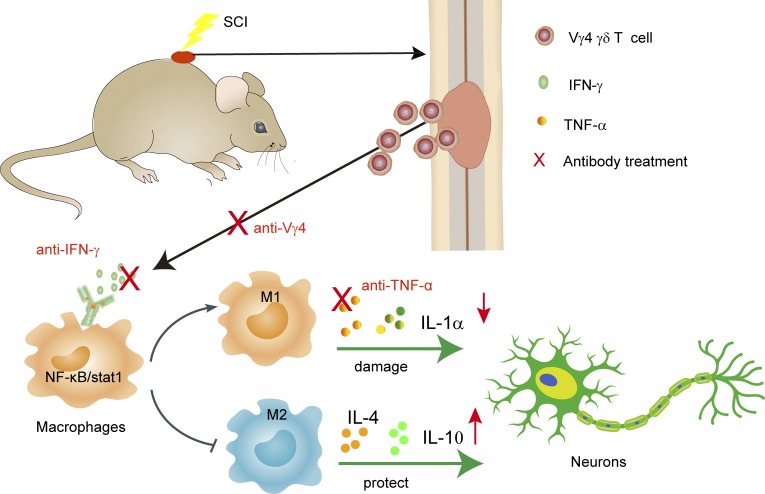
The role of γδ T cells in spinal cord injury remains unknown. Sun et al. report that Vγ4 γδ T cells produce IFN-γ, promote inflammatory cytokine production of macrophages, and are detrimental for functional recovery after SCI.
Abstract
Immune responses and neuroinflammation are critically involved in spinal cord injury (SCI). γδ T cells, a small subset of T cells, regulate the inflammation process in many diseases, yet their function in SCI is still poorly understood. In this paper, we demonstrate that mice deficient in γδ T cells (TCRδ−/−) showed improved functional recovery after SCI. γδ T cells are detected at the lesion sites within 24 hours after injury and are predominantly of the Vγ4 subtype and express the inflammatory cytokine IFN-γ. Inactivating IFN-γ signaling in macrophages results in a significantly reduced production of proinflammatory cytokines in the cerebrospinal fluid (CSF) of mice with SCIs and improves functional recovery. Furthermore, treatment of SCI with anti-Vγ4 antibodies has a beneficial effect, similar to that obtained with anti–TNF-α. In SCI patients, γδ T cells are detected in the CSF, and most of them are IFN-γ positive. In conclusion, manipulation of γδ T cell functions may be a potential approach for future SCI treatment.
Graphical Abstract
Introduction
Spinal cord injury (SCI) is one of the most serious public health problems worldwide and usually results in irreversible sensory, motor, and autonomic impairments (Rubiano et al., 2015). The clinical treatment for SCI is limited to minimizing secondary complications and maximizing residual functions. Experimental strategies have been designed to identify effective approaches that can alleviate secondary damage, reactivate spared circuitry, and rewire the spinal cord after injury (Ramer et al., 2014). Two defined phases are involved in SCI, namely the primary injury and a prolonged secondary injury. The primary injury mechanically damages local cells and the blood–spinal cord barrier (BSCB) and subsequently activates local neuroinflammation and immune responses, which are critically related to the secondary injury (Anwar et al., 2016).
After disruption of the BSCB by a mechanical force that directly destroys neural tissues and endothelial cell membranes, lymphocytes infiltrate the lesion site and drive inflammatory immune responses (Schnell et al., 1999). The inflammation alters the local microenvironment, promotes the release of nitric oxide and reactive oxygen species, and increases BSCB permeability, which further facilitates the entry of peripheral immune cells and leads to the secondary injury at the lesion site, as well as in the adjacent tissue. Nevertheless, numerous studies have reported neuroprotective and neuroregenerative roles of inflammatory responses that can eliminate tissue debris and induce the release of neurotrophic factors. Recent evidence supports the idea that inflammation has a dual role in SCI: deleterious in the acute injury phase and beneficial in the chronic recovery phase (Ankeny and Popovich, 2009).
IFN-γ is a pleiotropic cytokine that exhibits both pro- and antiinflammatory properties (Ottum et al., 2015; Becher et al., 2017). However, the roles of IFN-γ in SCI have been somewhat contradictory. It has been reported that IFN-γ has a protective effect by reducing the production of chondroitin sulfate proteoglycans and increasing the expression of neurotrophic factors (Fujiyoshi et al., 2010). Additionally, type 1 T helper (Th1) cells are required for the recruitment of tissue-repairing macrophages by secreting IL-10, which is IFN-γ dependent (Ishii et al., 2013). Yet, it was noted that IFN-γ could activate M1 macrophages, which are harmful to central nervous system (CNS) repairs (Kigerl et al., 2009). As recently reviewed, the dual effect of IFN-γ in neuroinflammation might depend on the administered dosage, disease phase, and its targets (Becher et al., 2017). Further studies are needed to study the essential source and the effects of IFN-γ in SCI.
TNF-α originates mainly from microglia and infiltrated monocytes/macrophages and can also be released by T cells (Kleine et al., 2003; Olmos and Lladó, 2014). Macrophages adopt two functionally antagonistic phenotypes: iNOS+ M1 and CD206+ M2 (Cusimano et al., 2012; Lech and Anders, 2013). The M1 phenotype is characterized by the excessive release of nitric oxide, IL-1, IL-6, and TNF-α, which leads to toxicity. On the contrary, M2 cells produce immunosuppressive cytokines (e.g., IL-10), stimulate the secretion of growth factors, and thus promote tissue remodeling and repair. Contrary to TNF-α, IFN-γ is mainly derived from T cells and facilitates the polarization of M1 macrophages (Gordon and Taylor, 2005; David and Kroner, 2011; Murray et al., 2014).
γδ T cells have several specific features and unique biological functions (Prinz et al., 2013; Wu et al., 2017) and can be divided into either IFN-γ or IL-17 producers (Jensen et al., 2008; Schmolka et al., 2015). We previously reported that γδ T cells predominantly produce IFN-γ, which is regulated by unique epigenetic and transcription programs (Chen et al., 2007). Peripheral γδ T cells are composed of two main subsets: Vγ1 and Vγ4 γδ T cells, which have divergent roles in different disease models (Heilig and Tonegawa, 1986; Ito et al., 1989; Pereira et al., 1995). Our group showed that Vγ4 γδ T cells, which produce IFN-γ, and Vγ1 γδ T cells, which suppress this function, play divergent roles in tumor immunity (He et al., 2010; Hao et al., 2011). Other groups similarly reported that γδ T cells could activate macrophages via IFN-γ (Holderness et al., 2013). In addition, γδ T cells were considered responsible for recruitment of monocytes/macrophages that boosted innate responses (Bonneville et al., 2010). Additionally, increasing evidence has indicated that γδ T cells play critical roles in regulating immune responses in the CNS (Shichita et al., 2009; Benakis et al., 2016; Malik et al., 2016). However, whether γδ T cells participate in immune responses after SCI is unknown.
In this paper, we show that γδ T cells, especially Vγ4 γδ T cells, play a detrimental role in a mouse model of SCI, probably by providing a critical source of IFN-γ, which induces macrophages to adopt the M1 phenotype with increased secretion of proinflammatory cytokines, such as TNF-α. Blocking IFN-γ or inactivating Vγ4 γδ T cells with antibodies had a similar effect as that of anti–TNF-α treatment and improved the functional recovery after SCI, which may lead to a new therapeutic approach to SCI.
Results
γδ T cell depletion contributes to functional recovery in a mouse SCI model
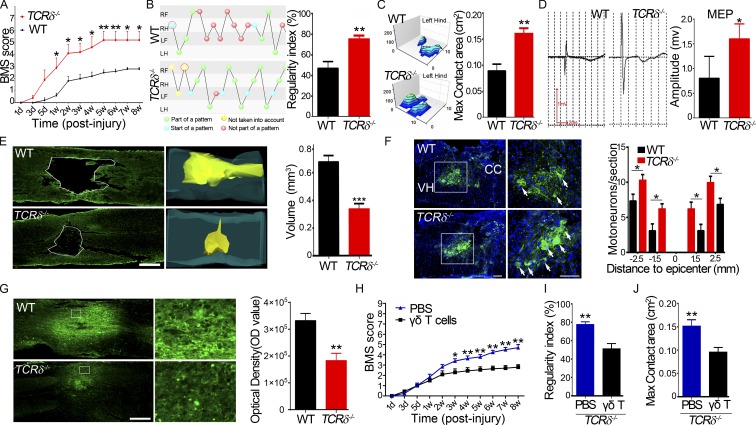
Knockout of the TCRδ gene (TCRδ−/− mice) caused a complete loss of γδ T cells, with no effect on the development of αβ T cells (Itohara et al., 1993), which, using flow cytometry, was confirmed by showing that few γδ T cells were detectable in the mutant spleen (Fig. S1 A). To study the role of γδ T cells in functional recovery after SCI, a moderate contusion at the level of the 11th thoracic vertebrae (T11) was made in TCRδ−/− and age-matched WT B6 mice, and the spontaneous recovery of hind limb movement was monitored using the Basso mouse score (BMS; Ung et al., 2007). Both TCRδ−/− and WT animals exhibited complete hind limb paralysis with a BMS score of 0 at 1 d after injury. TCRδ−/− mice recovered gradually: from 5 d after injury, their BMS index increased progressively and peaked at 5 wk after injury (mean of 5.25 ± 1.22, n = 8; Fig. 1 A). In contrast, functional recovery in WT mice was significantly slower, with a small increase in the BMS index of ∼2.5 at 2 wk after injury and no further improvements up to 8 wk after injury (Fig. 1 A). This significant difference was also apparent in an increased regularity index (improved walking steps) and enlarged hind max contact area in TCRδ−/− mice 8 wk after injury, compared with control animals (75.00 ± 10.60 vs. 47.00 ± 18.75 and 0.161 ± 0.029 vs. 0.089 ± 0.037, respectively, n = 8; Fig. 1, B and C). To confirm this, we stimulated the dura mater at the T6 level as reported previously (Baskin and Simpson, 1987) and recorded electromyography of biceps flexor cruris at 8 wk after injury. We found that the amplitudes of motor-evoked potentials (MEPs) were significantly higher in TCRδ−/− than in control mice (1.6 ± 0.86 vs. 0.8 ± 0.44 mV; P < 0.05, n = 8 in each group; Fig. 1 D), indicating a better recovery of electrophysiological functions of injured hind limbs in mutant mice than in control mice. To assess whether structures were preserved better in mutant mice after injury, we first measured the size of spinal cord lesions in serial horizontal sections at 8 wk after injury using anti–glial fibrillary acidic protein (GFAP) immunostaining and found that the lesion volume was significantly smaller in TCRδ−/− than in WT mice (0.33 ± 0.10 vs. 0.68 ± 0.11 mm3; P < 0.01, n = 6 animals in each group; Fig. 1 E). We then counted the number of surviving spinal motor neurons using anti–choline acetyltransferase (ChAT) immunostaining at five different levels: the injury site, as well as 1.5 mm and 2.5 mm rostral and caudal. There were no surviving motor neurons at the injury sites in both groups, but more motor neurons survived at the four distant sites in TCRδ−/− mice than in WT mice (Fig. 1 F). As SCI can induce an increase of nonphosphorylated forms of neurofilament H, detected by antibody SMI32 (Pitt et al., 2000), we stained sections with SMI32 and found that the expression in neurons was significantly higher in WT than in TCRδ−/− samples (Fig. 1 G). These results indicated that depletion of γδ T cells contributed to motor neuron survival and thereby promoted functional recovery after SCI. To test this hypothesis further, γδ T cells from WT mice were isolated and adoptively transferred into TCRδ−/− mice. Using flow cytometry, transferred γδ T cells were detectable in mutant spleens 48 h after transplantation (Fig. S1 A). Compared with mice treated with PBS, mice with reconstituted γδ T cells exhibited less desirable functional recovery, with significantly lower BMSs (Fig. 1 H), regularity index (Fig. 1 I), and hind max contact area (Fig. 1 J) after injury. These results suggested a detrimental role of γδ T cells in our mouse model of SCI.
