl. S5):56–68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 5.Gurusamy K.S., Kumar S., Davidson B.R., Fusai G. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD010244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilimoria K.Y., Bentrem D.J., Ko C.Y., Ritchey J., Stewart A.K., Winchester D.P., Talamonti M.S. Validation of the 6th edition ajcc pancreatic cancer staging system: Report from the national cancer database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardiere C., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Sohal D.P., Mangu P.B., Khorana A.A., Shah M.A., Philip P.A., O’Reilly E.M., Uronis H.E., Ramanathan R.K., Crane C.H., Engebretson A., et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016;34:2784–2796. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khorana A.A., Mangu P.B., Berlin J., Engebretson A., Hong T.S., Maitra A., Mohile S.G., Mumber M., Schulick R., Shapiro M., et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017;35:2324–2328. doi: 10.1200/JCO.2017.72.4948. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed M., Brace C.L., Lee F.T., Jr., Goldberg S.N. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrafiello G., Ierardi A.M., Fontana F., Petrillo M., Floridi C., Lucchina N., Cuffari S., Dionigi G., Rotondo A., Fugazzola C. Microwave ablation of pancreatic head cancer: Safety and efficacy. J. Vasc. Interv. Radiol. 2013;24:1513–1520. doi: 10.1016/j.jvir.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Cazzato R.L., Garnon J., Ramamurthy N., Koch G., Tsoumakidou G., Caudrelier J., Arrigoni F., Zugaro L., Barile A., Masciocchi C., et al. Percutaneous image-guided cryoablation: Current applications and results in the oncologic field. Med. Oncol. 2016;33:140. doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 13.Dubinsky T.J., Cuevas C., Dighe M.K., Kolokythas O., Hwang J.H. High-intensity focused ultrasound: Current potential and oncologic applications. AJR Am. J. Roentgenol. 2008;190:191–199. doi: 10.2214/AJR.07.2671. [DOI] [PubMed] [Google Scholar]
- 14.Chauffert B., Mornex F., Bonnetain F., Rougier P., Mariette C., Bouche O., Bosset J.F., Aparicio T., Mineur L., Azzedine A., et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 15.Hammel P., Huguet F., van Laethem J.L., Goldstein D., Glimelius B., Artru P., Borbath I., Bouche O., Shannon J., Andre T., et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 16.Loehrer P.J., Sr., Feng Y., Cardenes H., Wagner L., Brell J.M., Cella D., Flynn P., Ramanathan R.K., Crane C.H., Alberts S.R., et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K., Niu L., Mu F., Hu Y. Cryosurgery in combination with brachytherapy of iodine-125 seeds for pancreatic cancer. Gland Surg. 2013;2:91–99. doi: 10.3978/j.issn.2227-684X.2013.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 19.Scheffer H.J., Nielsen K., van Tilborg A.A., Vieveen J.M., Bouwman R.A., Kazemier G., Niessen H.W., Meijer S., van Kuijk C., van den Tol M.P., et al. Ablation of colorectal liver metastases by irreversible electroporation: Results of the COLDFIRE-I ablate-and-resect study. Eur. Radiol. 2014;24:2467–2475. doi: 10.1007/s00330-014-3259-x. [DOI] [PubMed] [Google Scholar]
- 20.Bimonte S., Leongito M., Granata V., Barbieri A., Del Vecchio V., Falco M., Nasto A., Albino V., Piccirillo M., Palaia R., et al. Electrochemotherapy in pancreatic adenocarcinoma treatment: Pre-clinical and clinical studies. Radiol. Oncol. 2016;50:14–20. doi: 10.1515/raon-2016-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantore M., Girelli R., Mambrini A., Frigerio I., Boz G., Salvia R., Giardino A., Orlandi M., Auriemma A., Bassi C. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br. J. Surg. 2012;99:1083–1088. doi: 10.1002/bjs.8789. [DOI] [PubMed] [Google Scholar]
- 22.Girelli R., Frigerio I., Giardino A., Regi P., Gobbo S., Malleo G., Salvia R., Bassi C. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch. Surg. 2013;398:63–69. doi: 10.1007/s00423-012-1011-z. [DOI] [PubMed] [Google Scholar]
