The brain is our computing machine that integrates stimuli from the environment and orchestrates responses to these stimuli. Here, Jonathan Kipnis proposes that the defining role of the immune system is to sense microorganisms and to inform the brain about them.
Abstract
The brain is our computing machine that integrates stimuli from the environment and orchestrates responses to these stimuli. Here, I propose that the defining role of the immune system is to sense microorganisms and to inform the brain about them.
This is a tale of two systems. Both the central nervous system and the immune system are composed of heterogeneous cell populations. Both encompass enormous variability and heterogeneity within each cell type. Both release and respond to neurotransmitters and cytokines. Both sense environmental stimuli. Both respond to deviations in homeostasis. Both use “synapses” for cell–cell interactions. Both generate and store memories. The two systems were believed to live separately from each other to ensure a person’s health. Interaction between them, when it occurred, was considered for decades as pathological. Recent works from numerous laboratories suggest that the time has come for reappraisal of these assumptions.
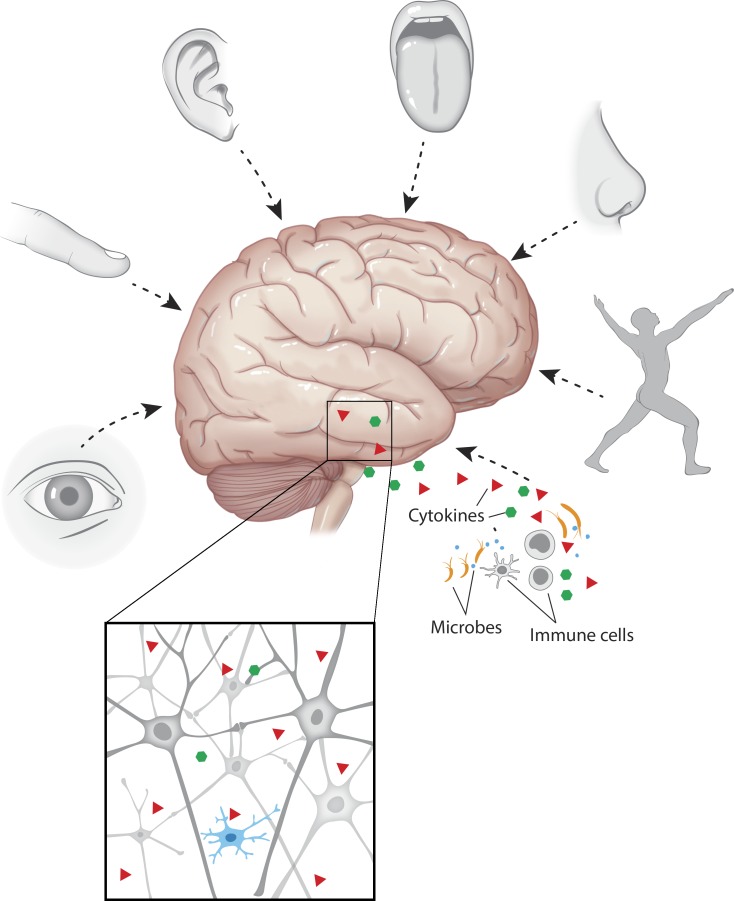
Why would nature disconnect two such vital systems from one another? We have evolved as multicellular organisms within an ocean of microorganisms. Presumably, the evolution of our immune system is what has allowed us to prevail. Our brain is a computing center, a supercomputer that constantly surveys our external and internal environments and responds to the plethora of cues they present (Fig. 1). We have five senses—visual, olfactory, gustatory, somatosensory, and auditory. In addition, the vagus nerve delivers information about our visceral organs to the brain, referred to by some as the sixth sense (Zagon, 2001; proprioception, a sense of position and movement, is also often referred to as the sixth sense; Smith, 2011). Senses are needed to report to the brain about the external (and internal) environment for it to compute activity to preserve the organism. But how does our supercomputer “sense” (and protect us from, when needed) the microorganisms that live within us (the commensals), surround us, or antagonistically invade us? Is it conceivable that the brain would give up on the ability to sense the world of microorganisms in which we survive?
Figure 1.
A schematic representation of the senses that are hardwired in the brain. The senses that protect the individual from external and internal perturbations through a contact delivery of information to the brain include the five senses, the proprioception, and the seventh sense—immune input. The peripheral immune cells detect microorganisms and deliver the information to the brain. Although neurons are the primary targets, when an excess of immune information is delivered (in pathology), microglia respond either as bystanders or as active players. (Note that arrows schematically indicate inputs of senses into the brain circuits but not the precise position of where each sense is being projected.)
I would like to propose that the defining role of the immune system is to sense the microorganisms and to deliver the necessary information about them to the brain. The immune response, therefore, should be hardwired in our brain, which makes the immune system our “seventh sense” (Fig. 1).