Figure 1.
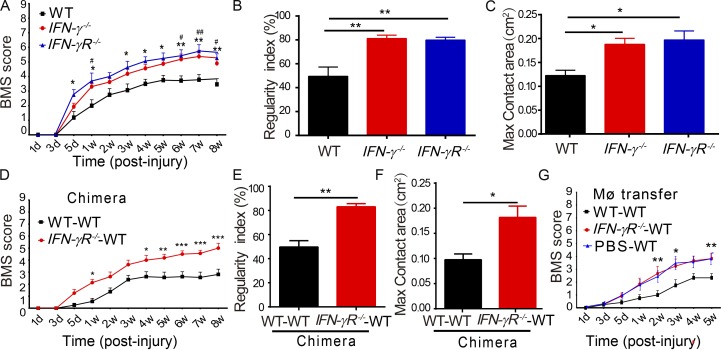
γδ T cells play a detrimental role in traumatic SCI. (A) BMSs of WT and TCRδ−/− mice at different time points after spinal cord contusion (P < 0.0001, n = 8; repeated measures ANOVA with Bonferroni’s post-hoc correction). (B and C) Locomotor functional recovery evaluated using the CatWalk XT automated quantitative gait analysis system. (B) Regularity index, P = 0.0024. (C) Hind max contact area, P = 0.0065. (D) Examples and comparison of amplitudes of MEP recordings 8 wk after surgery (P = 0.034). (B–D) n = 8; Student’s t test. (E) Representative injury sites in WT and TCRδ−/− animals 8 wk after surgery, labeled with anti-GFAP antibodies, and comparison of lesion volumes in both groups (P = 0.0004). Bar, 500 µm. (F) Survival of motor neurons immunostained with anti-ChAT antibodies in the spinal cord ventral horn at the eighth week after SCI and comparison of ventral horn neurons in both groups at various distances from the injury epicenter (P = 0.032). Bars, 250 µm. CC, central canal; VH, ventral horn; arrows indicate neurons. (E and F) n = 6; Student’s t-test. (G) Anti-SMI32 immunostaining of horizontal spinal sections disclosed more immunoreactivity in the WT than in the mutant 8 wk after surgery (P = 0.0028, n = 5; Student's t test). Bar, 500 µm. (F and G) The right panels are magnified from the boxed regions on the left. (H–J) Functional recovery of TCRδ−/− mice after reconstitution with WT γδ T cells or injection of PBS (n = 6). (H) P = 0.0018 (repeated measures ANOVA with Bonferroni’s post-hoc correction). (I) P = 0.0002 (Student's t test). (J) Hind max contact area, P = 0.0003 (Student's t test). Error bars represent mean ± SEM (where included). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
γδ T cells are recruited to the injury site and produce IFN-γ in situ
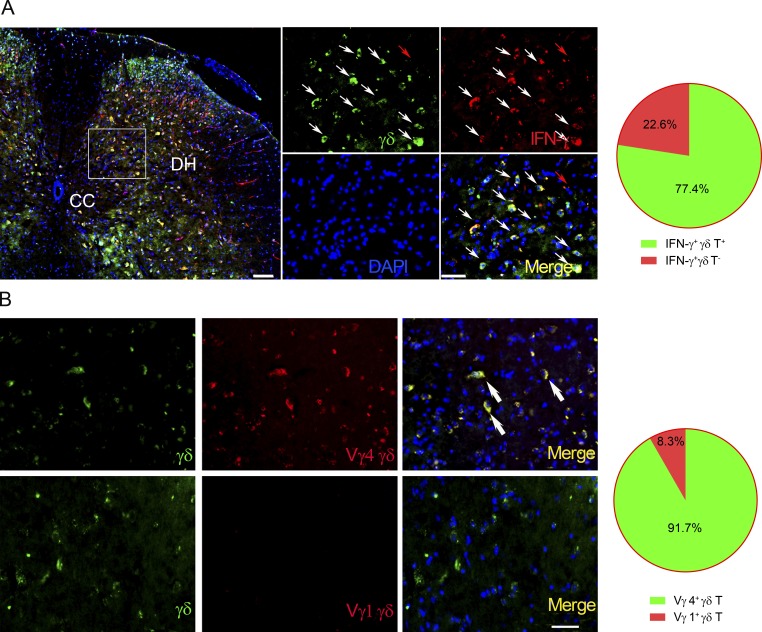
To determine whether γδ T cells are recruited into the injury site after SCI, we used TCRα+/−;TCRδEGFP/+ mice for an SCI model in which γδ T cells were specifically labeled with enhanced GFP (Prinz et al., 2006). As predicted, γδ T cells appeared at the injury site 24 h after injury and were quantified. Five slices per mouse and six mice per genotype were quantified. Sections matched in anatomy were serially chosen, and a 0.1-mm2 area per image was selected randomly (Fu et al., 2017). Most of the IFN-γ–positive cells were γδ T cells (77.4 ± 2.0%, n = 6; Fig. 2 A). Immunohistochemical staining with anti-Vγ1 and -Vγ4 antibodies showed that the majority of infiltrating γδ T cells (91.7 ± 2.3%, n = 6) were positive for Vγ4, but not for Vγ1 (Fig. 2 B). This suggested that Vγ4 γδ T cells might provide an early source of IFN-γ in SCI.
Figure 2.
γδ T cells are recruited to the injury site and express IFN-γ. Spinal cord transverse sections from WT mice 24 h after injury. (A) TCR δ-GFP mice were sacrificed at 24 h after SCI, and spinal cords were prepared for immunohistochemistry. Sections were stained and read by confocal microscopy. DAPI shows the nucleus. The percentage of γδ T cells and non-γδ T cells in total IFN-γ+ cells was calculated and is shown in the pie chart. White arrows point to IFN-γ–producing γδ T cells. Red arrows point to IFN-γ+ γδ TCR cells. CC, central canal; DH, dorsal horn. (B) Double immunofluorescence showed that most γδ T cells were Vγ4 positive (indicated by arrows), but not Vγ1 positive. Bars, 50 µm. n = 6 mice per group. One representative of at least two independent experiments is shown.
γδ T cell–derived IFN-γ hampers functional recovery
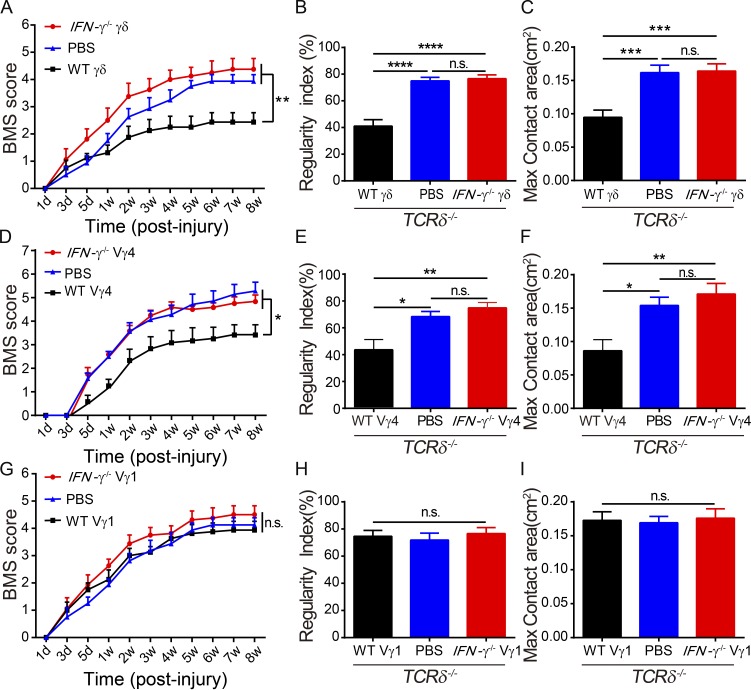
Based on the results reported (see previous paragraph), we hypothesized that γδ T cell–derived IFN-γ might be a detrimental mediator in functional recovery after SCI. To test this, TCRδ−/− mice were reconstituted with in vivo expanded γδ T cells derived from WT or IFN-γ−/− mice or injected with PBS 1 d before SCI. The transferred γδ T cells were detectable in the spleen of the recipient mice 24 h after transplantation (Fig. S1 A). We found that γδ T cells were well reconstituted (Fig. S1 B). TCRδ−/− mice that received γδ T cells from IFN-γ−/− mice had improved recovery compared with those that received γδ T cells from WT mice or those treated with PBS alone, as shown by measuring BMSs (Fig. 3 A), regularity indexes (Fig. 3 B), and greater max contact areas of hind limbs (Fig. 3 C). This was further supported by the observation of smaller lesions and an increased number of surviving motor neurons in the group reconstituted with IFN-γ−/− γδ T cells versus the group with WT γδ T cells (Fig. S2). These results indicated that IFN-γ might be a critical mediator involved in the detrimental effects of γδ T cells in SCI.
Figure 3.