- 23.Girelli R., Frigerio I., Salvia R., Barbi E., Tinazzi Martini P., Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br. J. Surg. 2010;97:220–225. doi: 10.1002/bjs.6800. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio M., Crosara S., De Robertis R., Butturini G., Salvia R., Paiella S., Bassi C., Mucelli R.P. Percutaneous Radiofrequency Ablation of Unresectable Locally Advanced Pancreatic Cancer: Preliminary Results. Technol. Cancer Res. Treat. 2017;16:285–294. doi: 10.1177/1533034616649292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frigerio I., Girelli R., Giardino A., Regi P., Salvia R., Bassi C. Short term chemotherapy followed by radiofrequency ablation in stage III pancreatic cancer: Results from a single center. J. Hepatobiliary Pancreat. Sci. 2013;20:574–577. doi: 10.1007/s00534-013-0613-3. [DOI] [PubMed] [Google Scholar]
- 26.Paiella S., Malleo G., Cataldo I., Gasparini C., De Pastena M., De Marchi G., Marchegiani G., Rusev B., Scarpa A., Girelli R., et al. Radiofrequency ablation for locally advanced pancreatic cancer: SMAD4 analysis segregates a responsive subgroup of patients. Langenbecks Arch. Surg. 2017 doi: 10.1007/s00423-017-1627-0. [DOI] [PubMed] [Google Scholar]
- 27.Lygidakis N.J., Sharma S.K., Papastratis P., Zivanovic V., Kefalourous H., Koshariya M., Lintzeris I., Porfiris T., Koutsiouroumba D. Microwave ablation in locally advanced pancreatic carcinoma—A new look. Hepatogastroenterology. 2007;54:1305–1310. [PubMed] [Google Scholar]
- 28.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1) Eur J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Chen X., Yang H., Wang X., Yuan D., Zeng Y., Wen T., Yan L., Li B. Tumour cryoablation combined with palliative bypass surgery in the treatment of unresectable pancreatic cancer: A retrospective study of 142 patients. Postgrad. Med. J. 2011;87:89–95. doi: 10.1136/pgmj.2010.098350. [DOI] [PubMed] [Google Scholar]
- 30.Song Z.G., Hao J.H., Gao S., Gao C.T., Tang Y., Liu J.C. The outcome of cryoablation in treating advanced pancreatic cancer: A comparison with palliative bypass surgery alone. J. Dig. Dis. 2014;15:561–569. doi: 10.1111/1751-2980.12170. [DOI] [PubMed] [Google Scholar]
- 31.Gao H.F., Wang K., Meng Z.Q., Chen Z., Lin J.H., Zhou Z.H., Wang P., Shi W.D., Sheng Y.H. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60:1906–1910. doi: 10.5754/hge13498. [DOI] [PubMed] [Google Scholar]
- 32.Li Y.J., Huang G.L., Sun X.L., Zhao X.C., Li Z.G. The combination therapy of high-intensity focused ultrasound with radiotherapy in locally advanced pancreatic carcinoma. World J. Surg. Oncol. 2016;14:60. doi: 10.1186/s12957-016-0809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sofuni A., Moriyasu F., Sano T., Itokawa F., Tsuchiya T., Kurihara T., Ishii K., Tsuji S., Ikeuchi N., Tanaka R., et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J. Gastroenterol. 2014;20:9570–9577. doi: 10.3748/wjg.v20.i28.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung H.Y., Jung S.E., Cho S.H., Zhou K., Han J.Y., Han S.T., Kim J.I., Kim J.K., Choi J.Y., Yoon S.K., et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40:1080–1086. doi: 10.1097/MPA.0b013e31821fde24. [DOI] [PubMed] [Google Scholar]
- 35.Wang K., Chen Z., Meng Z., Lin J., Zhou Z., Wang P., Chen L., Liu L. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int. J. Hyperther. 2011;27:101–107. doi: 10.3109/02656736.2010.525588. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H., Yang G., Wang D., Yu X., Zhang Y., Zhu J., Ji Y., Zhong B., Zhao W., Yang Z., et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21:447–452. doi: 10.1097/CAD.0b013e32833641a7. [DOI] [PubMed] [Google Scholar]
- 37.Ning Z.Y., Cheng C.S., Xie J., Chen Q.W., Xu L.T., Zhuang L.P., Zhang C.Y., Song L.B., Shi W.D., Zhu X.Y., et al. A retrospective analysis of survival factors of high intensity focused ultrasound (HIFU) treatment for unresectable pancreatic cancer. Discov. Med. 2016;21:435–445. [PubMed] [Google Scholar]