There are several examples of immune inputs affecting neural circuits. We have recently shown that IFN-γ, by directly affecting the inhibitory neuronal layers I/II, regulates circuits underlying social behavior (Filiano et al., 2016). IL-17 has been implemented in sensory function (Chen et al., 2017) and in social behavior (Shin Yim et al., 2017). The role of TNF and IL-1 in affecting neural circuits was demonstrated years ago (Stellwagen and Malenka, 2006; Prieto et al., 2015). This is just a partial list of immune signaling molecules affecting neuronal function.
Holding a conversation in a noisy place or with impaired hearing is difficult. Food tastes different when we cannot smell it or feel its texture. The brain receives such sensations as stimuli and computes its responses, but all relevant circuits are interconnected so that interference with one alters the function of others (and of the brain as a unit). Some circuits have more interconnections than others, and thus the impact of their disturbance will be more widespread. When we are sick, for example, whether we are suffering from a minor cold or a more serious infectious illness, we feel weak and sleepy, and our appetite is depressed. Sickness in children usually affects their behavior, making them more inclined to be comforted by cuddling, whereas the effect of a similar pathogen on adult patients may result in withdrawal behavior. Although the circuits modulating such behaviors are similar, the immune input (immune neuromodulation) in children and adults may differ sufficiently to change their behavioral manifestations from one extreme to the other, yet in all cases molecules derived from immune cells are implicated as potential modulators of brain function. Sickness behavior could thus be viewed, for example, as an overwhelming input into the brain via the seventh sense, resulting in interference with other circuits that receive the inputs. Similarly, an impaired or dysfunctional immune system could lead to abnormal consequences. Failure to properly sense the pertinent microorganisms (pathological, commensal, or both) might trigger an altered immune response, with adverse impact on brain function.
This unified theory of neuroimmune interactions could explain why the elimination of certain types of immune cells alters behaviors (Kipnis et al., 2004; Ziv et al., 2006; Derecki et al., 2010) in ways that are similar to those resulting from overactivation of the immune system (Dantzer and Kelley, 2007; Godbout et al., 2008; Moreau et al., 2008; Fu et al., 2010). It could also explain why microglia, the only parenchymal resident immune cells, are associated with many (if not all) neurodegenerative conditions (Prinz et al., 2017). Spillover of the immune signals aimed at neurons may be received by microglia. Microglia may respond to these signals and impact neural function or simply be an activated bystander that indicates an abnormal immune input but does not impact the progression of the disease.
The suggestions above are enigmatic because we have yet to recapitulate the neuroimmune connectome—a detailed map of connections, interactions, and interdependencies between different immune cell–derived molecules (mostly cytokines) and neural circuits. Once the connectome emerges through empirical (single-cell sequencing) and theoretical/mathematical modeling (clustering of cytokine receptors that may predict circuits most susceptible to particular immune inputs) approaches, it can be expected to yield a better understanding of the anatomical and functional organization of the seventh sense and its targeted circuits. By recognizing and unraveling the phenomenon of the neuro–immune axis as a structural code of the brain, we will get closer to understanding the essence—etiology, course, and potential therapies—of many neurological diseases.
Acknowledgments
I would like to thank S. Smith for editing the manuscript and Anita Impagliazzo for the art work. I also thank all the members of my lab for their valuable comments during multiple discussions of ideas presented here.
This work was supported by grants from the National Institutes of Health (AG034113, AG057496, and NS096967).
The author declares no competing financial interests.
References
- Chen C., et al. Nature. 2017 doi: 10.1038/nature20818. [DOI] [Google Scholar]
- Dantzer R., and Kelley K.W. Brain Behav. Immun. 2007 doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki N.C., et al. J. Exp. Med. 2010 doi: 10.1084/jem.20091419. [DOI] [Google Scholar]
- Filiano A.J., et al. Nature. 2016 doi: 10.1038/nature18626. [DOI] [Google Scholar]
- Fu X., et al. J. Neuroinflammation. 2010 doi: 10.1186/1742-2094-7-43. [DOI] [Google Scholar]
- Godbout J.P., et al. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301649. [DOI] [Google Scholar]
- Kipnis J., et al. Proc. Natl. Acad. Sci. USA. 2004 doi: 10.1073/pnas.0402268101. [DOI] [Google Scholar]
- Moreau M., et al. Brain Behav. Immun. 2008 doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto G.A., et al. Proc. Natl. Acad. Sci. USA. 2015 doi: 10.1073/pnas.1514486112. [DOI] [Google Scholar]
- Prinz M., et al. Nat. Immunol. 2017 doi: 10.1038/ni.3703. [DOI] [Google Scholar]
- Shin Yim Y., et al. Nature. 2017 doi: 10.1038/nature23909. [DOI] [Google Scholar]
- Smith R. Gesnerus. 2011 [PubMed] [Google Scholar]
- Stellwagen D., and Malenka R.C. Nature. 2006 doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Zagon A. Trends Neurosci. 2001 doi: 10.1016/S0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- Ziv Y., et al. Nat. Neurosci. 2006 doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]