γδ T cell–derived IFN-γ is critical in impairing recovery after SCI. (A–C) TCRδ−/− SCI mice were reconstituted with γδ T cells from WT or IFN-γ−/− animals or PBS alone, and evaluation of recovery after SCI. (D–F) Functional recovery of TCRδ−/− SCI mice reconstituted with Vγ4 γδ T cells from WT or IFN-γ−/− mice or PBS only. (G–I) Recovery after SCI of TCRδ−/− SCI mice reconstituted with Vγ1 γδ T cells from WT or IFN-γ−/− mice or PBS only. Error bars represent mean ± SEM. (A, D, and G) Two-way repeated measures ANOVA with Bonferroni’s post-hoc correction. (B, C, E, F, H, and I) One-way ANOVA with Tukey’s multiple comparisons test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. n.s., not significant. n = 8.
To define which subset of γδ T cells was the main source of IFN-γ, Vγ4 γδ T cells or Vγ1 γδ T cells from WT or IFN-γ−/− mice or PBS as a control were transferred into TCRδ−/− mice 1 d before SCI. TCRδ−/− mice reconstituted with Vγ4 γδ T cells from IFN-γ−/− mice or injected with PBS displayed better functional recovery than those reconstituted with WT Vγ4 γδ T cells, as shown in BMSs and CatWalk tests (Fig. 3, D–F). Interestingly, this effect was not obtained with Vγ1 γδ T cells: TCRδ−/− mice reconstituted with WT Vγ1 γδ T cells, IFN-γ−/− Vγ1 γδ T cells, or PBS had similar functional recoveries (Fig. 3, G–I). These results strongly suggest that Vγ4 γδ T cells are the earliest source of IFN-γ after SCI.
IFN-γ signaling is critical for the functional impairment after SCI
To further evaluate whether IFN-γ acts as a key mediator in γδ T cell–induced impairment of recovery, experimental SCI was performed in IFN-γ−/− and IFN-γR−/− mice. As expected, IFN-γ−/− and IFN-γR−/− mice recovered better than control mice, as indicated by higher BMSs, increased regularity indexes, and larger max contact areas of hind limbs (Fig. 4, A–C). These results were consistent with the previous findings that IFN-γ–stimulated M1 macrophages were detrimental to neurons in vitro (Kigerl et al., 2009).
Figure 4.
IFN-γ signaling in macrophages is essential for impairing neurological recovery from SCI. (A–C) After SCI, behavioral tests were assessed in WT, IFN-γ−/−, and IFN-γR−/− mice. (A) Statistical analysis comparing IFN-γ−/− and WT mice (*, **) or IFN-γR−/− and WT (#, ##). (B and C) Regularity index and hind max contact area with CatWalk test (C). (D–F) Irradiated WT mice were reconstituted with bone marrow from WT or IFN-γR−/− mice and tested for recovery. (G) BMSs were measured in irradiated WT mice injected with peritoneal macrophages prepared from WT or IFN-γR−/− mice or PBS alone. Error bars represent mean ± SEM. (A, D, and G) Two-way repeated measures ANOVA with Bonferroni’s post-hoc correction. (B, C, E, and F) One-way ANOVA with Tukey’s multiple comparisons test. * or #, P < 0.05; ** or ##, P < 0.01; ***, P < 0.001. n = 8. MØ, macrophages.
To determine whether macrophages were critical IFN-γ responders in vivo, a bone marrow chimera assay and adoptive transfer experiments were performed. For the chimera assay, bone marrow cells from WT or IFN-γR−/− mice were transplanted into irradiated WT mice, and chimeric mice were found to have ∼85% chimeric cells (Fig. S1 D), indicating a successful reconstitution. The chimeric mice that received IFN-γR−/− bone marrow cells exhibited better functional recovery than those that received WT bone marrow cells, as shown in BMSs and CatWalk tests (Fig. 4, D–F). This indicated that bone marrow–derived cells were the IFN-γ responders after SCI. For adoptive transfer experiments, peritoneal macrophages were collected from WT or IFN-γR−/− mice and transferred into WT mice by intravenous injections (Wang et al., 2015). Compared with mice that received WT macrophages, those that received IFN-γR−/− macrophages recovered better in terms of BMSs (Fig. 4 G) and CatWalk tests (Fig. S3). Thus, bone marrow–derived macrophages (BMDMs) are likely the main responders to IFN-γ signaling and impair recovery after SCI.
γδ T cell–derived IFN-γ regulates the production of proinflammatory cytokines
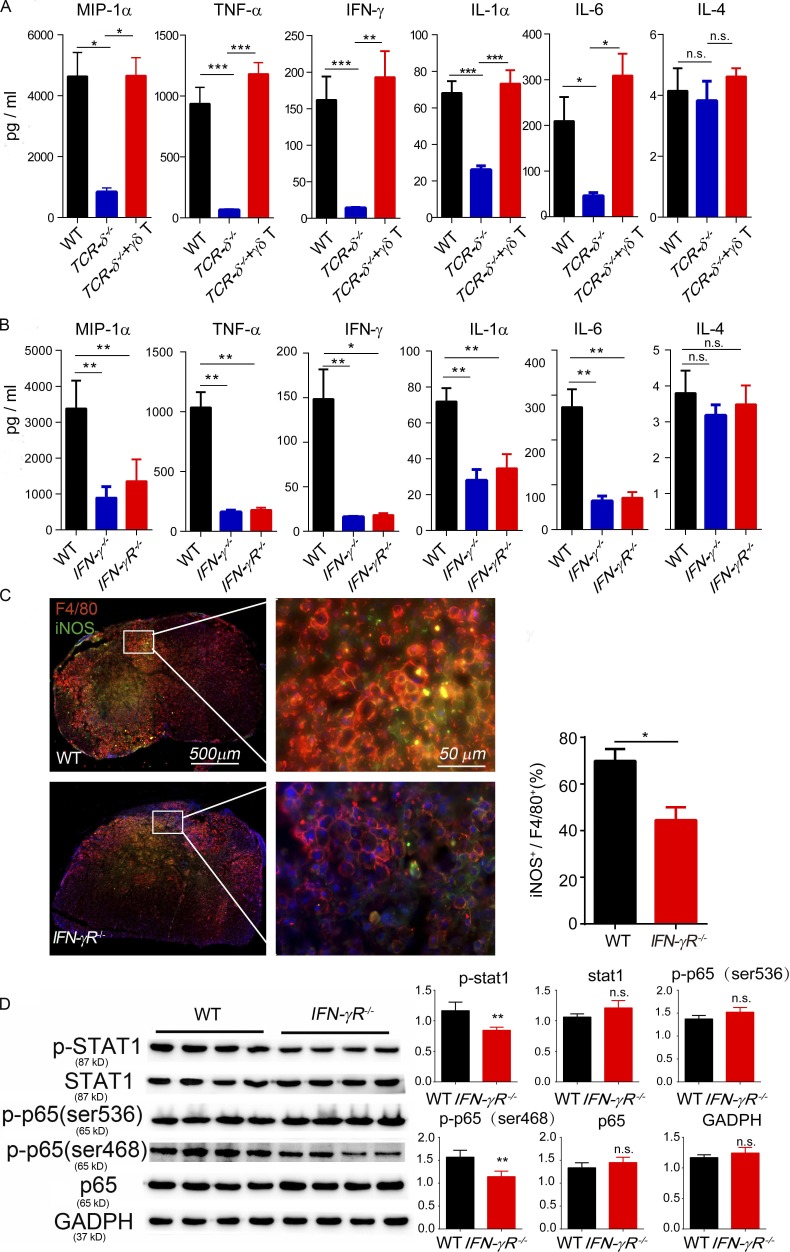
We then asked whether the depletion of γδ T cells or inactivation of IFN-γ functions influences the production of proinflammatory cytokines. Spinal cord tissues from the injury sites were collected 24 h after injury, and the levels of different cytokines were measured using a multiplex Luminex kit. In samples from TCRδ−/− mice, the levels of M1-related proinflammatory cytokines, including IL-1α, IL-6, TNF-α, IFN-γ, and MIP-1α, were significantly decreased when compared with samples from WT mice or from TCRδ−/− mice reconstituted with WT γδ T cells (Fig. 5 A and Fig. S4). These proinflammatory cytokines were also significantly reduced in lesioned spinal cord tissue from IFN-γ−/− and IFN-γR−/− mice (Fig. 5 B and Fig. S4). To test whether inactivation of IFN-γ signaling affects macrophage phenotypes, 24 h after SCI, tissue sections were immunostained with anti-F4/80, a generic macrophage marker, and with anti–nitric oxide synthase (iNOS), a marker of the M1 phenotype. The ratio of iNOS- to F4/80-positive cells was used to estimate the proportion of M1-activated macrophages. As shown in Fig. 5 C, most infiltrating macrophages in WT mice were iNOS positive, showing an M1 phenotype (Prieur et al., 2011). In contrast, the proportion of iNOS-positive cells was reduced in IFN-γR−/− mice (Fig. 5 C). Additionally, some Arg1-positive cells, presumably M2 macrophages, were present in spinal sections from IFN-γR−/− mice (Fig. S5). These results further supported the fact that γδ T cells produced early IFN-γ–stimulated proinflammatory M1 macrophages and induced detrimental effects in SCI.
Figure 5.
γδ T cells and IFN-γ influence the production of proinflammatory cytokines at the SCI site. Proinflammatory cytokines were analyzed in spinal samples at 1 d after SCI. (A) The levels of MIP-1α, TNF-α, IFN-γ, IL-1α, and IL-6 were significantly decreased in the TCRδ−/− group compared with the WT or TCRδ−/− plus γδ T cell reconstitution groups. IL-4 expression was comparable in the three groups (one-way ANOVA with Tukey’s multiple comparisons test). (B) In the IFN-γ−/− and IFN-γR−/− group, the levels of MIP-1α, TNF-α, IFN-γ, IL-1α, and IL-6 were significantly decreased compared with the WT group, but there were no significant differences between the IFN-γ−/− and IFN-γR−/− groups (one-way ANOVA with Tukey’s multiple comparisons test). (C) Spinal sections from WT mice 1 wk after SCI immunostained with anti-F4/80 (a generic marker for macrophages) and anti-iNOS (a maker for M1 macrophages). The ratio of iNOS-positive to F4/80-positive cells was significantly decreased in the IFN-γR−/− group compared with the WT (P = 0.0113). (D) Protein expression was analyzed by Western blot using BMDMs from WT and IFN-γR−/− mice followed with lipopolysaccharide stimulation. The phosphorylation of Stat1 and p65 was significantly decreased in the IFN-γR−/− group compared with the WT (Student's t test). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results (C) represent one of at least two repeated experiments; mean ± SEM; n = 4. n.s., not significant.