- 38.Shi Y., Ying X., Hu X., Shen H. Pain management of pancreatic cancer patients with high-intensity focused ultrasound therapy. Pak. J. Pharm. Sci. 2017;30:303–307. [PubMed] [Google Scholar]
- 39.Xiong L.L., Hwang J.H., Huang X.B., Yao S.S., He C.J., Ge X.H., Ge H.Y., Wang X.F. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10:123–129. [PubMed] [Google Scholar]
- 40.Zhao J., Zhao F., Shi Y., Deng Y., Hu X., Shen H. The efficacy of a new high intensity focused ultrasound therapy for locally advanced pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017 doi: 10.1007/s00432-017-2459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang K., Zhu H., Meng Z., Chen Z., Lin J., Shen Y., Gao H. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie. 2013;36:88–92. doi: 10.1159/000348530. [DOI] [PubMed] [Google Scholar]
- 42.Vidal-Jove J., Perich E., Del Castillo M.A. Ultrasound Guided High Intensity Focused Ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason. Sonochem. 2015;27:703–706. doi: 10.1016/j.ultsonch.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Comito T., Cozzi L., Clerici E., Franzese C., Tozzi A., Iftode C., Navarria P., D’Agostino G., Rimassa L., Carnaghi C., et al. Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Results of a Phase 2 Study. Technol. Cancer Res. Treat. 2017;16:295–301. doi: 10.1177/1533034616650778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dholakia A.S., Chaudhry M., Leal J.P., Chang D.T., Raman S.P., Hacker-Prietz A., Su Z., Pai J., Oteiza K.E., Griffith M.E., et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:539–546. doi: 10.1016/j.ijrobp.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurka M.K., Collins S.P., Slack R., Tse G., Charabaty A., Ley L., Berzcel L., Lei S., Suy S., Haddad N., et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat. Oncol. 2013;8:44. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herman J.M., Chang D.T., Goodman K.A., Dholakia A.S., Raman S.P., Hacker-Prietz A., Iacobuzio-Donahue C.A., Griffith M.E., Pawlik T.M., Pai J.S., et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koong A.C., Le Q.T., Ho A., Fong B., Fisher G., Cho C., Ford J., Poen J., Gibbs I.C., Mehta V.K., et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Polistina F., Costantin G., Casamassima F., Francescon P., Guglielmi R., Panizzoni G., Febbraro A., Ambrosino G. Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann. Surg. Oncol. 2010;17:2092–2101. doi: 10.1245/s10434-010-1019-y. [DOI] [PubMed] [Google Scholar]
- 49.Schellenberg D., Goodman K.A., Lee F., Chang S., Kuo T., Ford J.M., Fisher G.A., Quon A., Desser T.S., Norton J., et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 50.Schellenberg D., Kim J., Christman-Skieller C., Chun C.L., Columbo L.A., Ford J.M., Fisher G.A., Kunz P.L., Van Dam J., Quon A., et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Tozzi A., Comito T., Alongi F., Navarria P., Iftode C., Mancosu P., Reggiori G., Clerici E., Rimassa L., Zerbi A., et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat. Oncol. 2013;8:148. doi: 10.1186/1748-717X-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alagappan M., Pollom E.L., von Eyben R., Kozak M.M., Aggarwal S., Poultsides G.A., Koong A.C., Chang D.T. Albumin and Neutrophil-Lymphocyte Ratio (NLR) Predict Survival in Patients With Pancreatic Adenocarcinoma Treated With SBRT. Am. J. Clin. Oncol. 2016 doi: 10.1097/COC.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 53.Chang D.T., Schellenberg D., Shen J., Kim J., Goodman K.A., Fisher G.A., Ford J.M., Desser T., Quon A., Koong A.C. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 54.Chuong M.D., Springett G.M., Freilich J.M., Park C.K., Weber J.M., Mellon E.A., Hodul P.J., Malafa M.P., Meredith K.L., Hoffe S.E., et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Mahadevan A., Jain S., Goldstein M., Miksad R., Pleskow D., Sawhney M., Brennan D., Callery M., Vollmer C. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 56.Mahadevan A., Miksad R., Goldstein M., Sullivan R., Bullock A., Buchbinder E., Pleskow D., Sawhney M., Kent T., Vollmer C., et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:e615–e622. doi: 10.1016/j.ijrobp.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 57.Mellon E.A., Hoffe S.E., Springett G.M., Frakes J.M., Strom T.J., Hodul P.J., Malafa M.P., Chuong M.D., Shridhar R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979–985. doi: 10.3109/0284186X.2015.1004367. [DOI] [PubMed] [Google Scholar]