Transcription factor NF-κB is critical in regulating the expression of numerous proinflammatory cytokines such as TNF-α and IL-6, and its phosphorylation induces the production of these detrimental cytokines after SCI (Mattson and Camandola, 2001). To test whether the changes of cytokine levels observed upon IFN-γ inactivation were related to NF-κB signaling, we isolated macrophages from WT and IFN-γR−/− mice and stimulated them with lipopolysaccharide. Western blot analysis showed that the phosphorylation of NF-κB p65 (Ser 468) and Stat1 was significantly less in IFN-γR−/− mice when compared with WT control mice (Fig. 5 D).
Anti-Vγ4 treatment improves functional recovery after SCI
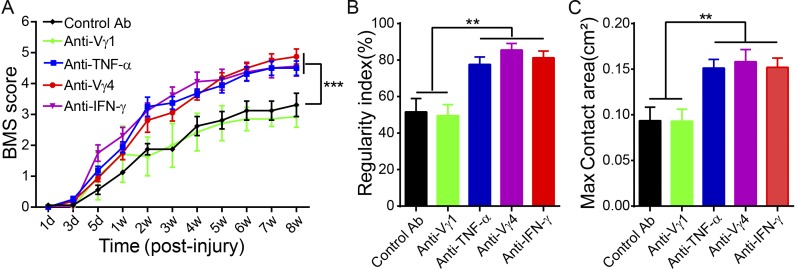
Having determined that Vγ4 γδ T cells are the main source of IFN-γ at the SCI injury site and that IFN-γ favors the production of proinflammatory cytokines that hinder recovery, we hypothesized that Vγ4 γδ T cells might serve as a potential target for therapy after SCI. To test this, WT mice with SCI were treated with anti-Vγ4 antibodies, and their neurological functional recoveries were monitored as described in the previous paragraph. Using flow cytometry analysis of spleen samples, we detected 21.5% Vγ4-positive cells and 49.5% Vγ1-positive cells among all γδ T cells from control antibody–treated mice; however, the percentages were reduced to 1.19% in anti-Vγ4 antibody–treated mice and 7.26% in anti-Vγ1–treated mice, respectively (Fig. S1 C), indicating that antibodies effectively inactivated Vγ4- or Vγ1-positive cells. Anti–TNF-α treatment, previously known to be beneficial in SCI, was used as a positive control. Treatment with anti-Vγ4, anti–IFN-γ, or anti–TNF-α antibodies had similar effects and resulted in significantly better recoveries when compared with treatment with control antibody or anti-Vγ1 antibodies, which yielded comparable results, as estimated in the BMS and CatWalk tests (Fig. 6, A–C).
Figure 6.
Anti-Vγ4 or anti–IFN-γ treatment contributes to functional recovery after SCI. WT SCI animals were treated with anti–IFN-γ, anti–TNF-α, anti-Vγ4, and anti-Vγ1 antibodies or isotype antibodies as control and underwent behavioral tests. (A–C) BMSs (A) were estimated at different time points after injury (P < 0.0001; two-way repeated measures ANOVA with Bonferroni’s post-hoc correction), and regularity index (B) and max contact area (C; one-way ANOVA with Tukey’s multiple comparisons test) were measured with CatWalk at the eighth week after injury. n = 8 mice per group; mean ± SEM; **, P < 0.01; ***, P < 0.001, all compared with the control antibody group.
Traumatic SCI in human subjects induces the infiltration of γδ T cells
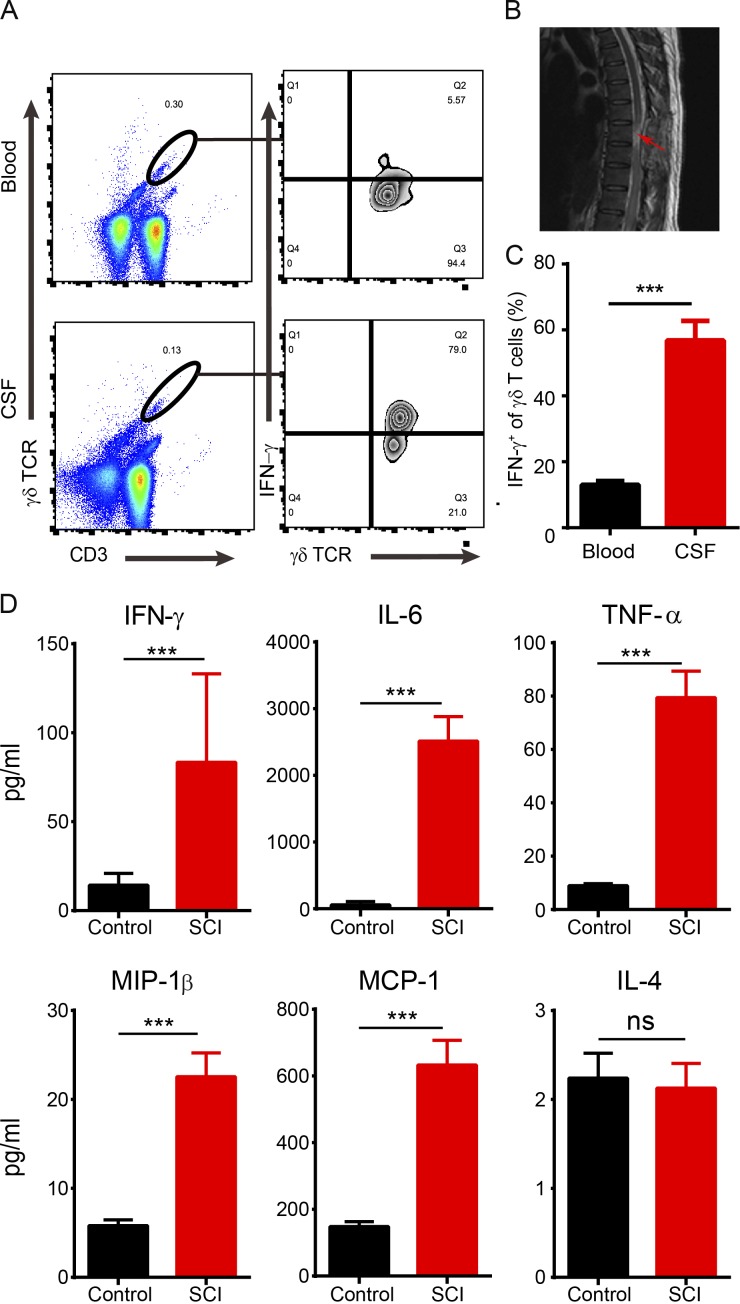
To investigate whether lesion infiltration with γδ T cells also occurs in patients with SCI, cerebrospinal fluid (CSF) samples were collected from 22 patients with thoracic or cervical segment injuries (Fig. 7 B and Table 1) and from 20 control donors without SCI. Using flow cytometry analysis, γδ T cells were detected in the CSF or the peripheral blood from patients (n = 15; Fig. 7 A), but not in controls (n = 3; Fig. S1 D). Interestingly, we found detectable levels of IFN-γ, but not IL-17, in the CSF and blood from SCI patients (Fig. 7 A and Fig. S1 D). Furthermore, the level of IFN-γ from CSF-derived γδ T cells was significantly higher than that from blood-derived γδ T cells (Fig. 7, B and C; n = 15 patients). Levels of proinflammatory cytokines IFN-γ, IL-6, TNF-α, MIP-1β, and MCP-1 were significantly increased in the CSF of SCI patients (n = 22) compared with controls (n = 20; Fig. 7 D). Our results from human samples thus confirm the conclusion from our animal studies that γδ T cells were the source of IFN-γ in SCI.
Figure 7.
Infiltration of γδ T cells and elevated levels of inflammatory cytokines in the CSF of SCI patients. (A) Using flow cytometry analysis, γδ T cells were enriched in the CSF and peripheral blood from SCI patients, and most of CSF-derived γδ T cells were positive for IFN-γ. (B) MRI image showing the injury site in a patient with SCI (arrow). (C) Statistical analysis showed a significantly higher ratio of IFN-γ+ γδ T cells in CSF than peripheral blood samples (P < 0.0001; n = 15). (D) The levels of IFN-γ, IL-6, TNF-α, MIP-1β, and MCP-1, but not IL-4, were significantly increased in the CSF from SCI patients compared with the control group (n = 20–22). Numbers represent frequencies (percentage) of cells in corresponding gates. Results (A and B) represent one of at least two repeated experiments. ***, P < 0.01. Mean ± SEM; Student's t test; ns, not significant.
Table 1. Patient demographics and baseline neurological status.
| ID | Mechanism of injury | Spinal injury | Age | Sex | ASIA grade | Level |
|---|---|---|---|---|---|---|
| yr | ||||||
| 1 | MVA | T9/T10 fracture-dislocation | 45 | M | A | Thoracic |
| 2 | Fall from standing height | T4 burst fracture | 21 | M | A | Thoracic |
| 3 | Diving | C6/7 fracture-dislocation | 36 | M | B | Cervical |
| 4 | MVA | C4/5 fracture-dislocation | 28 | F | A | Cervical |
| 5 | Fall from standing height | C5/6 flexion-distraction | 39 | M | B | Cervical |
| 6 | Fall from standing height | T9 and T10 burst fractures | 41 | F | B | Thoracic |
| 7 | MVA | T10 burst fracture | 32 | M | A | Thoracic |
| 8 | MVA | T1 burst fracture | 25 | M | A | Thoracic |
| 9 | MVA | T6 burst fracture | 33 | M | A | Thoracic |
| 10 | MVA | T12 burst fracture | 50 | M | A | Thoracic |
| 11 | Fall from standing height | T8 burst fracture | 46 | M | B | Thoracic |
| 12 | MVA | C6/7 fracture-dislocation | 33 | M | A | Cervical |
| 13 | MVA | T11/T11 fracture-dislocation | 26 | F | A | Thoracic |
| 14 | MVA | T6 and T7 burst fractures | 36 | M | A | Thoracic |
| 15 | MVA | T8 and T9 burst fractures | 42 | M | A | Thoracic |
| 16 | Fall from standing height | T8 and T9 burst fractures | 31 | M | A | Thoracic |
| 17 | Fall from standing height | C5/6 flexion-distraction | 30 | F | B | Cervical |
| 18 | Fall from standing height | C3/4 flexion-distraction | 37 | M | A | Cervical |
| 19 | MVA | C5/6 flexion-distraction | 36 | M | A | Cervical |
| 20 | MVA | T1 burst fracture | 35 | M | B | Thoracic |
| 21 | MVA | T10 burst fracture | 22 | F | A | Thoracic |
| 22 | MVA | T7 burst fracture | 28 | M | A | Thoracic |
| Totals | 34.18 ± 7.69 | 17 M, 5 F | 16 A, 6 B | 7 cervical,15 thoracic |
MVA, motor vehicle accident; M, male; F, female.
Discussion
Neurological impairment after SCI is a severe health problem that is aggravated by the infiltration of immune cells and the resulting inflammation at and around the injury site. Our research shows that γδ T cells, especially Vγ4 γδ T cells, are recruited at the injury site during the early phase after SCI, which impairs functional recovery. IFN-γ secreted by Vγ4 γδ T cells acts on BMDMs, triggering their production of TNF-α and other cytokines. Inactivation of Vγ4 γδ T cells or blocking of IFN-γ functions results in functional improvement comparable to that obtained with anti–TNF-α treatment, further confirming a detrimental role of Vγ4 γδ T cells in SCI. Detection of IFN-γ–producing γδ T cells in the CSF and peripheral blood of SCI patients lends further support to our hypothesis. Therefore, our studies demonstrate a previously unknown, physiopathological mechanism of neurological impairment after SCI and identify Vγ4 γδ T cells as novel potential therapeutic targets.
γδ T cells are a unique subset of T cells that play different roles in different disease models. For example, γδ T cells prevented the overproduction of inflammatory cytokines in the Listeria monocytogenes–infected mouse model (Skeen et al., 2001), whereas they provided a major source of inflammatory cytokines detrimental to the injury in an ischemic model (Shichita et al., 2009). They also harbored different roles in different tumor models (Gao et al., 2003; Daley et al., 2016). A striking observation in our study was that complete depletion of γδ T cells resulted in improved neurological recovery from experimental SCI in mice and that recovery in TCRδ−/− mice was very similar to that in IFN-γ−/− mice (Fig. 1 and Fig. 3), indicating that γδ T cells may provide the main source of IFN-γ in SCI. Indeed, γδ T cells, especially IFN-γ–positive Vγ4 γδ T cells, are recruited at the injury site (Fig. 2), and the reconstitution of TCRδ−/− mice with Vγ4 γδ T cells isolated from IFN-γ−/− mice, but not from WT mice (Fig. 3), enhanced neurological recoveries. This is in agreement with the idea that Vγ4 γδ T cells provide an early source of IFN-γ in SCI and play a detrimental role in neurological recoveries. However, we cannot exclude the possibility that γδ T cells might also contribute to the immunopathology upon injury. It also remains unclear which factors trigger the recruitment of Vγ4 γδ T cells at the injury sites, whether the IFN-γ–producing γδ T cells are selectively recruited, and how they are triggered to produce IFN-γ. SCI broke the BSCB, and this may have caused the release of different cytokines and chemokines. Further studies are needed to better identify the factors responsible for the recruitment and activation of γδ T cells in SCI.
The effect of IFN-γ on CNS inflammation and repair is still controversial. Using genetic animal models, we provide convincing evidence that the early production of IFN-γ by γδ T cells is detrimental for recovery after SCI and that BMDMs are key responders to IFN-γ. In addition, we found an early filtration of γδ T cells with the elevated level of IFN-γ in the CSF from SCI patients, further supporting our hypothesis that IFN-γ influences the outcome of neuronal injury. Furthermore, other studies using IFN-γ−/− mice also demonstrated a neurotoxic effect of IFN-γ after peripheral nerve injuries (Victório et al., 2012). A detrimental role of IFN-γ produced by Th1 cells has also been suggested in other CNS diseases (Smith et al., 2009; Silver et al., 2015). We are aware that our data appear contradictory to observations that administration of IFN-γ has a protective role after SCI (Fujiyoshi et al., 2010; Ishii et al., 2013). This apparent discrepancy might be caused by the different techniques used to produce and analyze SCI in mice. In this, it is worth noting that the putative protective role of IFN-γ in these experiments was not tested directly in IFN-γ−/− or IFN-γR−/− mice. The differences in the approaches and time points of interfering IFN-γ signaling might also have contributed to the discrepancies.
Macrophages are key regulators of balanced pro- and antiinflammatory responses. According to the microenvironment, macrophages can adopt two phenotypes: classically (M1) or alternatively (M2) activated macrophages (Murray et al., 2014). Macrophages adopt the M1 phenotype upon stimulation with IFN-γ, plus bacterial products such as lipopolysaccharide. IFN-γ induces secretion by M1 macrophages of inflammatory cytokines including TNF-α, IL-1, and IL-6 (David and Kroner, 2011). M2 macrophages, induced by Th2 cytokines such as IL-4 and IL-13, as well as by immune complexes, produce antiinflammatory cytokines, inhibit the inflammation response, and promote tissue repair (Gordon and Taylor, 2005). One important finding of our studies was that BMDMs are the responders to Vγ4-derived IFN-γ and are a main source of proinflammatory cytokines. This is supported by two sets of observations. First, IFN-γR−/− mice, chimeras with IFN-γR−/− bone marrow, as well as mice receiving adoptively transferred IFN-γR−/− peritoneal macrophages show similar functional recoveries after SCI (Fig. 4). However, we cannot exclude the possibility that the adoptively transferred macrophages might possess different abilities to traffic to the lesion sites. Second, levels of proinflammatory cytokines and numbers of M1 macrophages are reduced in IFN-γR−/− mice compared with WT mice (Fig. 5 A). We present consistent results that inflammatory cytokines in TCRδ−/−, IFN-γ−/−, and IFN-γR−/− mice are more significantly reduced than WT mice in the SCI model (Fig. 5, A and B), further supporting our notion that γδ T cells provide the earliest source of IFN-γ that triggers the production of inflammatory cytokines by macrophages in an IFN-γ–IFN-γR–dependent manner. By regulating the production of proinflammatory cytokines, the NF-κB pathway plays a central role in controlling inflammation (Libermann and Baltimore, 1990; Beg et al., 1993; Beg and Baltimore, 1996; Sumitomo et al., 1999; Luan et al., 2014; Yu et al., 2015), and we show reduced NF-κB p65 phosphorylation in IFN-γR−/− macrophages (Fig. 5 D). In the presence of IFN-γ, cytokines released from damaged spinal cords may trigger NF-κB activation and production of proinflammatory cytokines. This could result in a positive loop, accounting for the “secondary hit” of SCI. In contrast, a decrease in IFN-γ signaling may down-regulate the NF-κB pathway, interrupting that positive loop and thus showing improved neurological recovery.
Based on the fact that Vγ4 γδ T cells are the key source of IFN-γ, which then triggers detrimental inflammatory cytokine production by macrophages and increases neurological injury, it is certainly possible that Vγ4 γδ T cells are the potential target for in vivo intervention. Indeed, we used a Vγ4 γδ TCR–specific antibody (clone UC3) in vivo and showed that injection of neutralizing anti-Vγ4 antibodies has a beneficial effect of neurological recovery upon SCI (Fig. 6). Although there is evidence showing that anti-pan γδ TCR antibody (clone UC7) induces TCR internalization rather than depleting the cells (Koenecke et al., 2009), it remains unclear whether anti-Vγ1 (clone 2.11) or anti-Vγ4 antibodies deplete the corresponding subset of γδ T cells or just inactivate them. However, these two antibodies have been widely used in vivo to inactivate these two subsets of γδ T cells in many disease models (Huber et al., 2000; Hahn et al., 2004; Huang et al., 2009; Hao et al., 2011). Our results further emphasize that antibodies against Vγ4 γδ T cells inactivate the function of this subset of γδ T cells.
The inflammatory cytokine TNF-α is considered a therapeutic target in autoimmune diseases, and anti–TNF-α antibodies have been used successfully in Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis (Prescott et al., 2007; Kemény-Beke et al., 2010; Papoutsaki and Costanzo, 2013; Mozaffari et al., 2014; Ceccarelli et al., 2017; Rayen et al., 2017). It was also shown that treatment with anti–TNF-α is beneficial in animal SCI models (Genovese et al., 2006, 2007; Marchand et al., 2009; Bayrakli et al., 2012). In our model, the improved recovery observed in the absence of γδ T cells, especially Vγ4 γδ T cells and their production of IFN-γ, is very similar to the effect of anti–TNF-α treatment (Fig. 6), and this suggests that Vγ4 γδ T cells are a potential therapeutic target in SCI. This is further supported by our demonstration that human samples from SCI patients are enriched in IFN-γ–positive γδ T cells and inflammatory cytokines (Fig. 7). Further studies are needed to understand future links between γδ T cell–derived IFN-γ, the consequential production of inflammatory cytokines, and the mechanisms of functional recovery.
In summary, as revealed in our mouse model, γδ T cells, especially Vγ4 γδ T cells, play a detrimental role in the acute phase of SCI by providing IFN-γ that acts on BMDMs and directs them toward M1 phenotypes; these phenotypes in turn produced proinflammatory cytokines. Treatment with neutralizing Vγ4 antibodies shows a beneficial effect similar to that of anti–TNF-α. Therefore, Vγ4 γδ T cells should be considered as a novel therapeutic target for SCI.
Materials and methods
Mice
C57BL/6J, TCRδ−/−, IFN-γ−/−, IFN-γR−/−, and TCRα+/−;TCRδEGFP/+ mice were purchased from the Jackson Laboratory. All mice were females, aged 7–8 wk old, and weighed 17–22 g at the time of surgery. Animals were maintained under pathogen-free conditions in the animal facility at Jinan University. All procedures were approved by and performed in accordance with the Jinan University’s Institutional Laboratory Animal Care and Use Committee.
Reagents
PerCP-conjugated anti–mouse CD11c (clone N418) was purchased from BioLegend. PE-conjugated anti–mouse F4/80 (clone BM8), APC-conjugated anti–mouse CD86 (clone GL1), and FITC anti–mouse TNF-α (clone XT3.11) were purchased from Tianjin Sungene. GolgiPlugs were purchased from BD Biosciences. Anti–mouse CD3e mAb (clone 145-2C11), anti–mouse CD28 mAb (clone 37.51), and purified hamster IgG anti–mouse TCR Vγ1 (clone 2.11)/Vγ4 (clone UC3)/TCR-γ/δ (clone GL3) mAb were from Tianjin Sungene. The recombinant human TNF-α receptor IgG Fc fusion protein (rhTNFR:Fc) was obtained from Shanghai CP Guojian Pharmaceutical Co., Ltd. Antibodies used for γδ T cell flow cytometric analysis such as FITC anti–mouse TCR Vγ1 (clone 2.11), PE anti–mouse TCR Vγ4 (clone UC3-10A6), APC anti–mouse TCR-γ/δ (clone GL3), PerCP/Cy5.5 anti–human TCR-γ/δ (clone GL3), and FITC anti–human IFN-γ (clone B27) were purchased from Tianjin Sungene. PerCP/Cy5.5 anti–human TCR-γ/δ (clone B1), V500 anti–human CD3 (clone SP34-2), and V450 anti–mouse CD3 (clone 500A2) were from BD Biosciences, and PE anti–human IL-17A (clone BL168) was from BioLegend. The standard culture medium was RPMI 1640 (Euroclone) or DMEM (Hyclone; Thermo) containing 10% FCS (Euroclone), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin.