- 58.Moningi S., Dholakia A.S., Raman S.P., Blackford A., Cameron J.L., Le D.T., De Jesus-Acosta A.M., Hacker-Prietz A., Rosati L.M., Assadi R.K., et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann. Surg. Oncol. 2015;22:2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rwigema J.C., Parikh S.D., Heron D.E., Howell M., Zeh H., Moser A.J., Bahary N., Quinn A., Burton S.A. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am. J. Clin. Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 60.Song Y., Yuan Z., Li F., Dong Y., Zhuang H., Wang J., Chen H., Wang P. Analysis of clinical efficacy of CyberKnife® treatment for locally advanced pancreatic cancer. Onco Targets Ther. 2015;8:1427–1431. doi: 10.2147/OTT.S81939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X., Li F., Ju X., Cao F., Cao Y., Fang F., Qing S., Shen Y., Jia Z., Zhang H. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017;6:2263–2270. doi: 10.1002/cam4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Jiang Y., Li J., Tian S., Ran W., Xiu D. Intraoperative ultrasound-guided iodine-125 seed implantation for unresectable pancreatic carcinoma. J. Exp. Clin. Cancer Res. 2009;28:88. doi: 10.1186/1756-9966-28-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H., Wang J., Jiang Y., Li J., Tian S., Ran W., Xiu D., Gao Y. The investigation of 125I seed implantation as a salvage modality for unresectable pancreatic carcinoma. J. Exp. Clin. Cancer Res. 2013;32:106. doi: 10.1186/1756-9966-32-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu K.C., Niu L.Z., Hu Y.Z., He W.B., He Y.S., Li Y.F., Zuo J.S. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J. Gastroenterol. 2008;14:1603–1611. doi: 10.3748/wjg.14.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu K.C., Niu L.Z., Hu Y.Z., He W.B., He Y.S., Zuo J.S. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J. Dig. Dis. 2008;9:32–40. doi: 10.1111/j.1443-9573.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 66.Zou Y.P., Li W.M., Zheng F., Li F.C., Huang H., Du J.D., Liu H.R. Intraoperative radiofrequency ablation combined with 125 iodine seed implantation for unresectable pancreatic cancer. World J. Gastroenterol. 2010;16:5104–5110. doi: 10.3748/wjg.v16.i40.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kluger M.D., Epelboym I., Schrope B.A., Mahendraraj K., Hecht E.M., Susman J., Weintraub J.L., Chabot J.A. Single-Institution Experience with Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann. Surg. Oncol. 2016;23:1736–1743. doi: 10.1245/s10434-015-5034-x. [DOI] [PubMed] [Google Scholar]
- 68.Martin R.C., Kwon D., Chalikonda S., Sellers M., Kotz E., Scoggins C., McMasters K.M., Watkins K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy. Ann. Surg. 2015;262:486–494. doi: 10.1097/SLA.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 69.Martin R.C., McFarland K., Ellis S., Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J. Am. Coll. Surg. 2012;215:361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Martin R.C., McFarland K., Ellis S., Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: Potential improved overall survival. Ann. Surg. Oncol. 2013;20(Suppl. S3):S443–S449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 71.Belfiore G., Belfiore M.P., Reginelli A., Capasso R., Romano F., Ianniello G.P., Cappabianca S., Brunese L. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med. Oncol. 2017;34:38. doi: 10.1007/s12032-017-0887-4. [DOI] [PubMed] [Google Scholar]