Contusive SCI model
Contusive SCI was performed using a New York University impactor as described previously (Ma et al., 2015). In brief, mice were anesthetized with pentobarbital (50 mg/kg intraperitoneally) and underwent a laminectomy at the T11 and T12 level. After clamping the transverse processes of T11 and T12 to stabilize the spine, the exposed dorsal surface of the cord was subjected to a weight drop injury using a 10-g rod dropped from a height of 6.25 mm. After injury, the muscles and skin were closed in layers, and mice were placed in a temperature- and humidity-controlled chamber. Manual bladder emptying was performed three times daily until reflex bladder emptying was established.
Bone marrow chimeras
Bone marrow chimeras were prepared as described previously (Gao et al., 2003). In brief, recipients were irradiated twice at 450 rad with a 2-h interval (total of 900 rad). Donor bone marrow cells were injected intravenously into recipients (107 cells per mouse). Antibiotics were added to the drinking water for the first 4 wk after reconstitution. At 8 wk after reconstitution, the mice were used for SCI.
Tissue processing
To harvest spinal cords, mice were deeply anesthetized with 1.25% tribromoethanol, and the blood was drawn off through cardiac puncture. A 10-mm segment of cord centered on T11 was removed and quickly frozen in an ethanol–dry ice bath. Samples were stored at −80°C. The tissue samples were later homogenized in 200 µl of ice-cold cell lysis buffer (Beyotime) containing enzyme inhibitors and centrifuged at 10,000 g for 10 min at 4°C, and then the supernatants were frozen at −80°C. Protein levels were determined with the bicinchoninic acid protein assay as described by the manufacturer (Beyotime).
Immunohistochemistry
For immunofluorescence staining, spinal cord tissues were resected from mice 8 wk after surgery. Mice were deeply anesthetized with 1.25% tribromoethanol after thoracotomy, and 10 ml of normal saline was rapidly perfused through the left ventricle. This was followed by perfusion of 30 ml of 4% paraformaldehyde for fixation. A spinal cord segment measuring 5 mm in length was resected from the level of T11 and soaked in 4% paraformaldehyde for 24 h before being transferred into 30% sucrose solution until the tissue sank. After freezing, the tissues were cut into ∼15–20-µm sections (Leica). Slices were incubated with the following mouse-specific primary antibodies: 1:1,000 anti-GFAP (ab7260; Abcam), 1:200 anti-ChAT (AB144P; Millipore), 1:500 anti-SMI32 (NE1023; Merck), and 1:200 anti-iNOS (BD610328; Abcam). For visualization, 1:1,000 fluorescent Alexa Fluor 488 or 546 secondary antibodies (Invitrogen Vector) were used for both or single staining at room temperature for 2 h. After washing, the tissue slices were fixed with Vectashield containing DAPI and used for visualization. An inverted fluorescence microscope (Axio Observer A1; Carl Zeiss) was used to capture images and conduct further analysis. To determine the total number of neurons, the number of motor neurons in both spinal cord anterior horns was estimated.
Image analysis
The densities of γδ+ cells and IFN-γ+ cells were quantified and expressed as the numbers of marker+ cells divided by the area of outlined regions. A 0.1-mm2 area per image was selected randomly for quantification. Five slices per mouse and six mice per genotype were quantified. γδ+ cells and Vγ1 γδ+ or Vγ4 γδ+ cells were quantified and expressed as the numbers of marker+ cells divided by a 0.1-mm2 area per image. Five slices per mouse and six mice per genotype were quantified. Sections matched in anatomy were serially chosen (Fu et al., 2017).
For quantitative analysis of cavity volume, serial spinal cord sections stained with GFAP were three-dimensionally reconstructed. A total of five serial transverse spinal cord sections equally spaced 200 µm apart were used to create a three-dimensional image corresponding to a 1-cm-long spinal cord segment. The lesion cavity and the spinal cord were three-dimensionally reconstructed with a microscope attached to a Neurolucida system (MBF Bioscience). The Neurolucida software calculated the volumes of the cystic cavities automatically (Gu et al., 2010).
BMS
The BMS primary scoring system is based on a scale that ranges from 0 (complete paralysis) to 9 points (completely normal). The mice were placed on a flat surface and observed for 5 min. Hind limb motor functions were scored by the single-blind method using two independent blind observers. The mean score of both observers for two hind limbs was used as the BMS of the sample.
EthoVision-assisted open-field test
Mice were placed under illumination (500 lx) in one corner of a 50 × 50 × 35–cm box. The trajectories of mice were recorded for 15 min. Five mice from each group were continuously monitored. A video-tracking system (XT 7.0 EthoVision; Noldus) was used to analyze trajectories and total movements.
CatWalk-assisted gait analysis
Gait analysis of mice from each group was conducted using an automated quantitative gait analysis system (CatWalk XT; Noldus). Each mouse was subjected to at least three assessments, each of which required continuous walking on a glass plate, along a path that was 50 cm in length. The entire experiment was conducted in a dark, quiet environment. The CatWalk system automatically identified and tagged each paw print and then generated a series of parameters, including paw statistics, general parameters (mean speed and cadence), step sequence parameters, and bases of support. The walking coordination was assessed using the regularity index as described previously (Koopmans et al., 2005), calculated as the number of normal step sequence patterns multiplied by four and divided by the total amount of paw placements; that value is ∼100% in normal animals. The max contact area was indicated by the maximal area of contact between the paw and the walking floor.
Sample collection from patients
Patients sustaining acute SCI were recruited at The First Affiliated Hospital of Jinan University by neurosurgeons from May 2013 to June 2017. Inclusion criteria for the trial included American Spinal Injury Association (ASIA) grade A or B SCI upon presentation, spinal injury between C4 and T10 inclusive, within 48 h after injury and the ability to provide a valid, reliable neurological examination. Patients were excluded if they had concomitant head injuries, concomitant major trauma to the chest, pelvis, or extremities that required invasive intervention (e.g., chest tube, internal or external fixation), or if they were too sedated or intoxicated to undergo a valid neurological examination. The CSF from SCI patients was obtained via myelotomy. To obtain the control samples from non-SCI patients, we enrolled individuals with hip osteoarthritis who were undergoing hip replacements under spinal anesthesia. The anesthesiologist punctured the dura with a spinal needle, and a 1.5–2.0-ml sample of CSF was collected, after which the anesthetic agent was injected (Kwon et al., 2010). The clinical trial protocol was approved by the Human Ethics Committee at Jinan University, and the sample collection was approved by individual patients.
Cytokine analysis
The human CSF was analyzed on a Bio-Plex system (BioRad) using a 17-plex human cytokine kit that included IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-17, MCP-1 (MCAF), IFN-γ, GM-CSF, G-CSF, MIP-1β, and TNF-α (catalog no. M5000031YV). The mice spinal cords were analyzed with the Bio-Plex system using a 23-plex cytokine array kit that included Eotaxin, G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, KC, MCP-1 (MCAF), MIP-1α, MIP-1β, RANTES, and TNF-α (catalog no. M60009RDPD).
Bone marrow isolation, differentiation, and polarization
Mouse bone marrow cells were flushed from the femur of WT or IFN-γR1−/− mice (females, 8 wk old) and differentiated into BMDMs in DMEM with 10% FBS and 20 ng/ml M-CSF in a 6-well cell culture plate (106 cells/ml) for 6 d (Zhang et al., 2008). BMDMs were also washed and resuspended in fresh media and incubated for a further 1 h in the presence of 100 ng/ml Escherichia coli 0111:B4 LPS (Sigma-Aldrich) and then were washed and proceeded to the cell lysis and protein extraction for Western blot testing.
Western blot
BMDM lysates were obtained with radioimmunoprecipitation assay lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium orthovanadate, sodium fluoride, and EDTA; Beyotime), 2 mg/ml aprotinin, and 1 mM PMSF. Then they were subjected to 10,000 g centrifugation at 4°C for 20 min. The protein concentration was measured by a bicinchoninic acid protein assay kit. The immunoblotting was performed as described previously (Zhu et al., 2014; Zhao et al., 2017), and the following primary antibodies were used: anti-p65, p-p65 (Ser 468), p-p65 (Ser 536), STAT-1, p-STAT-1, and GAPDH, all from Cell Signaling Technology.
γδ T cell proliferation and transplantation
For expansion of Vγ1, Vγ4, and γδ T cells from WT or IFN-γ−/− mice in vitro, splenic γδ T cells were sorted and expanded with anti-Vγ1 anti-Vγ4 or anti-γδ antibodies as described previously (Hao et al., 2011). For polarization of T cells, the cells were cultured with 10 μg/ml of plate-coated anti-CD3, 1 μg/ml of soluble anti-CD28, and 2 ng/ml IL-2 in the neutral culture medium as described previously (He et al., 2010; Zhao et al., 2011). In cell transfer experiments, PBS, Vγ1, Vγ4, or γδ T cells (106 cells per mouse) from WT or IFN-γ−/− mice were transplanted into the TCRδ−/− mice (10 mice per group) with intravenous injection. 1 d after the transplantation, all mice received contusive SCI, and ethology evaluation followed.
Flow cytometry
Macrophages were put in a new well and treated with 1:1,000 GolgiPlug and 1 ng/ml ionomycin for 6 h and then were permeabilized with 0.5% (wt/vol) saponin for 30 min (37°C, 5% of CO2). The intracellular and cell surface staining were performed as the protocol described in the fixation/permeabilization kit (554714; BD). For cytokine staining, cells were collected and incubated with FITC anti–mouse TNF-α for 30 min at 4°C, washed with PBS, labeled with cell surface–staining marker PE-anti–mouse F4/80, APC-anti–mouse CD86, and PerCP-anti–mouse CD11c for 20 min at 4°C, and detected by FACS verse flow cytometry (BD). Data were analyzed using FlowJo (TreeStar).