- 72.Dunki-Jacobs E.M., Philips P., Martin R.C. Evaluation of resistance as a measure of successful tumor ablation during irreversible electroporation of the pancreas. J. Am. Coll. Surg. 2014;218:179–187. doi: 10.1016/j.jamcollsurg.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Lambert L., Horejs J., Krska Z., Hoskovec D., Petruzelka L., Krechler T., Kriz P., Briza J. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: General hospital cancer center experience. Neoplasma. 2016;63:269–273. doi: 10.4149/213_150611N326. [DOI] [PubMed] [Google Scholar]
- 74.Mansson C., Brahmstaedt R., Nilsson A., Nygren P., Karlson B.M. Percutaneous irreversible electroporation for treatment of locally advanced pancreatic cancer following chemotherapy or radiochemotherapy. Eur. J. Surg. Oncol. 2016;42:1401–1406. doi: 10.1016/j.ejso.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 75.Narayanan G., Hosein P.J., Beulaygue I.C., Froud T., Scheffer H.J., Venkat S.R., Echenique A.M., Hevert E.C., Livingstone A.S., Rocha-Lima C.M., et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J. Vasc. Interv. Radiol. 2017;28:342–348. doi: 10.1016/j.jvir.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 76.Paiella S., Butturini G., Frigerio I., Salvia R., Armatura G., Bacchion M., Fontana M., D’Onofrio M., Martone E., Bassi C. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: Results of a prospective study. Dig. Surg. 2015;32:90–97. doi: 10.1159/000375323. [DOI] [PubMed] [Google Scholar]
- 77.Scheffer H.J., Vroomen L.G., de Jong M.C., Melenhorst M.C., Zonderhuis B.M., Daams F., Vogel J.A., Besselink M.G., van Kuijk C., Witvliet J., et al. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology. 2017;282:585–597. doi: 10.1148/radiol.2016152835. [DOI] [PubMed] [Google Scholar]
- 78.Vogel J.A., Rombouts S.J., de Rooij T., van Delden O.M., Dijkgraaf M.G., van Gulik T.M., van Hooft J.E., van Laarhoven H.W., Martin R.C., Schoorlemmer A., et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann. Surg. Oncol. 2017;24:2734–2743. doi: 10.1245/s10434-017-5900-9. [DOI] [PubMed] [Google Scholar]
- 79.Yan L., Chen Y.L., Su M., Liu T., Xu K., Liang F., Gu W.Q., Lu S.C. A Single-institution Experience with Open Irreversible Electroporation for Locally Advanced Pancreatic Carcinoma. Chin. Med. J. Engl. 2016;129:2920–2925. doi: 10.4103/0366-6999.195476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y., Shi J., Zeng J., Alnagger M., Zhou L., Fang G., Long X., Pan Z., Li Y., Chen J., et al. Percutaneous Irreversible Electroporation for Ablation of Locally Advanced Pancreatic Cancer: Experience From a Chinese Institution. Pancreas. 2017;46:e12–e14. doi: 10.1097/MPA.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 81.Belfiore M.P., Ronza F.M., Romano F., Ianniello G.P., De Lucia G., Gallo C., Marsicano C., Di Gennaro T.L., Belfiore G. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: Our preliminary experience. Int. J. Surg. 2015;21(Suppl. S1):S34–S39. doi: 10.1016/j.ijsu.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 82.Huggett M.T., Jermyn M., Gillams A., Illing R., Mosse S., Novelli M., Kent E., Bown S.G., Hasan T., Pogue B.W., et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer. 2014;110:1698–1704. doi: 10.1038/bjc.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bown S.G., Rogowska A.Z., Whitelaw D.E., Lees W.R., Lovat L.B., Ripley P., Jones L., Wyld P., Gillams A., Hatfield A.W. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Granata V., Fusco R., Piccirillo M., Palaia R., Petrillo A., Lastoria S., Izzo F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015;18:230–236. doi: 10.1016/j.ijsu.2015.04.055. [DOI] [PubMed] [Google Scholar]