MEP recording
The MEPs were assayed by electromyography on the mice 8 wk after surgery as described previously (Ding et al., 2014), with minor modifications. A stimulation electrode was applied to the rostral extremity of the surgically exposed spinal cord region, and the recording electrode was placed in the biceps flexor cruris. A single square wave stimulus of 0.5 mA, 0.5 ms in duration, 2-ms time delay, and 1 Hz was used. The latency period was measured as the length of time from the stimulus to the onset of the first response wave. The amplitude was measured from the initiation point of the first response wave to its highest point. The peak-to-peak amplitude (P-P value) was calculated to estimate the nerve conduction function in the hind limb.
Statistical analysis
The SPSS 19.0 statistical software (IBM) was used to process and analyze the data and images. Data were presented as the mean ± SEM. For analyzing the differences in BMSs between the groups over time, a two-way repeated measures ANOVA with Bonferroni’s post-hoc correction was performed. Other data were analyzed using Student’s t test or one-way ANOVA with Tukey’s multiple comparison test. All tests were two sided, and the level of significance was set at 0.05.
Online supplemental material
Fig. S1 contains data showing the validation of γδ T cell transfer, of γδ T cell depletion after antibody treatment, of TCR-δ−/− mice, and of chimeric experiments and the infiltration of γδ T cells after SCI. Fig. S2 shows morphological changes in spinal cords of TCRδ−/− mice reconstituted after SCI with γδ T cells from WT or IFN-γ−/− mice. Fig. S3 includes the results of CatWalk tests in WT mice reconstituted after SCI with different macrophages. Fig. S4 shows the levels of inflammatory cytokines in different mice 24 h after SCI, analyzed using the Bio-Plex system. Fig. S5 shows M2 macrophages present in the lesion site in IFN-γR−/− mouse after SCI.
Supplementary Material
Acknowledgments
We wish to thank Hezuo Lü for technical support with animal models, Xin Sun, Panpan Yu and Yiwen Ruan for critical comments, and Andre M. Goffinet for revising the manuscript.
This work is supported by the National Natural Science Foundation of China (grant 31400770 to Z. Yin and grant 81571186 to L. Zhou), the 111 Project (grant B14036 to L. Zhou and grant B16021 to Z. Yin), the National Basic Research Program of China (973 Program; grant 2014CB542205 to L. Zhou), the Science & Technology Planning and Key Technology Innovation Projects of Guangdong (grant 2014B050504006 to L. Zhou), the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (grant IRT-15R13 to Y. Zhao), and the Guangdong Innovative and Entrepreneurial Research Program (grant 201301s0105240297 to Z. Yin).
The authors declare no competing financial interests.
Author contributions: Z. Yin, L. Zhou, and K.-F. So designed experiments; G. Sun, S. Yang, and G. Cao performed research; Q. Wang, J. Hao, Q. Wen, and Z. Liu assisted in animal models and data analysis; Z. Li, S. Zhou, and Y. Zhao contributed to study design; G. Sun, S. Yang, and H. Yang prepared the manuscript; Z. Yin and L. Zhou revised the manuscript.
References
- Ankeny D.P., and Popovich P.G.. 2009. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 158:1112–1121. 10.1016/j.neuroscience.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar M.A., Al Shehabi T.S., and Eid A.H.. 2016. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 10:98 10.3389/fncel.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin D.S., and Simpson R.K. Jr. 1987. Corticomotor and somatosensory evoked potential evaluation of acute spinal cord injury in the rat. Neurosurgery. 20:871–877. 10.1227/00006123-198706000-00009 [DOI] [PubMed] [Google Scholar]
- Bayrakli F., Balaban H., Ozum U., Duger C., Topaktas S., and Kars H.Z.. 2012. Etanercept treatment enhances clinical and neuroelectrophysiological recovery in partial spinal cord injury. Eur. Spine J. 21:2588–2593. 10.1007/s00586-012-2319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Spath S., and Goverman J.. 2017. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17:49–59. 10.1038/nri.2016.123 [DOI] [PubMed] [Google Scholar]
- Beg A.A., and Baltimore D.. 1996. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 274:782–784. 10.1126/science.274.5288.782 [DOI] [PubMed] [Google Scholar]
- Beg A.A., Finco T.S., Nantermet P.V., and Baldwin A.S. Jr. 1993. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol. Cell. Biol. 13:3301–3310. 10.1128/MCB.13.6.3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C., Brea D., Caballero S., Faraco G., Moore J., Murphy M., Sita G., Racchumi G., Ling L., Pamer E.G., et al. 2016. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22:516–523. 10.1038/nm.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., O’Brien R.L., and Born W.K.. 2010. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10:467–478. 10.1038/nri2781 [DOI] [PubMed] [Google Scholar]
- Ceccarelli F., Massafra U., Perricone C., Idolazzi L., Giacomelli R., Tirri R., Russo R., Pistone G., Ruscitti P., Parisi S., et al. 2017. Anti-TNF treatment response in rheumatoid arthritis patients with moderate disease activity: a prospective observational multicentre study (MODERATE). Clin. Exp. Rheumatol. 35:24–32. [PubMed] [Google Scholar]
- Chen L., He W., Kim S.T., Tao J., Gao Y., Chi H., Intlekofer A.M., Harvey B., Reiner S.L., Yin Z., et al. 2007. Epigenetic and transcriptional programs lead to default IFN-gamma production by gammadelta T cells. J. Immunol. 178:2730–2736. 10.4049/jimmunol.178.5.2730 [DOI] [PubMed] [Google Scholar]
- Cusimano M., Biziato D., Brambilla E., Donegà M., Alfaro-Cervello C., Snider S., Salani G., Pucci F., Comi G., Garcia-Verdugo J.M., et al. 2012. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 135:447–460. 10.1093/brain/awr339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D., Zambirinis C.P., Seifert L., Akkad N., Mohan N., Werba G., Barilla R., Torres-Hernandez A., Hundeyin M., Mani V.R.K., et al. 2016. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell. 166:1485–1499.e15. 10.1016/j.cell.2016.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., and Kroner A.. 2011. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12:388–399. 10.1038/nrn3053 [DOI] [PubMed] [Google Scholar]
- Ding Y., Qu Y., Feng J., Wang M., Han Q., So K.F., Wu W., and Zhou L.. 2014. Functional motor recovery from motoneuron axotomy is compromised in mice with defective corticospinal projections. PLoS One. 9:e101918 10.1371/journal.pone.0101918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Zou M.M., Zhu J.W., Zhang Y., Chen W.J., Cheng M., Liu C.F., Ma Q.H., and Xu R.X.. 2017. TRIM32 affects the recovery of motor function following spinal cord injury through regulating proliferation of glia. Oncotarget. 8:45380–45390. 10.18632/oncotarget.17492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyoshi T., Kubo T., Chan C.C., Koda M., Okawa A., Takahashi K., and Yamazaki M.. 2010. Interferon-γ decreases chondroitin sulfate proteoglycan expression and enhances hindlimb function after spinal cord injury in mice. J. Neurotrauma. 27:2283–2294. 10.1089/neu.2009.1144 [DOI] [PubMed] [Google Scholar]
- Gao Y., Yang W., Pan M., Scully E., Girardi M., Augenlicht L.H., Craft J., and Yin Z.. 2003. γδ T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 198:433–442. 10.1084/jem.20030584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T., Mazzon E., Crisafulli C., Di Paola R., Muià C., Bramanti P., and Cuzzocrea S.. 2006. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J. Pharmacol. Exp. Ther. 316:1006–1016. 10.1124/jpet.105.097188 [DOI] [PubMed] [Google Scholar]
- Genovese T., Mazzon E., Crisafulli C., Esposito E., Di Paola R., Muià C., Di Bella P., Meli R., Bramanti P., and Cuzzocrea S.. 2007. Combination of dexamethasone and etanercept reduces secondary damage in experimental spinal cord trauma. Neuroscience. 150:168–181. 10.1016/j.neuroscience.2007.06.059 [DOI] [PubMed] [Google Scholar]
- Gordon S., and Taylor P.R.. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953–964. 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- Gu W., Zhang F., Xue Q., Ma Z., Lu P., and Yu B.. 2010. Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology. 30:205–217. 10.1111/j.1440-1789.2009.01063.x [DOI] [PubMed] [Google Scholar]
- Hahn Y.-S., Taube C., Jin N., Sharp L., Wands J.M., Aydintug M.K., Lahn M., Huber S.A., O’Brien R.L., Gelfand E.W., and Born W.K.. 2004. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J. Immunol. 172:2894–2902. 10.4049/jimmunol.172.5.2894 [DOI] [PubMed] [Google Scholar]
- Hao J., Dong S., Xia S., He W., Jia H., Zhang S., Wei J., O’Brien R.L., Born W.K., Wu Z., et al. 2011. Regulatory role of Vγ1 γδ T cells in tumor immunity through IL-4 production. J. Immunol. 187:4979–4986. 10.4049/jimmunol.1101389 [DOI] [PubMed] [Google Scholar]
- He W., Hao J., Dong S., Gao Y., Tao J., Chi H., Flavell R., O’Brien R.L., Born W.K., Craft J., et al. 2010. Naturally activated V γ 4 γ δ T cells play a protective role in tumor immunity through expression of eomesodermin. J. Immunol. 185:126–133. 10.4049/jimmunol.0903767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig J.S., and Tonegawa S.. 1986. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 322:836–840. 10.1038/322836a0 [DOI] [PubMed] [Google Scholar]
- Holderness J., Hedges J.F., Ramstead A., and Jutila M.A.. 2013. Comparative biology of γδ T cell function in humans, mice, and domestic animals. Annu. Rev. Anim. Biosci. 1:99–124. 10.1146/annurev-animal-031412-103639 [DOI] [PubMed] [Google Scholar]
- Huang Y., Jin N., Roark C.L., Aydintug M.K., Wands J.M., Huang H., O’Brien R.L., and Born W.K.. 2009. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J. Immunol. 183:849–855. 10.4049/jimmunol.0804104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S.A., Graveline D., Newell M.K., Born W.K., and O’Brien R.L.. 2000. V γ 1+ T cells suppress and V γ 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunol. 165:4174–4181. 10.4049/jimmunol.165.8.4174 [DOI] [PubMed] [Google Scholar]
- Ishii H., Tanabe S., Ueno M., Kubo T., Kayama H., Serada S., Fujimoto M., Takeda K., Naka T., and Yamashita T.. 2013. ifn-γ-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis. 4:e710 10.1038/cddis.2013.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bonneville M., Takagaki Y., Nakanishi N., Kanagawa O., Krecko E.G., and Tonegawa S.. 1989. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc. Natl. Acad. Sci. USA. 86:631–635. 10.1073/pnas.86.2.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A.R., Hooper M.L., Farr A., and Tonegawa S.. 1993. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 72:337–348. 10.1016/0092-8674(93)90112-4 [DOI] [PubMed] [Google Scholar]