- 85.Granata V., Fusco R., Setola S.V., Piccirillo M., Leongito M., Palaia R., Granata F., Lastoria S., Izzo F., Petrillo A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017;23:4767–4778. doi: 10.3748/wjg.v23.i26.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tempero M.A., Malafa M.P., Al-Hawary M., Asbun H., Bain A., Behrman S.W., Benson A.B., Binder E., Cardin D.B., Cha C., et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017;15:1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 87.Giardino A., Girelli R., Frigerio I., Regi P., Cantore M., Alessandra A., Lusenti A., Salvia R., Bassi C., Pederzoli P. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB Oxford. 2013;15:623–627. doi: 10.1111/hpb.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varadhachary G.R., Tamm E.P., Abbruzzese J.L., Xiong H.Q., Crane C.H., Wang H., Lee J.E., Pisters P.W., Evans D.B., Wolff R.A. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 89.de Geus S.W.L., Eskander M.F., Kasumova G.G., Ng S.C., Kent T.S., Mancias J.D., Callery M.P., Mahadevan A., Tseng J.F. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer. 2017 doi: 10.1002/cncr.30856. [DOI] [PubMed] [Google Scholar]
- 90.Petrelli F., Comito T., Ghidini A., Torri V., Scorsetti M., Barni S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:313–322. doi: 10.1016/j.ijrobp.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 91.Bohoudi O., Bruynzeel A.M.E., Senan S., Cuijpers J.P., Slotman B.J., Lagerwaard F.J., Palacios M.A. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother. Oncol. 2017 doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 92.Strunk H.M., Henseler J., Rauch M., Mucke M., Kukuk G., Cuhls H., Radbruch L., Zhang L., Schild H.H., Marinova M. Clinical Use of High-Intensity Focused Ultrasound (HIFU) for Tumor and Pain Reduction in Advanced Pancreatic Cancer. Rofo. 2016;188:662–670. doi: 10.1055/s-0042-105517. [DOI] [PubMed] [Google Scholar]
- 93.Vroomen L., Petre E.N., Cornelis F.H., Solomon S.B., Srimathveeravalli G. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences? Diagn. Interv. Imaging. 2017;98:609–617. doi: 10.1016/j.diii.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Ansari D., Kristoffersson S., Andersson R., Bergenfeldt M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: A systematic review of safety and efficacy. Scand. J. Gastroenterol. 2017 doi: 10.1080/00365521.2017.1346705. [DOI] [PubMed] [Google Scholar]
- 95.Martin R.C., Durham A.N., Besselink M.G., Iannitti D., Weiss M.J., Wolfgang C.L., Huang K.W. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J. Surg. Oncol. 2016;114:865–871. doi: 10.1002/jso.24404. [DOI] [PubMed] [Google Scholar]
- 96.Giardino A., Innamorati G., Ugel S., Perbellini O., Girelli R., Frigerio I., Regi P., Scopelliti F., Butturini G., Paiella S., et al. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology. 2017 doi: 10.1016/j.pan.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Niu L., Chen J., He L., Liao M., Yuan Y., Zeng J., Li J., Zuo J., Xu K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149. doi: 10.1097/MPA.0b013e3182965dde. [DOI] [PubMed] [Google Scholar]
- 98.Rovere-Querini P., Manfredi A.A. Tumor destruction and in situ delivery of antigen presenting cells promote anti-neoplastic immune responses: Implications for the immunotherapy of pancreatic cancer. JOP. 2004;5:308–314. [PubMed] [Google Scholar]
- 99.Vatner R.E., Cooper B.T., Vanpouille-Box C., Demaria S., Formenti S.C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kanda M., Matthaei H., Wu J., Hong S.M., Yu J., Borges M., Hruban R.H., Maitra A., Kinzler K., Vogelstein B., et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maitra A., Adsay N.V., Argani P., Iacobuzio-Donahue C., De Marzo A., Cameron J.L., Yeo C.J., Hruban R.H. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod. Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 103.Noonan A.M., Farren M.R., Geyer S.M., Huang Y., Tahiri S., Ahn D., Mikhail S., Ciombor K.K., Pant S., Aparo S., et al. Randomized phase 2 trial of the oncolytic virus pelareorep (reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 2016;24:1150–1158. doi: 10.1038/mt.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sze D.Y., Reid T.R., Rose S.C. Oncolytic virotherapy. J. Vasc. Interv. Radiol. 2013;24:1115–1122. doi: 10.1016/j.jvir.2013.05.040. [DOI] [PubMed] [Google Scholar]