- Jensen K.D., Su X., Shin S., Li L., Youssef S., Yamasaki S., Steinman L., Saito T., Locksley R.M., Davis M.M., et al. 2008. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 29:90–100. 10.1016/j.immuni.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemény-Beke A., Szekanecz Z., Szántó S., Bodnár N., Módis L. Jr., Gesztelyi R., Zsuga J., Szodoray P., and Berta A.. 2010. Safety and efficacy of etanercept therapy in ankylosing spondylitis patients undergoing phacoemulsification surgery. Rheumatology (Oxford). 49:2220–2221. 10.1093/rheumatology/keq288 [DOI] [PubMed] [Google Scholar]
- Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G.. 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29:13435–13444. 10.1523/JNEUROSCI.3257-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T.O., Zwerenz P., Zöfel P., and Shiratori K.. 2003. New and old diagnostic markers of meningitis in cerebrospinal fluid (CSF). Brain Res. Bull. 61:287–297. 10.1016/S0361-9230(03)00092-3 [DOI] [PubMed] [Google Scholar]
- Koenecke C., Chennupati V., Schmitz S., Malissen B., Förster R., and Prinz I.. 2009. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur. J. Immunol. 39:372–379. 10.1002/eji.200838741 [DOI] [PubMed] [Google Scholar]
- Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., and Joosten E.A.. 2005. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma. 22:214–225. 10.1089/neu.2005.22.214 [DOI] [PubMed] [Google Scholar]
- Kwon B.K., Stammers A.M., Belanger L.M., Bernardo A., Chan D., Bishop C.M., Slobogean G.P., Zhang H., Umedaly H., Giffin M., et al. 2010. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma. 27:669–682. 10.1089/neu.2009.1080 [DOI] [PubMed] [Google Scholar]
- Lech M., and Anders H.J.. 2013. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta. 1832:989–997. 10.1016/j.bbadis.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Libermann T.A., and Baltimore D.. 1990. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 10:2327–2334. 10.1128/MCB.10.5.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H., Zhang Q., Wang L., Wang C., Zhang M., Xu X., Zhou H., Li X., Xu Q., He F., et al. 2014. OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-mediated ERK1/2/NF-κB pathway in RAW264.7 cells. J. Interferon Cytokine Res. 34:526–536. 10.1089/jir.2013.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.F., Chen Y.J., Zhang J.X., Shen L., Wang R., Zhou J.S., Hu J.G., and Lü H.Z.. 2015. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav. Immun. 45:157–170. 10.1016/j.bbi.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Malik S., Want M.Y., and Awasthi A.. 2016. The Emerging Roles of Gamma-Delta T Cells in Tissue Inflammation in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 7:14 10.3389/fimmu.2016.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F., Tsantoulas C., Singh D., Grist J., Clark A.K., Bradbury E.J., and McMahon S.B.. 2009. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur. J. Pain. 13:673–681. 10.1016/j.ejpain.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Mattson M.P., and Camandola S.. 2001. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 107:247–254. 10.1172/JCI11916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari S., Nikfar S., Abdolghaffari A.H., and Abdollahi M.. 2014. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin. Biol. Ther. 14:583–600. 10.1517/14712598.2014.885945 [DOI] [PubMed] [Google Scholar]
- Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. 2014. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 41:14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G., and Lladó J.. 2014. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014:861231 10.1155/2014/861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottum P.A., Arellano G., Reyes L.I., Iruretagoyena M., and Naves R.. 2015. Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front. Immunol. 6:539 10.3389/fimmu.2015.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsaki M., and Costanzo A.. 2013. Treatment of psoriasis and psoriatic arthritis. BioDrugs. 27(S1, Suppl 1):3–12. 10.1007/BF03325637 [DOI] [PubMed] [Google Scholar]
- Pereira P., Gerber D., Huang S.Y., and Tonegawa S.. 1995. Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. J. Exp. Med. 182:1921–1930. 10.1084/jem.182.6.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt D., Werner P., and Raine C.S.. 2000. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6:67–70. 10.1038/71555 [DOI] [PubMed] [Google Scholar]
- Prescott K., Costner M., Cohen S., and Kazi S.. 2007. Tumor necrosis factor-alpha inhibitor associated ulcerative colitis. Am. J. Med. Sci. 333:137–139. 10.1097/MAJ.0b013e3180312362 [DOI] [PubMed] [Google Scholar]
- Prieur X., Mok C.Y., Velagapudi V.R., Núñez V., Fuentes L., Montaner D., Ishikawa K., Camacho A., Barbarroja N., O’Rahilly S., et al. 2011. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 60:797–809. 10.2337/db10-0705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz I., Sansoni A., Kissenpfennig A., Ardouin L., Malissen M., and Malissen B.. 2006. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat. Immunol. 7:995–1003. 10.1038/ni1371 [DOI] [PubMed] [Google Scholar]
- Prinz I., Silva-Santos B., and Pennington D.J.. 2013. Functional development of γδ T cells. Eur. J. Immunol. 43:1988–1994. 10.1002/eji.201343759 [DOI] [PubMed] [Google Scholar]
- Ramer L.M., Ramer M.S., and Bradbury E.J.. 2014. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 13:1241–1256. 10.1016/S1474-4422(14)70144-9 [DOI] [PubMed] [Google Scholar]
- Rayen J., Currie T., Gearry R.B., Frizelle F., and Eglinton T.. 2017. The long-term outcome of anti-TNF alpha therapy in perianal Crohn’s disease. Tech. Coloproctol. 21:119–124. 10.1007/s10151-016-1578-4 [DOI] [PubMed] [Google Scholar]
- Rubiano A.M., Carney N., Chesnut R., and Puyana J.C.. 2015. Global neurotrauma research challenges and opportunities. Nature. 527:S193–S197. 10.1038/nature16035 [DOI] [PubMed] [Google Scholar]
- Schmolka N., Wencker M., Hayday A.C., and Silva-Santos B.. 2015. Epigenetic and transcriptional regulation of γδ T cell differentiation: Programming cells for responses in time and space. Semin. Immunol. 27:19–25. 10.1016/j.smim.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Schnell L., Fearn S., Klassen H., Schwab M.E., and Perry V.H.. 1999. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur. J. Neurosci. 11:3648–3658. 10.1046/j.1460-9568.1999.00792.x [DOI] [PubMed] [Google Scholar]
- Shichita T., Sugiyama Y., Ooboshi H., Sugimori H., Nakagawa R., Takada I., Iwaki T., Okada Y., Iida M., Cua D.J., et al. 2009. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 15:946–950. 10.1038/nm.1999 [DOI] [PubMed] [Google Scholar]
- Silver J., Schwab M.E., and Popovich P.G.. 2015. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 7:a020602 10.1101/cshperspect.a020602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen M.J., Rix E.P., Freeman M.M., and Ziegler H.K.. 2001. Exaggerated proinflammatory and Th1 responses in the absence of gamma/delta T cells after infection with Listeria monocytogenes. Infect. Immun. 69:7213–7223. 10.1128/IAI.69.12.7213-7223.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A., Zhang R., Varma A.K., Das A., Ray S.K., and Banik N.L.. 2009. Estrogen partially down-regulates PTEN to prevent apoptosis in VSC4.1 motoneurons following exposure to IFN-gamma. Brain Res. 1301:163–170. 10.1016/j.brainres.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo M., Tachibana M., Nakashima J., Murai M., Miyajima A., Kimura F., Hayakawa M., and Nakamura H.. 1999. An essential role for nuclear factor kappa B in preventing TNF-alpha-induced cell death in prostate cancer cells. J. Urol. 161:674–679. 10.1016/S0022-5347(01)61993-1 [DOI] [PubMed] [Google Scholar]
- Ung R.V., Lapointe N.P., Tremblay C., Larouche A., and Guertin P.A.. 2007. Spontaneous recovery of hindlimb movement in completely spinal cord transected mice: a comparison of assessment methods and conditions. Spinal Cord. 45:367–379. 10.1038/sj.sc.3101970 [DOI] [PubMed] [Google Scholar]
- Victório S.C., Cartarozzi L.P., Hell R.C., and Oliveira A.L.. 2012. Decreased MHC I expression in IFN γ mutant mice alters synaptic elimination in the spinal cord after peripheral injury. J. Neuroinflammation. 9:88 10.1186/1742-2094-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cao K., Sun X., Chen Y., Duan Z., Sun L., Guo L., Bai P., Sun D., Fan J., et al. 2015. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia. 63:635–651. 10.1002/glia.22774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wu P., Qiu F., Wei Q., and Huang J.. 2017. Human γδT-cell subsets and their involvement in tumor immunity. Cell. Mol. Immunol. 14:245–253. 10.1038/cmi.2016.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.-J., Zhang D.-M., Jia L.-L., Qi J., Song X.-A., Tan H., Cui W., Chen W., Zhu G.-Q., Qin D.-N., and Kang Y.M.. 2015. Inhibition of NF-κB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicol. Appl. Pharmacol. 284:315–322. 10.1016/j.taap.2015.02.023 [DOI] [PubMed] [Google Scholar]
- Zhang X., Goncalves R., and Mosser D.M.. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 10.1002/0471142735.im1401s83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Hao J., Ni Y., Luo W., Liang R., Cao G., Zhao Y., Wang P., Zhao L., Tian Z., et al. 2011. Vγ4 γδ T cell-derived IL-17A negatively regulates NKT cell function in Con A-induced fulminant hepatitis. J. Immunol. 187:5007–5014. 10.4049/jimmunol.1101315 [DOI] [PubMed] [Google Scholar]
- Zhao R., Liu Y., Wang H., Yang J., Niu W., Fan S., Xiong W., Ma J., Li X., Phillips J.B., et al. 2017. BRD7 plays an anti-inflammatory role during early acute inflammation by inhibiting activation of the NF-κB signaling pathway. Cell. Mol. Immunol. 14:830–841. 10.1038/cmi.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu E., Wang X., Zheng B., Wang Q., Hao J., Chen S., Zhao Q., Zhao L., Wu Z., and Yin Z.. 2014. miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORγt and STAT3. J. Immunol. 192:5599–5609. 10.4049/jimmunol.1303488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.